3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(13):3229-3241. doi:10.7150/ijms.112264 This issue Cite
Research Paper
Cardiovascular and brain effects of liraglutide in transthyretin amyloidosis (ATTR) mice models
Department of Endocrinology and Metabolism, Peking University People's Hospital, Beijing, China.
*Contributed equally to this work.
Received 2025-2-16; Accepted 2025-6-10; Published 2025-7-10
Abstract
Aim: The effects of glucagon-like peptide-1 receptor agonists (GLP-1RAs) in hereditary transthyretin amyloidosis (ATTRv) remain uncertain. This study aims to investigate whether liraglutide interacts with transthyretin protein (TTR) and thereby exerts therapeutic effects for ATTRv.
Methods: High throughput screening was conducted to characterize the drug targets of liraglutide, and microscale thermophoresis was used to observe direct binding of liraglutide to TTR. Humanized RBP4/TTR (normal)and RBP4/TTRVal50Met (ATTRv) mice were constructed, and treated with liraglutide (0.3mg/kg/d) or placebo for 28 days. Fasting plasma glucose, intraperitoneal glucose tolerance test (IPGTT), and plasma brain natriuretic peptide (BNP) were measured. Brain and cardiac tissues were processed with western blot, enzyme-linked immunosorbent assay (ELISA), real-time quantitative polymerase chain reaction (PCR), and pathological staining to evaluate the lesion status in corresponding organs.
Results: Liraglutide exhibited high affinity and direct combination ability to TTR. In ATTRv mice, liraglutide significantly decreased the contents of TTR protein in brain compared with placebo. However, the cardiovascular prognosis measurements including heart failure (plasma BNP concentrations), cardiac fibrosis (the relative expression levels of Cola1 and TGFβ1 in cardiac tissues), and pathological changes (right ventricular collagen percentage, ventricular septum thickness, left ventricular wall thickness, and left ventricular internal diameter) were statistically comparable between mice receiving liraglutide and placebo treatment.
Conclusion: Liraglutide could decrease the deposition of TTR in brain tissues, while it did not improve cardiovascular outcomes in ATTRv mice compared to placebo. More researches regarding the mechanisms and therapeutic effects of GLP-1RAs to ATTRv are still required.
Keywords: ATTRv, liraglutide, brain, heart failure, transthyretin amyloidosis polyneuropathy, transthyretin cardiac amyloidosis
Introduction
Transthyretin (TTR) serves as an important carrying protein for thyroxine (T4) and retinol binding protein 4 (RBP4), which is mainly synthesized in liver. [1,2] Mostly, TTR exists in serum by the form of tetramers. [2] When the structure of TTR monomers is disrupted, the stability of TTR tetramers would severely decline, which promotes the dissociation of TTR tetramers, aggregation of TTR monomers, and subsequent formation of amyloid in tissues, leading to transthyretin amyloidosis (ATTR). [3]
ATTR was considered a relative rare disease precedingly. While with the improvements of diagnostic methods, the prevalence of ATTR increased correspondingly. So far, there are two ATTR subtypes namely age-related ATTR (ATTR wild type, ATTRwt) and hereditary transthyretin amyloidosis (ATTR variant, ATTRv). [1] ATTRwt is primarily mediated by aging-related oxidative stress and cells injury, with a median onset age of 70-75 and prevalence more than 25%. [4] For ATTRv, the estimated global prevalence reached 10,186 people (range 5526-38,468), [5] of which more than 150 potential mutation sites have been identified. ATTR comprises a wide range of clinical manifestations including polyneuropathy (ATTR-PN) and cardiomyopathy (ATTR-CM), as well as mixed phenotypes. [6] For ATTRv, of all the mutation sites, p. Val50Met mutation is the most frequent currently, primarily causing a neuropathic phenotype and mixed phenotypes. [7, 8]
Glucagon-like peptide-1 receptor agonist (GLP-1RA) represents a novel class of anti-hyperglycemic medication, which exerts glucose lowering effects by stimulating insulin secretion, suppressing glucagon release, inhibiting hepatic gluconeogenesis, and restraining appetite. [9] In addition to its hypoglycemic effect, GLP-1RA also affects central nervous system, reaching the pathological regions of several neurodegenerative diseases especially Alzheimer's disease (AD) or Parkinson's disease (PD), exerting neuroprotective effects. [10] It was demonstrated that liraglutide could introduce neuroprotective effects in patients at high risk of AD, possibly by improving glucose metabolism and functional connectivity in brain cells. [11] Meanwhile, it was also indicated that liraglutide could reduce the load of amyloid-beta plaques in the hippocampus and decrease astrogliosis in the cerebral cortex of mice models with AD. [12] This effect may be mediated through the activation of the phosphoinositol 3-kinase/Akt (PI3K/Akt) pathway, which enhanced the presence of amyloid-beta transporters in cerebrospinal fluid, promoting the and clearance of amyloid-beta. [13]
Meanwhile, in respect to the cardiovascular effects of GLP-1RAs, since several cardiovascular outcome trials (CVOTs) have demonstrated that the cardio-protective effects of GLP-1RAs were independent with glycemic control or weight loss [14-16], GLP-1RAs have been proved with specific cardiovascular benefits. [17,18]
However, whether GLP-1RAs would contribute to the treatment of neurological and cardiovascular lesions in ATTRv remain unexplored. The aim of this study is to investigate whether liraglutide would treat amyloidosis-related neural and cardiovascular conditions caused by ATTRv, and to further characterize the corresponding underlying mechanisms.
Materials and methods
Materials
TTR protein was purchased from sinobiological company (12091-H08H, Beijing, China). Liraglutide was purchased from MedChemExpress company (HY-P0014, New Jersey, USA).
High throughput targets screening
To validate whether liraglutide would mediate brain and cardiovascular benefits for ATTRv by potentially interacting with TTR or other targets, we implemented high throughput targets screening for liraglutide. The screening platform was Discovery Studio 2017R2 (DS; BIOVIA-Dassault Systemes). The receptor and ligand package generation module within DS was utilized to generate Multi-Target Binding Motifs (MBMS). The optimized form of liraglutide was then linked to its respective potential target through ligand binding, when the ligand score would be subsequently calculated. These computational analyses were performed on an Intel® Xeon® E5-2699 v3 2.30 GHz octadeca-core processor running Windows 8.1 operating system.
Microscale Thermophoresis
To characterize the direct interaction between liraglutide and TTR, we employed microscale thermophoresis using the Monolith NT.115 instrument (NanoTemper Technologies). Fluorescent labeling of TTR proteins was performed following the manufacturer's protocol.
Different concentrations of liraglutide, ranging from 1.0E-10 M to 1.0E-4 M (10 nM to 10 μM) on a logarithmic scale, were prepared in binding buffer. After incubation, samples were loaded into Monolith NT.115 Series Capillaries (Nanotemper, Germany) and subjected to microthermophoresis using Medium MST-Power and 20% of Excitation-Power. Equilibrium dissociation constant (Kd) values were calculated from repeated measurements using the mass action equation provided by the NanoTemper software. During the experiment, the migration rate of the fluorescently labeled TTR protein in response to a temperature gradient was measured to assess thermophoresis. The migration rate of the labeled TTR is inversely proportional to its binding affinity to liraglutide.
Establishment of Animal Models
This study was ethically approved by the Institutional Animal Care and Use Committee (IACUC) of Peking University People's Hospital (No.2020PHE008). All procedures were performed in accordance with the guidelines of National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. [19]
Humanized RBP4/TTR (normal) and RBP4/TTRVal50Met (ATTRv) mice models were constructed in the Laboratory of Department of Endocrinology, Peking University People's Hospital and Beijing Cyagen Biological Science Co., LTD [20]. The model construction procedures, which utilized CRISPR-Cas9 mRNA to knock in humanized RBP4/TTR or RBP4/TTRVal50Met genes into C57BL/6 zygotes followed by multi-generation breeding, were detailed in our previous research. For this study, 16-week-old male mice (range 13-19 weeks) with (Rbp4[KI/KI], Ttr[KI/KI]) and (Rbp4[KI/KI], Ttr[p.V50M][KI/KI]) genotypes were randomly assigned into two groups. Normal RBP4/TTR mice (n=6) received placebo treatment, while RBP4/TTRVal50Met (ATTRv) mice were further randomized to receive either liraglutide (n=6) or placebo (n=6) treatment for a total of 28 days. Liraglutide was administered via intraperitoneal injection at a dose of 0.3mg/kg/day dissolved in normal saline, with a final solution volume of 10ml/kg/day based on the body weight of each mouse. When placebo administration was an intraperitoneal injection of equivalent dose of normal saline (10ml/kg/day). Day 0 (D0) was defined as the initiation day of the experiment, followed by daily administration until the completion at day 28 (week 4). Mice were housed at a constant temperature of 26℃ with a 12:12 hours light-dark cycle, with accommodations for 3-5 mice per cage. Unlimited water and fodders supplies were provided to each mouse. At the end of treatment (week 4), all the mice were sacrificed by cervical dislocation disposal. Cardiac, liver, and brain tissues were immediately excised and rapidly frozen in dry ice to preserve their integrity. The frozen tissues were then transferred and stored at -80°C to maintain their frozen state for subsequent analysis.
Fasting plasma glucose and intraperitoneal glucose tolerance test (IPGTT)
Fasting plasma glucose and IPGTT were measured at D0, Week 2, and Week 4. Before the assay, mice should fast for 12 hours. Blood sample collection was implemented with the tail cutting methods. Fasting blood glucose level was tested priorly, and after acclimatizing for 30 minutes, an intraperitoneal injection of glucose (5% solution) was administered with the dose of 10ml/kg. Blood glucose measurements were repeated at the timing of 15 min, 30 min, 60 min, 90 min, and 120 min post-injection. Blood glucose was measured with automatic glucometer (Yuwell Medical Equipment Co., Ltd, China).
Pathological staining
At week 4, cardiac specimens from each mouse were fixed with paraformaldehyde (PFA) followed by paraffin embedding. Cardiac tissues were then separated as right ventricle, ventricular septum, and left ventricle. Masson's trichrome staining was performed on the right ventricular myocardium to investigate ratio of collagen fiber deposition in the right ventricle, while hematoxylin-eosin (HE) staining was used for ventricular septum and left ventricle to measure the required parameters including ventricular septum thickness, left ventricular wall thickness, and left ventricular internal diameter. Brain tissue from mice was also fixed and paraffin embedded for pathological biopsy. Congo red staining was utilized to assess the percentage of amyloidosis in brain tissue sections. The area of Congo red-positive regions was measured as a percentage of the total section area using Image-Pro Plus 6.0 software (Media Cybernetics Inc., Rockville MD).
Immunoblotting
At week 4, western blots were implemented to determine the protein levels of brain natriuretic peptide (BNP), transforming growth factor-beta (TGF-β), and collagen type I alpha 1 (COL1A1) in cardiac tissues (β-tubulin for internal reference), as well as the protein levels of TTR in liver and brain tissues (β-actin for internal reference). The samples were homogenized in lysis buffer (mixture of Tris-HCl pH 8.0, 50 mmol/L of sodium chloride, 1.0% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and protease inhibitors), and the protein concentration was determined using a bicinchoninic acid (BCA) protein assay kit (Beyotime Biotechnology Co., Ltd, China) after homogenate centrifugation. To ensure accurate protein concentration measurement, a portion of the non-boiled samples (50 μL) was taken, boiled with loading buffer, and then measured. Following concentration determination, equal amounts of protein (50 μg) from each sample were adjusted and loaded onto SDS-PAGE gels.
For liver tissue samples, which were aimed at assessing TTR synthesis levels, proteins were denatured by heating in loading buffer at 95°C for 10 minutes. This denaturation step was necessary to linearize the proteins for accurate quantification of TTR expression. Non-reducing SDS-PAGE was performed without heat denaturation for brain tissue samples. This approach allowed us to differentiate between monomeric and multimeric forms of TTR, providing insights into the native state of TTR in the brain.
Equal amounts of 50 μg of protein were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12% or 15% polyacrylamide gels). The gel was then transferred onto a polyvinylidene difluoride (PVDF) membrane using a transfer membrane apparatus (Millipore Trading Co., Ltd, USA) at 4 °C, applying a voltage of 100V/100mA for two hours. After blocking the membrane with tris buffered saline tween (TBST) blocking solution containing 5% skim milk powder, the samples were incubated overnight at 4 °C with the corresponding primary antibodies: BNP (Affinity Biosciences Co., Ltd, Australia), TGF-β (Huaan Bioscience Co., Ltd, China), COL1A1 (Abcam Trading Co., LTD., China), β-tubulin (Beijing Solarbio Science & Technology Co., Ltd, China), TTR (Abmart, China), and β-actin (Abmart, China). The membrane was then incubated with horseradish peroxidase (HRP) conjugated secondary antibodies (Beyotime Biotechnology Co., Ltd) (diluted with TBST blocking solution: Tris 10mM, NaCl 150mM, 0.05% Tween-20, pH 7.5) for 2 hours at 37 °C on a shaker.
To ensure equal protein loading across lanes, we used pre-stained molecular weight markers to monitor the transfer and to standardize the amount of protein loaded per lane. Detection of protein bands was achieved using a chemiluminescent substrate (ECL), and the signals were captured using a chemiluminescence imaging system (ImageQuant LAS 4000). The protein contents were measured with grey-scale scanning software (ImageJ 1.48, NIH) and were normalized to the corresponding internal references.
Enzyme-linked immunosorbent (ELISA) assays
Serum BNP concentrations were measured by enzyme-linked immunosorbent assay (ELISA) at week 0, 2 and 4 after treatment. Blood sample was collected from the tail vein of mice. BNP was measured by mouse BNP kit (Wuhan Hualian Biotechnology Co., LTD., China), and all steps were performed in accordance with the instructions of each manufacturer.
Real-time quantitative polymerase chain reaction (RT-qPCR)
The gene expression levels of TTR, RBP4 in liver tissues were quantified using RT-qPCR. The RNA was extracted from liver tissues by Trizol (Invitrogen Trading Co., Ltd, USA) according to the protocols. RNA samples were reversely transcribed into cDNA thereafter and then into RT-qPCR amplification (Roche LightCycler® 480II, Roche Trading Co., Ltd, Switzerland) using the SYBR GREEN dye method. Specific primers for TTR, RBP4, and the housekeeping gene HPRT were designed to evaluate the mRNA levels. CT (cycle threshold) was defined as the minimum cycle turns for nucleic acid fluorescence signal to be detectable. Following the reaction, expression quantities of the above genes were calculated and rectified with HPRT as relative quantities through 2-ΔΔCT methods.
TTR Stability Assay
TTR stability assay was performed to evaluate the influence of liraglutide on TTR tetramer stability. TTR monomers protein (3.6μM) were added to the experimental plate, when control plate was with equal quantities of normal saline. Both experimental and control plates were then incubated with liraglutide mixture (pH adjusted to 4.4) at 30°C for 25 minutes. The eventual concentrations of liraglutide were 0, 1.8, 3.6, 5.4 and 7.2 μM. The samples were further incubated at a temperature of 37°C for a period of 72 hours, when optical density was measured using UV-visible spectrometer at a wavelength of 245nm. The solution turbidity can indicate amyloid fibril formation. [21] In general, turbidity can be expressed as optical density, and turbidity is in correspondence with particular optical density at each wavelength. [22] Therefore, we calculated the difference in optical density between the experimental group and the control group at the same concentration of liraglutide to assess the depolymerization of TTR tetramer and the formation of TTR fibrils. An increase in optical density indicates an increase in the formation of TTR fibrils, which is a result of TTR depolymerization.
Statistical analysis
The results of western blots were assessed and calculated through measurements performed using Image J software (NIH, Bethesda, MD, USA). Subsequently, data visualization was accomplished by creating graphs with Prism 8 software (GraphPad Software Inc., San Diego, CA, USA). Statistical analyses were implemented with IBM SPSS Version 26.0. For comparisons involving more than two groups, analysis of variance (ANOVA) was used, followed by Bonferroni's test for multiple comparisons. For comparisons between two groups, Student's t-test was used. Results were presented in the format of mean ± standard deviation (SD). Statistical significance was considered at P < 0.05.
Results
In our study, humanized RBP4/TTR mice (mice with humanized transthyretin and retinol binding protein 4) and RBP4/TTRVal50Met mice (mice with hereditary transthyretin amyloidosis) were established and divided into three groups by different genotypes and treatment strategies. A total of 18 mice were included and all experiment procedures were completed. Baseline characteristics were balanced among 3 treatment groups (Table S1).
TTR interacted with liraglutide
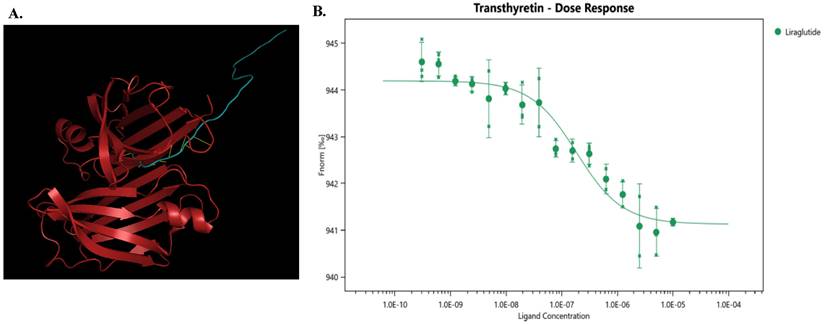
High-throughput screening was used to identify potential targets of liraglutide, and TTR showed a ligand score of 2.98349 (Figure 1A), suggesting that TTR might be a promising target for liraglutide.
To further investigate the interaction between liraglutide and TTR, we conducted microscale thermophoresis experiments. With an increasing concentration of liraglutide, the TTR migration rate gradually increased, following an 'S'-shaped fitting curve. Moreover, the equilibrium dissociation constant (Kd) for liraglutide was 1.569E-07± 6.37E-08, indicating a direct combination between liraglutide and TTR (Figure 1B).
Fasting glucose and intraperitoneal glucose tolerance test
In ATTRv mice, no significant difference was found in fasting plasma glucose at week 0, 2, 4 among liraglutide and placebo groups (Table S1). On Day 29, the intraperitoneal glucose tolerance test (IPGTT) revealed a significant difference in glucose tolerance between the ATTRv placebo group and the RBP4/TTR liraglutide-treated group. As depicted in Figure 2A, the IPGTT curve for the ATTRv placebo group (red line) showed higher blood glucose levels across all time points compared to the RBP4/TTR liraglutide-treated group (black line). This was further confirmed by the area under the curve (AUC) analysis presented in Figure 2B, where the RBP4/TTR liraglutide-treated group exhibited a significantly lower AUC value (p < 0.05) compared to the ATTRv placebo group. These results indicate that liraglutide treatment significantly improves glucose tolerance in RBP4/TTR mice compared to the ATTRv placebo group. More relevant results were exhibited in Figure 2A-2B and Table S1.
The effects of liraglutide on TTR monomers deposition and amyloidosis in mouse brain
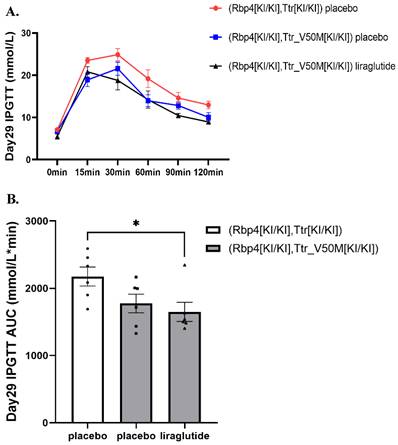
It was indicated that in mice with placebo treatment, the TTR protein levels in the brain tissue of ATTRv mice increased compared with humanized RBP4/TTR mice (p=0.014). In addition, liraglutide treatment in ATTRv mice led to a reduction in the monomeric TTR protein levels in brain tissue, as compared to placebo (p=0.009) (Figure 3A-B). While for following Congo red staining of brain tissue, there was no notable variance observed in the area affected by amyloidosis across all treatment groups (Table S1 and Figure 3C).
(A) The model diagram of liraglutide and TTR, made by Pymol. Red indicates TTR protein, blue indicates liraglutide, and yellow indicates mutual binding. This model aids in understanding how liraglutide interacts with TTR, offering insights into its potential therapeutic effects at the molecular level. (B) Liraglutide and TTR microscale thermography experiment. Kd(M): 1.569E-07 ± 6.37E-08; Signal to Noise: 10.7. The determined equilibrium dissociation constant (Kd) is 1.569E-07 ± 6.37E-08 M, indicating a strong binding affinity between liraglutide and TTR. The signal-to-noise ratio of 10.7 suggests high reliability of the experimental results.
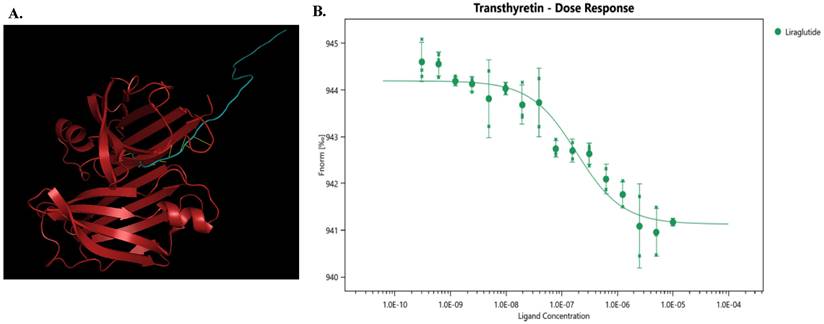
(A) The intraperitoneal glucose tolerance test (IPGTT) curve of mice on Day29 0-120min (n=6). The curve shows blood glucose levels at various time points, assessing glucose tolerance in mice. (B) Histogram of Day29 IPGTT area under the curve (AUC) data of mice (*: p<0.05; n=6). Statistically significant differences are marked with asterisks (: p<0.0.5), indicating significant variations in glucose tolerance among different treatment groups.
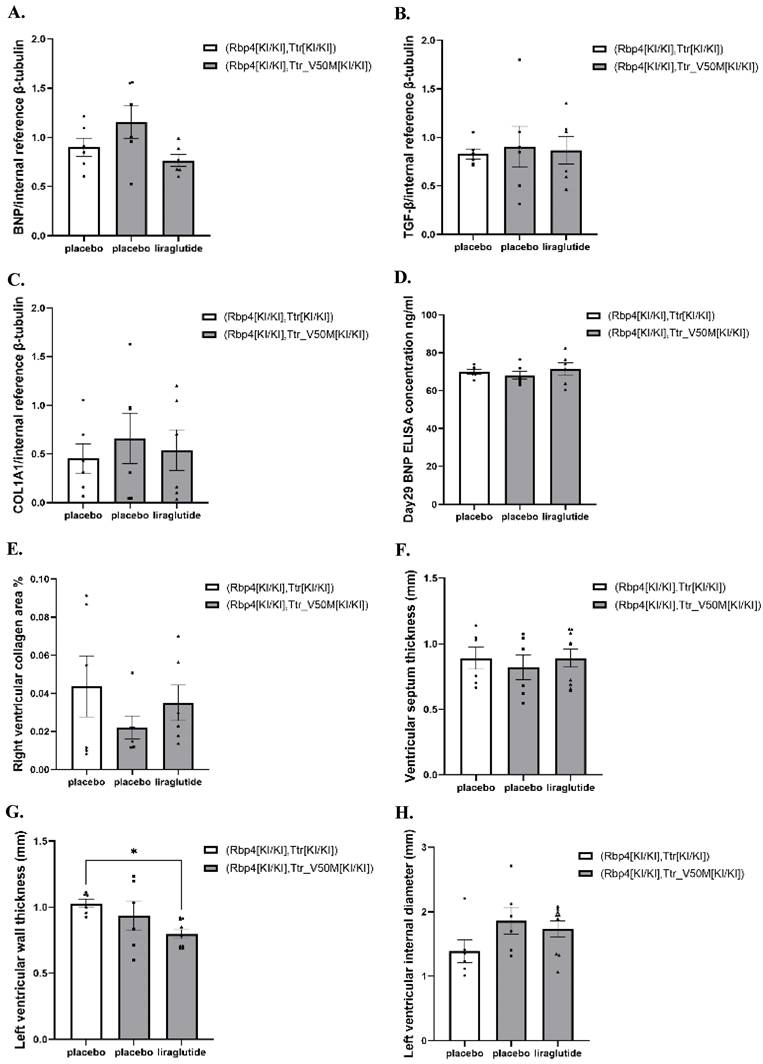
The effects of liraglutide on cardiac amyloidosis and heart failure in mice
In both normal and ATTRv mice, at week 4, the protein levels of BNP, TGF-β, and COL1A1 in the cardiac tissues did not exhibit significant changes (Table S1, Figure 4A-C and Figure S1-S2). Meanwhile, at week 4, in both normal and ATTRv mice, the plasma BNP concentrations were comparable among all treatment groups (Table S1 and Figure 4D). In addition, the pathological inspection indicated that the right ventricular collagen fiber deposition area, interventricular septum thickness, left ventricular wall thickness, and left ventricular diameter were comparable among ATTRv mice & liraglutide, ATTRv mice & placebo, and normal mice & placebo groups (Table S1 and Figure 4E-H).
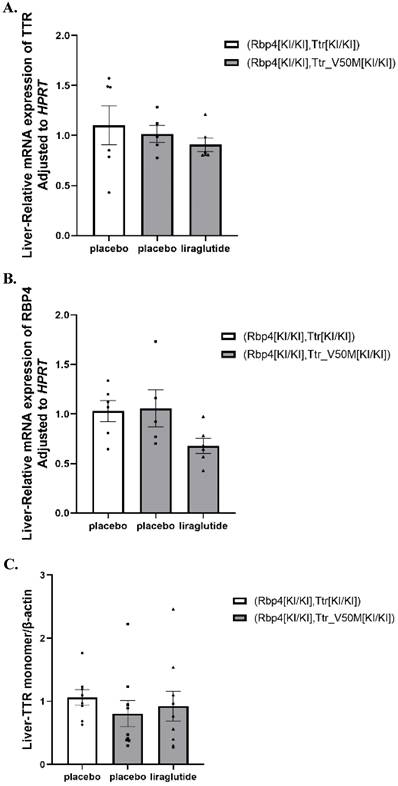
The effects of liraglutide on synthesis of TTR and RBP4 in mouse liver
No significant difference in the gene expression of TTR, RBP4, and the protein levels of TTR in the liver among all 3 treatment groups were identified (Table S1, Figure 5A-C and Figure S3-S4).
(A) Non-reducing SDS-PAGE of TTR monomer in mice brain (β-actin was boiled and used with SDS-PAGE). β-actin, boiled as an internal control, was used with SDS-PAGE to ensure consistent protein denaturation and quantification. (B) Results of TTR monomer protein expression in mice brain (*: p<0.05; **: p<0.01; n=6). Statistically significant differences are marked with asterisks (: p<0.05; **: p<0.01), indicating significant variations in TTR monomer expression among different treatment groups. (C) Congo Red positive area percentage of mouse brain tissue. Each experiment was repeated three times (n=6). Congo Red staining detects amyloid protein deposits, and the percentage of positive area reflects the severity of amyloidosis.
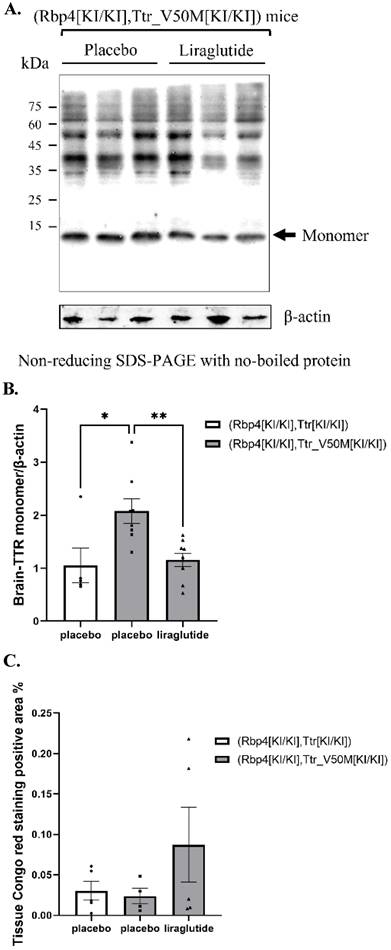
(A) Results of BNP protein expression in mice heart (n=6). (B) Results of TGF-β protein expression in mice heart (n=6). (C) Results of COL1A protein expression in mice heart (n=6). (D) Results of Day29 BNP concentration in mice serum (n=6). (E) Results of collagen fiber deposition area in the right ventricle of mice (n=6). (F) Results of ventricular septum thickness (n=6). (G) Results of left ventricular wall thickness (n=6). (H) Results of left ventricular internal diameter (n=6). These results assess the effects of liraglutide on cardiac function and structure.
(A) Results of TTR mRNA expression in mice liver (n=6). (B) Results of RBP4 mRNA expression in mice liver (n=6). (C) Results of TTR monomer protein expression in mice liver (n=6). These results evaluate the effects of liraglutide on liver TTR and RBP4 synthesis. These results evaluate the effects of liraglutide on liver TTR and RBP4 synthesis.
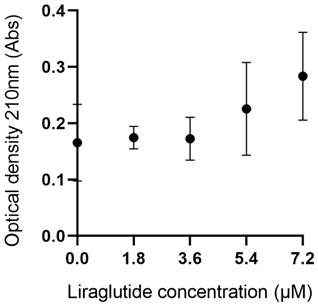
The effects of liraglutide on TTR stability
To assess the effect of liraglutide on TTR stability, we measured the optical density (OD) at 210 nm of TTR tetramers in the presence of various concentrations of liraglutide (0.0, 1.8, 3.6, 5.4, and 7.2 μM). As shown in Figure 6, the mean OD values were 0.166 at 0.0 μM, 0.175 at 1.8 μM, 0.173 at 3.6 μM, 0.226 at 5.4 μM, and 0.284 at 7.2 μM. Statistical analysis revealed no significant differences in TTR stability across the tested liraglutide concentrations (p > 0.05), indicating that the stability of TTR tetramers was not significantly influenced by the increase in liraglutide concentration.
Results of TTR stability experiment. The experiment measures changes in optical density of TTR tetramers in the presence of various concentrations of liraglutide, assessing the impact of liraglutide on TTR stability.
Discussion
As far as we know, this research utilized liraglutide in ATTR animal models for the first time, which is an emerging attempt to investigate the protective effects and underlying mechanisms of liraglutide in cardiovascular and nervous conditions raised by ATTR, and is a novel exploration for drug therapy in ATTR.
In this study, we found that TTR was a potential target of liraglutide, and successfully demonstrated that TTR had high affinity for liraglutide with direct binding. These discoveries provided novel insights for the potential mechanisms of liraglutide remedying ATTR, and would promote further pre-clinical and clinical investigations on this topic.
As mentioned above, liraglutide exhibited protective effects on several neuro-degenerative diseases. [10] Studies have found that liraglutide can enhance the Wnt/β-catenin signaling pathway in mouse brain tissue, then promote the expression of neurogenic markers and anti-apoptotic factors, and improve cognitive impairment. [23] In addition, liraglutide can also reduce the oxidative stress of neuronal mitochondria, then reduce the neuronal inflammatory response, and play a role in brain injury repair. [24,25] However, for ATTR-related central neural diseases (brain amyloidosis and degenerative lesions), no previous research evaluating the therapeutic potential of liraglutide has been conducted, and whether liraglutide would improve the prognosis in patients with ATTR related remains unclear. According to the results, we found a decrease in TTR monomer contents in ATTRv mice under liraglutide treatment compared with placebo, indicating improvement of brain TTR monomer deposition in protein levels. However, no significant decrease in amyloidosis area of ATTRv mice brain tissue were observed by Congo red stained sections, meaning that the evidence of brain amyloidosis improvement in histological levels remains lacking. Previous studies have identified nonfibrillar, Congo Red-negative transthyretin aggregates in early-stage familial amyloid polyneuropathy, indicating the presence of preamyloidogenic TTR species in nerves before fibril formation, whereas mature fibrils do not exhibit such cytotoxicity [26-28]. And this research suggests that the accumulation of nonfibrillar, Congo Red-negative TTR cytotoxic deposits—below the detectability threshold of Congo Red—may underlie early neuropathology [26-28].
This is further supported by evidence that prealbumin (TTR) is a common component of neurotic plaques in cerebral amyloidosis and that nonfibrillar, Congo Red-negative preamyloidogenic TTR species in nerves induce cytotoxic effects (including oxidative stress) in both animal models and human studies prior to the appearance of congophilic fibrillar amyloid [29-32]. Notably, it has also been suggested that amyloidosis area in pathological sections does not fully reflect the progression and severity of ATTR-PN. [33] Our research results aligned with these observations. Since liraglutide exhibited direct combining ability to TTR, it might improve the overall neural prognosis in patients with ATTR. More studies examining the specific effects of liraglutide on the pathological changes and neurological function in patients with ATTR are warranted in the future.
Notably, while the present study explored liraglutide's potential in modulating TTR metabolism for central nervous system pathologies, its broader therapeutic profile as a GLP-1RA—which has been extensively linked to cardiovascular protection via mechanisms such as anti-inflammation, endothelial function improvement, and atherosclerotic plaque stabilization—raises questions about whether its systemic effects on receptor signaling could underlie benefits across diverse organ systems, including both neural and cardiovascular tissues.
Previous research indicates that GLP-1RAs offer substantial cardiovascular advantages for individuals with or without T2DM, primarily by lowering the incidence of MACE (major adverse cardiovascular events) rates, which encompasses MI (myocardial infarction), stroke, and cardiovascular death. [34] Among the eight CVOTs (cardiovascular outcome trials) conducted on T2DM patients, five have reported positive outcomes [35-39], while three indicate neutral cardiovascular effects. [40-42] The HARMONY trial is particularly noteworthy as it implies that the cardiovascular benefits of GLP-1RAs might not be contingent upon weight reduction. [36]
GLP-1RAs also hold promise in the management of heart failure, especially HFpEF (heart failure with preserved ejection fraction), a condition prevalent among obese individuals and those with T2DM. [43] A meta-analysis encompassing over 60,000 T2DM patients from GLP-1RA CVOTs has revealed an 11% reduction in heart failure-related hospitalizations. [34] In a specific trial, semaglutide was found to alleviate HF-related symptoms and enhance exercise capacity in individuals with obesity, HFpEF, and T2DM. [44]
In the context of coronary artery disease, a pooled analysis of GLP-1RA CVOTs involving T2DM patients has indicated a 10% decrease in nonfatal MI. [34] This suggests that GLP-1RAs may possess anti-atherogenic properties that could influence various pathways associated with plaque development and progression.[45]
The SCALE Diabetes program and STEP 2 trial both have revealed that semaglutide enables a significant proportion of participants to achieve prediabetic HbA1c levels and improves various cardiovascular risk factors. [46,47] As a result, major diabetes associations have updated their guidelines to recommend GLP-1RAs for T2DM patients with established cardiovascular disease (CVD) or high-risk indicators, aiming to reduce the risk of MACE. [48-50]
The efficacy of GLP-1RA in improving cardiometabolic health in patients living with T2DM has spurred significant efforts toward developing next-generation therapies that surpass the effectiveness of GLP-1RA alone. [51] Novel dual agonists (targeting GLP-1R/GIPR such as tirzepatide) and triple agonists (targeting GLP-1R/GIP/GCG such as retatrutide) have demonstrated enhanced effects on weight loss and metabolic health outcomes in obesity, T2DM, and CVD. [52] In the SURPASS trials, tirzepatide has showed more pronounced reductions in TG, LDL-C, VLDL-C, and systolic blood pressure compared to semaglutide, while also lowering inflammatory markers in a Phase 2 trial. [53-57] Retatrutide, in a Phase 2 trial, has showed a dose-dependent decrease in systolic blood pressure. [58] Ongoing trials like SURPASS-CVOT and TRIUMPH-2/3 aim to systematically evaluate the safety profiles and cardiometabolic benefits of these agonists in T2DM and CVD populations. [59] The primary endpoints will focus on time to first occurrence of MACE and other relevant clinical outcomes, helping clarify whether their benefits arise from enhanced weight loss or direct receptor-mediated effects on metabolic organs. [59-61]
As clinical evidence robustly supports the cardiometabolic benefits of GLP-1RAs across diverse patient populations, their mechanistic basis, which encompasses both systemic metabolic improvements and direct cellular interactions, has become a focal point of research. GLP-1RAs mediate their effects through the GLP-1 (glucagon-like peptide 1) class B G protein-coupled receptor, which is broadly expressed in metabolic tissues such as pancreatic β-cells and cardiovascular structures including cardiac myocytes and vascular smooth muscle cells. [62-65] The cardiovascular advantages of GLP-1RAs may derive from dual pathways: indirect benefits via improved glycemic control and weight management in type 2 diabetes patients, and direct interactions with the cardiovascular system, supported by GLP-1 receptor expression in cardiac and vascular cell types. [64,65] In preclinical investigations using atherosclerosis-susceptible mouse models, GLP-1RAs such as semaglutide and liraglutide have been shown to mitigate atherosclerotic plaque progression independent of weight loss or cholesterol reduction, potentially through anti-inflammatory mechanisms like inhibition of Toll-like receptor-induced inflammation. [66-68] Furthermore, in nondiabetic hypertensive mice, liraglutide normalizes blood pressure, reduces cardiac hypertrophy, vascular fibrosis, and endothelial dysfunction in a GLP-1 receptor-dependent manner, while protecting against myocardial ischemia-reperfusion injury via endothelial GLP-1 receptor signaling; this was validated by blunted cardioprotective effects in mice with endothelial-specific ablation of the GLP-1 receptor gene. [65, 69-71]
Though preclinical and clinical evidence strongly supports GLP-1RA-mediated cardiovascular protection through multi-mechanistic pathways in diverse models, the specific effects of liraglutide on cardiovascular endpoints in TTR-related cardiac pathologies including myocardial fibrosis and heart failure remain underexplored. In our study, several correlative indexes were also measured to evaluate the cardiovascular outcomes in mice with liraglutide treatment. It is well known that TGF-β serves as a major cytokine whose activation aggravates cardiac fibrosis and hypertrophy. [26] Similarly, COL1A1 promotes pro-collagen synthesis and collagen fibers formation extracellularly, which could also be used as an indicator for cardiac fibrosis. [27] Meanwhile, BNP as a recognized marker, is also an authentic measurement for degrees of heart failure [28,72], and pathological sections can be used to observe the presence of cardiac mal-remodeling. Therefore, we observed the contents of TGF-β, COL1A1, BNP proteins in heart tissues, and investigated pathological sections of the cardiac of mice to observe the therapeutic effect of liraglutide in respect to myocardial fibrosis and heart failure. Serum BNP was also measured for evaluation of heart failure progression and severity. However, the results revealed no significant difference in the degree of cardiac fibrosis and the severity of heart failure after liraglutide treatment. This observation might be attributed to the relatively short observation period in our study, which could have been insufficient to detect relevant cardiovascular outcomes in the mice. Therefore, further researches investigating the efficacy of liraglutide in ATTR-CM with longer study periods and more outcome evaluating aspects are still required.
Moreover, TTR proteins are mainly synthesized in the liver, and exendin-4 (a GLP-1 analogue) was elucidated with the ability to influence the expression of multiple microRNAs in liver cells and thereby correspondingly adjust hepatic metabolism and biological synthesis [73]. In order to investigate whether liraglutide affects the synthesis process of TTR, we conducted several in-depth investigations. The results showed that compared with placebo, the effects of liraglutide on liver TTR synthesis in ATTRv mice did not reach a statistical difference, and the regulatory role of GLP-1RA in TTR synthesis of liver needs further research.
The instability of TTR tetramer was also thought to be a key step in the formation of ATTR related amyloidosis, which has promoted the discovery and development of TTR tetramer stabilizers. The clinical efficacy of TTR tetramer stabilizers diflunisal and tafamidis in treatment of ATTR has been confirmed in previous studies [1,74], and we therefore further explored whether liraglutide could similarly influence the stability of TTR tetramers. By stability assay, we observed comparable optical density of TTR tetramers solution before and after incubation with liraglutide, which indicated that liraglutide might not significantly influence the stability of TTR tetramers. While since TTR tetramers showed high affinity and direct bond to liraglutide, further researches elucidating the underlying biological effects of their combination should be carried out.
Our study presents several limitations that should be considered when interpreting the findings. Firstly, the use of only male mice in our experiments precludes the assessment of potential gender differences in response to liraglutide treatment. Future studies should include both male and female mice to provide a more comprehensive understanding of the drug's effects across sexes. Secondly, since the dose or duration of liraglutide treatment may not be optimal for observing beneficial effects in this model, the results should be interpreted with caution, and additional researches with varying treatment regimens are warranted. Thirdly, the observation period in our study was insufficient to detect relevant cardiovascular outcomes in the mice, which may have contributed to the lack of significant findings in cardiac fibrosis and heart failure severity. Meanwhile, since the findings in this study were primarily negative, we did not implement subsequent explorations of promising downstream pathways involved in liraglutide for TTR deposition. Nevertheless, as the brain TTR contents were reduced after liraglutide treatment in ATTR mice models, it is meaningful to further investigate the underlying pathways. More researches regarding the potential mechanisms for GLP-1RA improving ATTR are still warranted.
Despite these limitations, our study provides valuable insights as the first investigation into the effects of liraglutide on brain and cardiovascular conditions associated with ATTRv. The findings highlight the need for further research to validate our observations and to explore the potential efficacy and safety of GLP-1RAs in the treatment of ATTR.
Conclusion
In this study, TTR was found to be a potential target of liraglutide, and the direct interaction between them was also observed. In ATTRv mice, liraglutide significantly reduced the contents of TTR monomers in brain tissues compared with placebo, while the specific benefits towards neural function or pathological changes warrant further exploration. However, there was no evidence that liraglutide improved the cardiovascular prognosis of mice with ATTRv. More researches to validate these findings and demonstrate the underlying mechanisms are still required.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
Funding
This work was supported by the National Natural Science Foundation of China (No. 81970698) and the Natural Science Foundation of Beijing Municipality (No. 7202216). The funding agencies had no roles in the study design, data collection or analysis, decision to publish, or preparation of the manuscript.
Ethics approval
This study was ethically approved by the Institutional Animal Care and Use Committee (IACUC) of Peking University People's Hospital (No.2020PHE008).
Availability of data and material
All available data in this study were summarized in the manuscript and supplementary materials.
Authorship
Mengqing Zhang made substantial contributions to perform the experiment, analysis of data and draft the manuscript. Zonglin Li contributed to perform the experiment, analysis of data and was in charge of drafting the manuscript. Xiaoling Cai was in charge of the conception, design and analysis of data, and solved discrepancies during the experiments and manuscript drafting. Fang Lv contributed to performing the experiment. Xin Wen was involved in the design and conduct of the experiment. Chengcheng Guo was involved in performing the experiment. Chu Lin was involved in drafting the manuscript. Linong Ji directed all processes in the experiments and manuscript writing. Each author participated sufficiently in the work to take responsibility for appropriate portions of the content, and agreed to publish the manuscript publicly.
Competing Interests
L.J. has received fees for lecture presentations and for consulting from AstraZeneca, Merck, Metabasis, MSD, Novartis, Eli Lilly, Roche, Sanofi-Aventis, and Takeda. All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding authors) and declare no other support from any organization for the submitted work other than that described above.
References
1. Ueda M. Transthyretin: Its function and amyloid formation. Neurochem Int. 2022;155:105313 doi:10.1016/j.neuint.2022.105313
2. Vieira M, Saraiva MJ. Transthyretin: a multifaceted protein. Biomol Concepts. 2014;5(1):45-54 doi:10.1515/bmc-2013-0038
3. Griffin JM, Rosenthal JL, Grodin JL, Maurer MS, Grogan M, Cheng RK. ATTR Amyloidosis: Current and Emerging Management Strategies: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncology. 2021;3(4):488-505 doi:10.1016/j.jaccao.2021.06.006
4. Porcari A, Fontana M, Gillmore JD. Transthyretin cardiac amyloidosis. Cardiovasc Res. 2023;118(18):3517-3535 doi:10.1093/cvr/cvac119
5. Schmidt HH, Waddington-Cruz M, Botteman MF. et al. Estimating the global prevalence of transthyretin familial amyloid polyneuropathy. Muscle Nerve. 2018;57(5):829-837 doi:10.1002/mus.26034
6. Ioannou A, Fontana M, Gillmore JD. Patisiran for the Treatment of Transthyretin-mediated Amyloidosis with Cardiomyopathy. Heart Int. 2023;17(1):27-35 doi:10.17925/HI.2023.17.1.27
7. Knight DS, Zumbo G, Barcella W. et al. Cardiac Structural and Functional Consequences of Amyloid Deposition by Cardiac Magnetic Resonance and Echocardiography and Their Prognostic Roles. JACC Cardiovasc Imaging. 2019;12(5):823-833 doi:10.1016/j.jcmg.2018.02.016
8. Dardiotis E, Kyriakides T. Drug and Gene Therapy for Treating Variant Transthyretin Amyloidosis (ATTRv) Neuropathy. Curr Neuropharmacol. 2023;21(3):471-481 doi:10.2174/1570159X21666221108094736
9. Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab. 2021;46:101102 doi:10.1016/j.molmet.2020.101102
10. Salameh TS, Rhea EM, Talbot K, Banks WA. Brain uptake pharmacokinetics of incretin receptor agonists showing promise as Alzheimer's and Parkinson's disease therapeutics. Biochem Pharmacol. 2020;180:114187 doi:10.1016/j.bcp.2020.114187
11. Watson KT, Wroolie TE, Tong G. et al. Neural correlates of liraglutide effects in persons at risk for Alzheimer's disease. Behav Brain Res. 2019;356:271-278 doi:10.1016/j.bbr.2018.08.006
12. Holubová M, Hrubá L, Popelová A. et al. Liraglutide and a lipidized analog of prolactin-releasing peptide show neuroprotective effects in a mouse model of β-amyloid pathology. Neuropharmacology. 2019;144:377-387 doi:10.1016/j.neuropharm.2018.11.002
13. Wiciński M, Socha M, Malinowski B. et al. Liraglutide and its Neuroprotective Properties-Focus on Possible Biochemical Mechanisms in Alzheimer's Disease and Cerebral Ischemic Events. Int J Mol Sci. 2019;20(5):1050 doi:10.3390/ijms20051050
14. Pedrosa MR, Franco DR, Gieremek HW. et al. GLP-1 Agonist to Treat Obesity and Prevent Cardiovascular Disease: What Have We Achieved so Far? Curr Atheroscler Rep. 2022;24(11):867-884 doi:10.1007/s11883-022-01062-2
15. Hernandez AF, Green JB, Janmohamed S. et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet Lond Engl. 2018;392(10157):1519-1529 doi:10.1016/S0140-6736(18)32261-X
16. Khat DZ, Husain M. Molecular Mechanisms Underlying the Cardiovascular Benefits of SGLT2i and GLP-1RA. Curr Diab Rep. 2018;18(7):45 doi:10.1007/s11892-018-1011-7
17. Buse JB, Wexler DJ, Tsapas A. et al. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(2):487-493 doi:10.2337/dci19-0066
18. Anholm C, Kumarathurai P, Pedersen LR. et al. Liraglutide in combination with metformin may improve the atherogenic lipid profile and decrease C-reactive protein level in statin treated obese patients with coronary artery disease and newly diagnosed type 2 diabetes: A randomized trial. Atherosclerosis. 2019;288:60-66 doi:10.1016/j.atherosclerosis.2019.07.007
19. National Research Council. Guide for the Care and Use of Laboratory Animals. 8th ed. 2011 doi:10.17226/12910
20. Li Z, Lv F, Wen X. et al. Dapagliflozin treatment and cardiovascular outcome in RBP4/TTRVal30Met (transthyretin cardiac amyloidosis) mice. ESC Heart Fail. 2024;11(1):179-188 doi:10.1002/ehf2.14567
21. Bulawa CE, Connelly S, Devit M. et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci U S A. 2012;109(24):9629-9634 doi:10.1073/pnas.1121005109
22. Wang H, Xiang H, Xiong T, Feng J, Zhang J, Li X. A straightforward approach utilizing an exponential model to compensate for turbidity in chemical oxygen demand measurements using UV-vis spectrometry. Front Microbiol. 2023;14:1224207 doi:10.3389/fmicb.2023.1224207
23. Zhao Y, Yu J, Ping F. et al. Insulin and liraglutide attenuate brain pathology in diabetic mice by enhancing the Wnt/β-catenin signaling pathway. Exp Ther Med. 2022;24(1):439 doi:10.3892/etm.2022.11366
24. Custódio G, Massutti AM, da Igreja MR. et al. Does liraglutide alleviate inflammation in brain-dead donors? A randomized clinical trial. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. Published online November 9. 2023 doi:10.1097/LVT.0000000000000298
25. Duarte AI, Candeias E, Alves IN. et al. Liraglutide Protects Against Brain Amyloid-β(1-42) Accumulation in Female Mice with Early Alzheimer's Disease-Like Pathology by Partially Rescuing Oxidative/Nitrosative Stress and Inflammation. Int J Mol Sci. 2020 21(5). doi:10.3390/ijms21051746
26. Mendes Sousa M, Cardoso I, Fernandes R, Guimarães A, Saraiva MJ. Deposition of Transthyretin in Early Stages of Familial Amyloidotic Polyneuropathy: Evidence for Toxicity of Nonfibrillar Aggregates. Am J Pathol. 2001;159(6):1993-2000 doi:10.1016/S0002-9440(10)63050-7
27. Shirahama T, Skinner M, Westermark P. et al. Senile cerebral amyloid. Prealbumin as a common constituent in the neuritic plaque, in the neurofibrillary tangle, and in the microangiopathic lesion. Am J Pathol. 1982;107(1):41-50
28. Koike H, Misu K, Sugiura M. et al. Pathology of early- vs late-onset TTR Met30 familial amyloid polyneuropathy. Neurology. 2004;63(1):129-138 doi:10.1212/01.wnl.0000132966.36437.12
29. Andersson K, Olofsson A, Nielsen EH, Svehag SE, Lundgren E. Only amyloidogenic intermediates of transthyretin induce apoptosis. Biochem Biophys Res Commun. 2002;294(2):309-314 doi:10.1016/S0006-291X(02)00465-5
30. Sousa MM, Fernandes R, Palha JA, Taboada A, Vieira P, Saraiva MJ. Evidence for early cytotoxic aggregates in transgenic mice for human transthyretin Leu55Pro. Am J Pathol. 2002;161(5):1935-1948 doi:10.1016/S0002-9440(10)64469-0
31. Fiszman ML, Di Egidio M, Ricart KC. et al. Evidence of oxidative stress in familial amyloidotic polyneuropathy type 1. Arch Neurol. 2003;60(4):593-597 doi:10.1001/archneur.60.4.593
32. Ebenezer GJ, Liu Y, Judge DP. et al. Cutaneous nerve biomarkers in transthyretin familial amyloid polyneuropathy. Ann Neurol. 2017;82(1):44-56 doi:10.1002/ana.24972
33. Luigetti M, Romozzi M, Bisogni G. et al. hATTR Pathology: Nerve Biopsy Results from Italian Referral Centers. Brain Sci. 2020 10(11). doi:10.3390/brainsci10110780
34. Sattar N, Lee MMY, Kristensen SL. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9(10):653-662 doi:10.1016/S2213-8587(21)00203-5
35. Gerstein HC, Colhoun HM, Dagenais GR. et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet Lond Engl. 2019;394(10193):121-130 doi:10.1016/S0140-6736(19)31149-3
36. Mafham M, Preiss D. HARMONY or discord in cardiovascular outcome trials of GLP-1 receptor agonists? Lancet. 2018Oct27;392(10157):1489-1490 doi: 10.1016/S0140-6736(18)32348-1
37. Sp M, Sc B, A C. et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016 375(19). doi:10.1056/NEJMoa1607141
38. Marso SP, Daniels GH, Brown-Frandsen K. et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375(4):311-322 doi:10.1056/NEJMoa1603827
39. Gerstein HC, Sattar N, Rosenstock J. et al. Cardiovascular and Renal Outcomes with Efpeglenatide in Type 2 Diabetes. N Engl J Med. 2021;385(10):896-907 doi:10.1056/NEJMoa2108269
40. Holman RR, Bethel MA, Mentz RJ. et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2017;377(13):1228-1239 doi:10.1056/NEJMoa1612917
41. Pfeffer MA, Claggett B, Diaz R. et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med. 2015;373(23):2247-2257 doi:10.1056/NEJMoa1509225
42. Ruff CT, Baron M, Im K, O'Donoghue ML, Fiedorek FT, Sabatine MS. Subcutaneous infusion of exenatide and cardiovascular outcomes in type 2 diabetes: a non-inferiority randomized controlled trial. Nat Med. 2022;28(1):89-95 doi:10.1038/s41591-021-01584-3
43. Redfield MM, Borlaug BA. Heart Failure with Preserved Ejection Fraction: A Review. JAMA. 2023;329(10):827-838 doi:10.1001/jama.2023.2020
44. Kosiborod MN, Petrie MC, Borlaug BA. et al. Semaglutide in Patients with Obesity-Related Heart Failure and Type 2 Diabetes. N Engl J Med. 2024;390(15):1394-1407 doi:10.1056/NEJMoa2313917
45. Lecis D, Prandi FR, Barone L. et al. Beyond the Cardiovascular Effects of Glucagon-like Peptide-1 Receptor Agonists: Body Slimming and Plaque Stabilization. Are New Statins Born? Biomolecules. 2023;13(12):1695 doi:10.3390/biom13121695
46. Davies MJ, Bergenstal R, Bode B. et al. Efficacy of Liraglutide for Weight Loss Among Patients with Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial. JAMA. 2015;314(7):687-699 doi:10.1001/jama.2015.9676
47. Davies M, Færch L, Jeppesen OK. et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet Lond Engl. 2021;397(10278):971-984 doi:10.1016/S0140-6736(21)00213-0
48. Sattar N, Lee MMY, Kristensen SL, Branch KRH, Del Prato S, Khurmi NS, Lam CSP, Lopes RD, McMurray JJV, Pratley RE, Rosenstock J, Gerstein HC. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021Oct;9(10):653-662 doi: 10.1016/S2213-8587(21)00203-5
49. Mancini GBJ, O'Meara E, Zieroth S. et al. 2022 Canadian Cardiovascular Society Guideline for Use of GLP-1 Receptor Agonists and SGLT2 Inhibitors for Cardiorenal Risk Reduction in Adults. Can J Cardiol. 2022;38(8):1153-1167 doi:10.1016/j.cjca.2022.04.029
50. Yang X, Su G, Zhang T. et al. Comparison of admission glycemic variability and glycosylated hemoglobin in predicting major adverse cardiac events among type 2 diabetes patients with heart failure following acute ST-segment elevation myocardial infarction. J Transl Intern Med. 2024;12(2):188-196 doi:10.2478/jtim-2024-0006
51. Campbell JE, Müller TD, Finan B, DiMarchi RD, Tschöp MH, D'Alessio DA. GIPR/GLP-1R dual agonist therapies for diabetes and weight loss-chemistry, physiology, and clinical applications. Cell Metab. 2023;35(9):1519-1529 doi:10.1016/j.cmet.2023.07.010
52. Lyons SA, Beaudry JL. Synergistic Combinations of Gut- and Pancreas-Hormone-Based Therapies: Advancements in Treatments for Metabolic Diseases. Endocrinology. 2023;164(11):bqad153 doi:10.1210/endocr/bqad153
53. Frías JP, Davies MJ, Rosenstock J. et al. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N Engl J Med. 2021;385(6):503-515 doi:10.1056/NEJMoa2107519
54. Dahl D, Onishi Y, Norwood P. et al. Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients with Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial. JAMA. 2022;327(6):534-545 doi:10.1001/jama.2022.0078
55. Del Prato S, Kahn SE, Pavo I. et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet Lond Engl. 2021;398(10313):1811-1824 doi:10.1016/S0140-6736(21)02188-7
56. Ludvik B, Giorgino F, Jódar E. et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet Lond Engl. 2021;398(10300):583-598 doi:10.1016/S0140-6736(21)01443-4
57. Wilson JM, Lin Y, Luo MJ. et al. The dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist tirzepatide improves cardiovascular risk biomarkers in patients with type 2 diabetes: A post hoc analysis. Diabetes Obes Metab. 2022;24(1):148-153 doi:10.1111/dom.14553
58. Rosenstock J, Frias J, Jastreboff AM. et al. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: a randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet Lond Engl. 2023;402(10401):529-544 doi:10.1016/S0140-6736(23)01053-X
59. Morissette A, Mulvihill EE. Cardioprotective benefits of metabolic surgery and GLP-1 receptor agonist-based therapies. Trends Endocrinol Metab TEM. 2025;36(4):316-329 doi:10.1016/j.tem.2024.07.012
60. Marso SP, Bain SC, Consoli A. et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375(19):1834-1844 doi:10.1056/NEJMoa1607141
61. Liu Z, Huang Y, Yang Y. et al. Analysis and prediction of research hotspots and trends in heart failure research. J Transl Intern Med. 2024;12(3):263-273 doi:10.2478/jtim-2023-0117
62. Ast J, Arvaniti A, Fine NHF. et al. Super-resolution microscopy compatible fluorescent probes reveal endogenous glucagon-like peptide-1 receptor distribution and dynamics. Nat Commun. 2020;11(1):467 doi:10.1038/s41467-020-14309-w
63. Baggio LL, Yusta B, Mulvihill EE. et al. GLP-1 Receptor Expression Within the Human Heart. Endocrinology. 2018;159(4):1570-1584 doi:10.1210/en.2018-00004
64. McLean BA, Wong CK, Kaur KD, Seeley RJ, Drucker DJ. Differential importance of endothelial and hematopoietic cell GLP-1Rs for cardiometabolic versus hepatic actions of semaglutide. JCI Insight. 2021;6(22):e153732 doi:10.1172/jci.insight.153732
65. McLean BA, Wong CK, Kabir MG, Drucker DJ. Glucagon-like Peptide-1 receptor Tie2+ cells are essential for the cardioprotective actions of liraglutide in mice with experimental myocardial infarction. Mol Metab. 2022;66:101641 doi:10.1016/j.molmet.2022.101641
66. Rakipovski G, Rolin B, Nøhr J. et al. The GLP-1 Analogs Liraglutide and Semaglutide Reduce Atherosclerosis in ApoE-/- and LDLr-/- Mice by a Mechanism That Includes Inflammatory Pathways. JACC Basic Transl Sci. 2018;3(6):844-857 doi:10.1016/j.jacbts.2018.09.004
67. Punjabi M, Kosareva A, Xu L. et al. Liraglutide Lowers Endothelial Vascular Cell Adhesion Molecule-1 in Murine Atherosclerosis Independent of Glucose Levels. JACC Basic Transl Sci. 2023;8(2):189-200 doi:10.1016/j.jacbts.2022.08.002
68. Wong CK, McLean BA, Baggio LL. et al. Central glucagon-like peptide 1 receptor activation inhibits Toll-like receptor agonist-induced inflammation. Cell Metab. 2024;36(1):130-143.e5 doi:10.1016/j.cmet.2023.11.009
69. Helmstädter J, Frenis K, Filippou K. et al. Endothelial GLP-1 (Glucagon-Like Peptide-1) Receptor Mediates Cardiovascular Protection by Liraglutide In Mice With Experimental Arterial Hypertension. Arterioscler Thromb Vasc Biol. 2020;40(1):145-158 doi:10.1161/atv.0000615456.97862.30
70. Noyan-Ashraf MH, Momen MA, Ban K. et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58(4):975-983 doi:10.2337/db08-1193
71. Noyan-Ashraf MH, Shikatani EA, Schuiki I. et al. A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation. 2013;127(1):74-85 doi:10.1161/CIRCULATIONAHA.112.091215
72. Li Z, Wu B, Chen J. et al. WWP2 protects against sepsis-induced cardiac injury through inhibiting cardiomyocyte ferroptosis. J Transl Intern Med. 2024;12(1):35-50 doi:10.2478/jtim-2024-0004
73. Khalifa O, Ouararhni K, Errafii K, Alajez NM, Arredouani A. Targeted MicroRNA Profiling Reveals That Exendin-4 Modulates the Expression of Several MicroRNAs to Reduce Steatosis in HepG2 Cells. Int J Mol Sci. 2023 24(14). doi:10.3390/ijms241411606
74. Coelho T, Waddington Cruz M, Chao CC. et al. Characteristics of Patients with Hereditary Transthyretin Amyloidosis-Polyneuropathy (ATTRv-PN) in NEURO-TTRansform, an Open-label Phase 3 Study of Eplontersen. Neurol Ther. 2023;12(1):267-287 doi:10.1007/s40120-022-00414-z
Author contact
![]() Corresponding authors: Xiaoling Cai, MD; E-mail: dr_junelcom. Linong Ji, Professor; Department of Endocrinology and Metabolism, Peking University People's Hospital, No.11 Xizhimen South Street, Xicheng District, Beijing, China, 100044; E-mail: jilnedu.cn; Tel: 8610-88324108; Fax: 8610-88325534.
Corresponding authors: Xiaoling Cai, MD; E-mail: dr_junelcom. Linong Ji, Professor; Department of Endocrinology and Metabolism, Peking University People's Hospital, No.11 Xizhimen South Street, Xicheng District, Beijing, China, 100044; E-mail: jilnedu.cn; Tel: 8610-88324108; Fax: 8610-88325534.

 Global reach, higher impact
Global reach, higher impact