Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(13):3220-3228. doi:10.7150/ijms.115222 This issue Cite
Research Paper
Soluble endoglin reflects endothelial dysfunction in myocardial infarction patients: a retrospective observational study
1. Department of Biological and Medical Sciences, Faculty of Pharmacy in Hradec Králové, Charles University in Prague, Czech Republic.
2. Cardiology Department, Cardiac Centre, Institute for Clinical and Experimental Medicine, Prague, Czech Republic.
3. Department of Immunology and Allergology, University Hospital Hradec Králové and Charles University, Faculty of Medicine in Hradec Králové, Czech Republic.
4. Department of PISA, Cardiac Centre, Institute for Clinical and Experimental Medicine, Prague, Czech Republic.
Received 2025-4-7; Accepted 2025-6-11; Published 2025-7-4
Abstract
Acute manifestations of ischemic heart disease are among the most serious and fatal consequences of atherosclerotic processes. In this study, we hypothesized that a soluble proprotein convertase subtilisin/kexin type 9 (PCSK9), soluble bone morphogenetic protein 4 (BMP-4), soluble E-selectin (sE-selectin), soluble endoglin (sENG) and soluble endocan (Endocan) would differ from healthy controls in myocardial infarction (MI) patients admitted to the hospital without any previous history of cardiovascular disease and with no cardioprotective drugs taken before admission. The study was conducted using a cross-sectional design.
We analyzed data from 79 patients (mean age 54.1 ± 8.9, 18% of women) admitted for the first manifestation of MI and with no history of cardioprotective treatment use before the event. As a control group, we analyzed 17 age-matched healthy volunteers (mean age 51.5 ± 8.6, 47% of women). In addition to routinely obtaining clinical and laboratory data, we analyzed plasma concentrations of the aforementioned biomarkers using ELISA and Luminex analyses.
Patients with MI did not differ from healthy controls in total cholesterol, LDL, non-HDL, and triglyceride levels. PCSK9, BMP-4, and sE-selectin levels did not differ significantly between the MI and the control group. Patients with MI had significantly higher sENG and Endocan levels than the control group. In addition, levels of sENG were significantly higher in patients with higher body mass index (BMI) and in smokers.
We demonstrated that sENG could serve as a biomarker reflecting endothelial dysfunction in MI patients without prior treatment for cardiovascular risk factors.
Keywords: myocardial infarction, soluble endoglin, soluble endocan.
Introduction
Worldwide, cardiovascular diseases continue to place a heavy load on overall mortality, with acute coronary syndrome frequently being the first symptom to manifest [1]. This occurs due to the rupture of atherosclerotic plaques within the coronary arteries, leading to myocardial ischemia, necrosis, and subsequent clinical presentations. Thus, early diagnosis and risk assessment are essential for directing prompt therapies and enhancing patient outcomes [2].
The endothelium plays an essential role in coronary artery disease. In the presence of cardiovascular risk factors like hypertension, hyperlipidemia, or hyperglycemia, it changes its quiescence towards the activated state with expression of adhesion molecules and inflammatory markers that alter the permeability and vasoactive properties of the endothelium [3]. For this reason, endothelial dysfunction (ED) has been strongly associated with the increased risk of myocardial infarction (MI) [3,4].
Several markers of ED have been studied in relation to endothelial dysfunction, with some shown to be more or less specific in the context of various vascular pathologies. However, currently, there is no “golden standard” circulating biomarker for coronary artery disease [3]. We introduce possibly novel and interesting endothelial dysfunction and inflammation biomarkers related to therapy-naïve individuals with no symptomatic cardiovascular disease suffering MI episodes.
Proprotein convertase subtilisin/kexin type 9 is a critical player in cholesterol metabolism related to endothelial dysfunction, inflammation, and atherosclerosis [5]. Moreover, it was proposed as an adverse factor for cardiovascular risk beyond dyslipidemia [6]. Bone morphogenetic proteins (BMPs) are members of the Transforming Growth Factor β (TGFβ) superfamily, whereby BMP-4 is evidenced as a critical regulator in vascular biology. Its crucial role in leukocyte recruitment during vascular inflammation [7], as well as its potential involvement in hypertension development, have been demonstrated [8]. Several adhesion molecules, including E-selectin, are expressed during the inflammatory response of vascular endothelium. Moreover, studies report increased levels of sE-selectin as circulatory markers reflecting the burden of coronary artery disease [3].
Endoglin (ENG, CD105) is a 180 kDa transmembrane glycoprotein considered a co-receptor for ligands of the TGFβ superfamily [9]. There are two different isoforms of membrane ENG expressed by various cells (endothelial cells, macrophages, smooth muscle cells) and soluble endoglin (sENG) circulating in plasma or cell culture medium [10]. sENG is the N-terminal cleavage product of the extracellular domain of ENG formed by the activity of matrix metalloproteinases [11-13] that is released into the circulation. In addition to matrix metalloproteinases, thrombin has recently been described to generate sEng [14]. sENG can be detected and used as a biomarker in various cardiovascular and metabolic disorders, such as preeclampsia [15], hypercholesterolemia [16], familial hypercholesterolemia [17], atherosclerosis [18], arterial hypertension [19], septic shock disease [20], and liver alterations [21]. Moreover, based on our experimental data, increased sENG levels were demonstrated in the development of early aortic endothelial dysfunction [22] and liver sinusoidal endothelial dysfunction during NASH progression [21] and correlated with total cholesterol levels and progression of atherosclerosis in vivo [23], suggesting their importance in the development of endothelial dysfunction. In addition, we demonstrated that high levels of sENG aggravate aortic endothelial dysfunction [24] and endothelial inflammation [25], suggesting their potentially harmful effects on vascular endothelium.
However, sENG level changes in acute coronary syndrome (ACS) and coronary artery disease (CAD) show controversial data. For instance, Cruz-Gonzalez et al. found sENG levels in acute myocardial infarction patients to be lower than those of healthy individuals [26]. On the contrary, sENG levels were higher in patients with ruptured plaque and unstable plaque than those with stable plaque, suggesting that sENG reflects the plaque stability and risk of acute coronary syndrome [27].
Endocan, or Endothelial Cell-Specific Molecule-1 (ESM-1), a dermatan sulfate proteoglycan, is expressed primarily by the vascular endothelium and has been found freely circulating in the bloodstream as a soluble endocan (Endocan) [28]. Indeed, elevated plasma levels of Endocan may reflect endothelial activation and dysfunction [29]. Moreover, Endocan was shown to be an independent predictor of heart failure [30], a surrogate marker for hypertension [31], coronary artery disease, coronary slow flow [32], angina, and subclinical atherosclerosis [33].
Our previous extensive experimental studies, as well as those of others, have indicated a strong potential negative impact of high sENG levels on endothelial dysfunction development [24] and liver metabolism [34]. In this study, we focused on the human population. Specifically, we addressed whether there were any differences in the above-mentioned biomarkers of endothelial dysfunction between patients with MI and controls. In addition, we analyzed the potential association of these biomarkers with traditional lipid risk factors and smoking habits.
Materials and Methods
Data from patients recruited for the AMBITION (Institute for Clinical and Experimental Medicine Acute Myocardial Infarction Registry) registry were analyzed. This registry collects clinical and laboratory data from all consecutive patients hospitalized for MI at a tertiary heart center since June 2017 [35]. This observational study was approved by the ethical committee of Faculty Thomayer Hospital and Institute for Clinical and Experimental Medicine, Prague, Czech Republic, on June 13, 2018, under registration number G-18-52. The data from this project were accessed and analyzed (blinded for any personal information of participants) from 20.11.2023 to 14.5.2024, together with measuring soluble endoglin and other factors from available frozen blood samples. The methodology of this study has been previously described [36]. Briefly, in the hospital, all patients underwent detailed interviews regarding their health status, with additional information obtained from medical records and laboratory studies. Definitions of cardiovascular risk factors already present before MI were as follows: patients were considered smokers if they reported smoking at least one cigarette per day 12 months before developing MI; a history of diabetes was defined as the use of oral antidiabetic drugs or insulin at the time of hospital admission (for this study, patients treated by drugs including insulin were excluded); arterial hypertension was defined as self-reported hypertension and/or use of antihypertensive medications upon admission (for this study, patients treated by drugs were excluded). The purpose of this study is to examine persons with a history of diabetes and hypertension without any drug treatment. The Institutional Review Board of the Prague-based Institute for Clinical and Experimental Medicine approved the study, and all participants signed informed consent. The investigation conformed to the principles outlined in the Declaration of Helsinki. The exclusion criteria were unwillingness to sign informed consent and age above 65.
The project was a retrospective study involving plasma samples from 79 patients admitted with MI with neither a previous history of cardiovascular disease nor a history of treatment by cardioprotective drugs. The patients were hospitalized at the Department of Cardiology at the Institute for Clinical and Experimental Medicine (Prague, Czech Republic). The control group comprised samples from 17 age-matched and healthy volunteers of both genders recruited from preventive medical inspections to assess differences in the study parameters. Clinical data of both groups, including age, sex, diabetes mellitus, hypertension, and smoking habits, were collected. The study protocol was approved by the local institutional review board, and informed consent was obtained from all patients. The clinical characteristics of both groups of subjects in the study are summarized in Table 1.
Blood samples and analyses
All patients' blood for laboratory analyses was collected in the morning after admission in fasting status. Lipid parameters were analyzed using a fully automated enzymatic method (COBAS MIRA S analyzer, Roche Diagnostics, Basel, Switzerland) with enzymatic kits produced by the same manufacturer. For this study, only lipid parameters were used as follows: total cholesterol, LDL, HDL, triglycerides, and non-HDL cholesterol.
For this study, data from patients under 65 years old who were hospitalized for MI between 2020 and 2023 were analyzed. The Institutional Review Board of the Prague-based Institute for Clinical and Experimental Medicine approved the study, and all participants signed informed consent. The investigation conformed to the principles outlined in the Declaration of Helsinki. The exclusion criteria were unwillingness to sign informed consent and age above 65. Blood samples were collected the morning after hospital admission with MI or during regular medical inspections in case of healthy controls. The blood was taken in EDTA-containing tubes and centrifuged within 30 minutes at 1500G for 15 min at room temperature. Plasma samples were aliquoted and stored at -80°C before the biochemical and proteomic analysis.
Clinical data of controls and myocardial infarction patients
| Controls (n=17) | MI patients (n=79) | p | |
|---|---|---|---|
| Age | 51.5 ±8.6 | 54.1 ±8.9 | 0.1599 |
| Women (%) | 47 | 18 | 0.0142* |
| Smoking status (%) | 18 | 65 | 0.0005*** |
| Diabetes mellitus (%) | 0 | 8.9 | 0.3464 |
| Hypertension (%) | 18 | 8.9 | 0.3753 |
| Total cholesterol (mmol/L) | 5.6 ±0.7 | 5.5 ±1.1 | 0.6123 |
| Triglycerides (mmol/L) | 1.2 ±0.3 | 1.6 ±1.2 | 0.0985 |
| LDL (mmol/L) | 3.6 ±0.6 | 3.9 ±1.1 | 0.0836 |
| HDL (mmol/L) | 1.6 ±0.4 | 1.2 ±0.4 | <0.0001**** |
| non-HDL (mmol/L) | 4.0 ±0.6 | 4.3 ±1.2 | 0.1477 |
| BMI | 28.2 ±4.4 | 26.6 ±3.6 | 0.1116 |
Values are means ± SD, significant values marked with asterisks, abbreviations:
MI - myocardial infarction patients, LDL - low-density lipoproteins, HDL - high-density lipoproteins, BMI - body mass index.
Analysis of biomarkers of endothelial dysfunction
The concentrations of soluble endoglin (CD105) were assessed in plasma samples by sandwich enzyme-linked immunosorbent assay technique (ELISA) using the Quantikine Human Endoglin/CD105 ELISA kit (R&D Systems, MN, USA) according to the manufacturer's instructions. Samples were undiluted. The sensitivity of the kit was 0.007 ng/mL. The absorbance values were measured at 450 nm with a Multiskan RC ELISA reader (Thermo Fisher Scientific, MA, USA). The levels of Endocan, PCSK9, BMP-4, and sE-selectin were determined using the Human Premixed Multi-Analyte Magnetic Luminex Kit (R&D Systems, MN, USA), according to the manufacturer's protocol.
Statistical analysis
The statistical analysis was performed using GraphPad Prism software version 9.2 (GraphPad Software Inc., San Diego, CA, USA). Clinical data are expressed as mean ± standard deviation (SD). Laboratory data of endothelial dysfunction markers are presented as median with interquartile range (IQR). In the first place, a normality test was performed in all data, and based on these, non-parametric tests were used for further analyses. Direct group-group comparisons were carried out using a non-parametric Mann-Whitney test. Correlations were determined using Spearman´s r and p-value. Linear regression analyses were determined using the Beta coefficient, 95% CI, and p-value. P-values < 0.05 were considered statistically significant.
Results
Patients with MI did not differ from healthy controls in parameters of total cholesterol, LDL, triglycerides, and non-HDL levels
As shown in Table 1, there was a significantly lower representation of women and a higher representation of smokers in the MI group. Analysis of lipid parameters revealed no differences between MI patients and controls on the level of total cholesterol, LDL, non-HDL cholesterol, and triglycerides in plasma. Both groups also did not differ in BMI values. In contrast, the group of MI patients had significantly decreased levels of HDL compared to controls.
Patients with MI had significantly increased levels of sENG and Endocan in plasma compared to controls
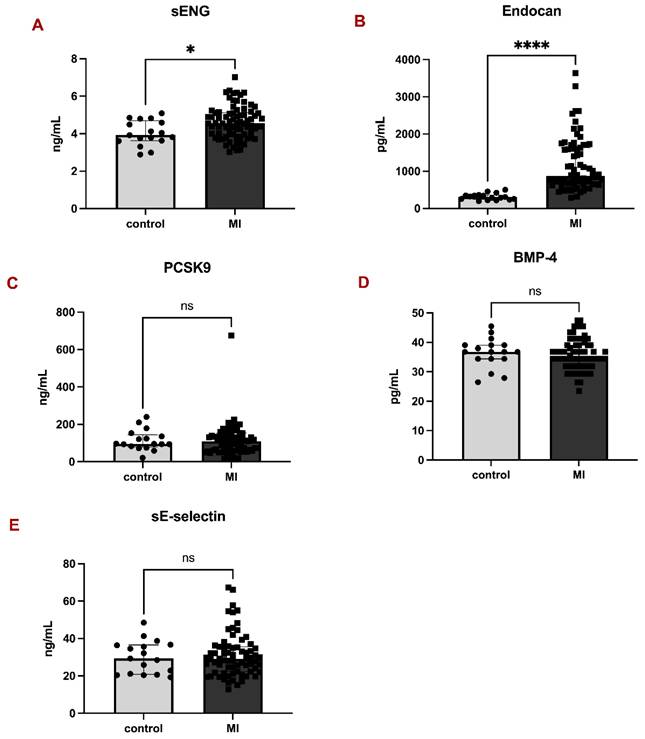
Analysis of plasma markers revealed significantly higher levels of sENG in MI patients compared to controls: 4.56 (3.97-5.12) vs 3.92 (3.62-4.69), p = 0.0113 (Fig. 1A). Similarly, the difference between MI patients and controls in Endocan levels proved significant: 868.4 (654.4-1631.0) vs 308.3 (245.1-358.0), p < 0.0001 (Fig. 1B). On the contrary, there were no significant differences in the levels of PCSK9 (Fig. 1C), BMP-4 (Fig. 1D), and sE-selectin (Fig. 1E) between the two groups.
sENG levels were significantly higher in BMI at risk and smoking MI patients
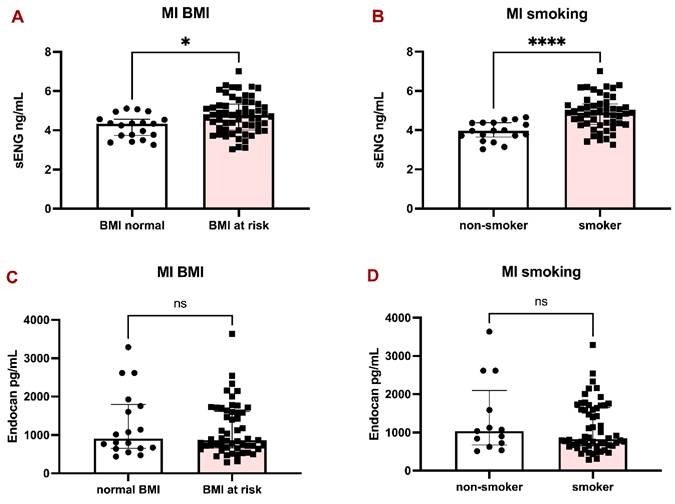
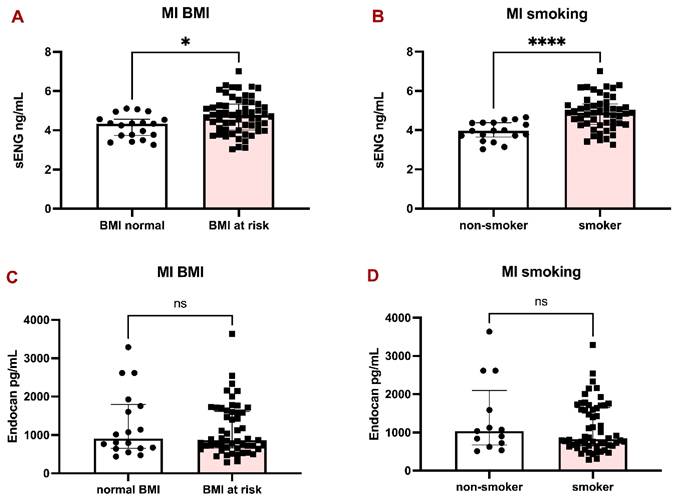
The patients with MI were divided into two groups according to BMI (over 25 kg/m2 considered at risk) and smoking habits. sENG was significantly higher in patients with BMI at risk (Fig. 2A) and in smokers (Fig. 2B), compared to Endocan, which did not significantly differ between BMI groups (Fig. 2C) and in smokers (Fig. 2D).
Since there was a considerably higher representation of smokers in the MI group (Table 1), we decided to perform further evaluation. A simple linear regression analysis initially showed that patients with MI had significantly higher plasma sENG levels compared to non-MI individuals (β = 0.59 ng/mL, 95% CI [0.15, 1.03], p = 0.009). However, given that smoking is a known risk factor for both MI and endothelial dysfunction, we conducted a multiple linear regression analysis adjusting for smoking status.
After adjustment for smoking, the association between MI and sENG levels was no longer statistically significant (β = 0.25 ng/mL, 95% CI [-0.19, 0.69], p = 0.256), whereas smoking remained a strong independent predictor of sENG levels (β = 0.71 ng/mL, 95% CI [0.37, 1.05], p <0.0001). The adjusted model explained 22% of the variance in sENG levels (R² = 0.22), compared to 7% (R² = 0.07) in the unadjusted model.
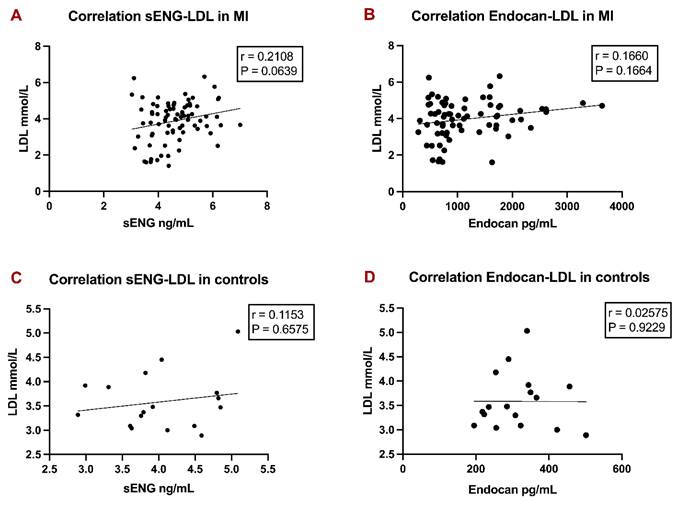
LDL levels gave only a weak correlation with sENG and Endocan levels in the cohort of MI patients
The last evaluation searched for possible correlations between sENG and Endocan and traditional risk factors for MI. We found no correlation of total cholesterol with sENG (r=0.01902; P=0.8687) or with Endocan (r=0.1974; P=0.0989), no correlation of triglycerides with sENG (r= -0.03969; P=0.7301) or with Endocan (r=0.06089; P=0.6139), no correlation of HDL with sENG (r= -0.06275; P=0.5852) or with Endocan (r=0.03664; P=0.7616) and no correlation of non-HDL with sENG (r=0.1785; P=0.1179) or with Endocan (r=0.1770; P=0.1398).
We found only a weak, non-significant correlation between LDL levels with sENG (Fig. 3A) in MI patients and LDL levels with Endocan (Fig. 3B) in MI patients. Both correlations also turned insignificant in controls (Fig. 3C, 3D). Data is presented as correlation coefficients and p-values in Figure 3.
Discussion
In our study, we demonstrated for the first time that plasma sENG levels were substantially higher in survivors of MI than in healthy controls. Moreover, in the survivors, sENG levels were higher in smokers than in non-smokers, indicating an association of sENG with this robust risk factor of endothelial dysfunction and indirect evidence that sENG might be an important marker for severe vascular damage.
Despite important scientific advances, MI remains at the top of death causes worldwide [1]. Searching for highly sensitive predictive indicators of these pathologies is, therefore, of great importance. Markers of endothelial dysfunction seem to be reasonable in evaluating the burden of coronary artery disease [3], and might bring an early diagnostic and prognostic tool for MI [2]. Some already established and novel markers of ED have been studied. However, we specifically focused on the levels of sENG, Endocan, PCSK9, BMP-4, and sE-selectin.
In this study, we hypothesized that the above-mentioned circulating biomarkers of endothelial dysfunction would differ in patients admitted for the first myocardial infarction compared to healthy controls. We included a specific group of patients with MI with no previous symptomatic cardiovascular disease and with no cardiovascular therapy before the coronary event. The reason for selecting this specific cohort was based on the assumption that several groups of drugs, being frequently taken in cardio-metabolic disorders, might significantly modify the values of circulating ED markers and the state of the endothelium due to their pleiotropic effects [37,38]. Accordingly, these patients were compared to a group of healthy volunteers who were not undergoing any cardio-metabolic treatment.
Parameters of endothelial dysfunction in controls and patients with myocardial infarction. Bar graphs representing the levels of sENG (A), Endocan (B), PCSK9 (C), BMP-4 (D), and sE-selectin (E) between controls and MI patients. All data are shown as median with interquartile range. Mann-Whitney test, *p < 0.05, ****p < 0.0001.
sENG and Endocan levels in the plasma of MI patients with respect to BMI and smoking habits. Bar graphs representing the levels of sENG in MI patients according to BMI (A) and smoking habits (B). Bar graphs representing the levels of Endocan in MI patients according to BMI (C) and smoking habits (D). All data are shown as median with interquartile range. Mann-Whitney test, *p < 0.05, ****p < 0.0001.
Correlation analyses of LDL with sENG and Endocan levels in MI patients and controls. Linear regression graphs of LDL with sENG in MI patients (A), LDL with Endocan in MI patients (B), LDL with sENG in controls (C), and LDL with Endocan in controls (D) were designed. Spearman r values were defined. P-values < 0.05 were considered statistically significant.
There were no differences in total cholesterol, triglycerides, LDL, and non-HDL levels between the MI and the control group. In contrast, there was a significant difference in HDL levels. This phenomenon may be partially attributed to the generally suboptimal values observed in patients with MI and the higher proportion of women in the control group. In general, these results indicate that the main atherogenic lipid parameters might not be sensitive enough to predict MI in patients with no previous history of cardiovascular events.
In the initial comparison, only sENG and Endocan reached statistical significance between the MI group and the control group. Notably, several papers have focused on the role of sENG in MI with discrepant observations [39-42]. Interestingly, sENG may not be only a potential circulating biomarker of MI. It was demonstrated that high levels of sENG could aggravate endothelial dysfunction [22] and induce inflammatory markers expression [25]. This suggests that high levels of sENG might even promote the progression or manifestation of MI by aggravating endothelial dysfunction and inflammation [43]. It is also of interest to mention that sENG regulates the expression of angiogenesis-related proteins, leading to anti-angiogenic effects [44]. In addition, elevated levels of sEng were associated with dysregulated angiogenesis and vascular remodeling in the brain [45]. Indeed, vascular remodeling, including neoangiogenesis, occurs following the acute stage of myocardial infarction [46]. Therefore, we propose that elevated levels of sENG may influence this angiogenic process in the heart, an aspect that warrants investigation in future prospective studies.
Endocan is a biomarker considered a potential prognostic tool for coronary artery disease [2]. A meta-analysis found that Endocan levels in patients with coronary artery disease were higher than in the control group, suggesting its potential role as a marker reflecting atherosclerosis progression [47]. Interestingly, similarly to sENG, Endocan is not only a biomarker of MI. It is widely accepted that Endocan is involved in the development of atherosclerosis, primarily by contributing to endothelial dysfunction [29]. Also, data from this study indicate a possible benefit of Endocan levels evaluation in therapy-naïve patients, reflecting endothelial dysfunction in the risk group.
Our study also assessed the relationship between BMI at risk, smoking habits, and sENG and Endocan levels in MI patients. The analyses revealed that only sENG levels, but not Endocan levels, were significantly higher in patients with a BMI over 25 kg/m2, and an even more pronounced increase was found in smokers. It should be emphasized that our MI group included a significantly higher number of smokers compared to the control group. Our multivariable analysis also proved smoking as an independent predictor of high sENG levels. This is an interesting finding if we consider the contribution of smoking to endothelial dysfunction, upregulation of matrix metalloproteinases (MMPs) and plaque instability [48], and the role of MMPs in endoglin shedding and elevated sENG levels [12,49].
We also found a weak, non-significant correlation of sENG with LDL, indicating that sENG is relatively independent of LDL in MI patients. This is in line with the paper showing no correlation between LDL and sENG in patients with already established chronic coronary artery disease undergoing cardiac catheterization [50].
Strengths and limitations of the study
The strength of our study lies in the robust experimental data that strongly support the biological plausibility of our findings in a well-defined patient group, indicating that sENG may also have the potential to cause vascular damage in humans.
Nevertheless, we are fully aware of the limitations, including the retrospective/cross-sectional design of this study, which does not allow us to reliably establish cause-and-effect relationships. The next limitation is that the samples for sENG measurements were collected during the acute phase of MI and may have been influenced by the acute pathophysiological changes occurring during this period. Another limitation is that we did not have a completely matched control group in this study. However, the main atherogenic lipid parameters were not different between the patients and the control group, and HDL cholesterol is recently not considered an anti-atherogenic lipid factor [51].
An important limitation of the study might be the gender distribution within the MI and control groups and its potential impact on the relevance of sENG levels. However, we took this aspect into account from the very beginning. Initially, we performed a statistical analysis, which showed no significant differences in sENG levels between men and women, either in the MI group or in the control group. In addition, the difference in sENG levels between MI patients and controls remained statistically significant when analyzing men and women separately. Therefore, we chose to include both sexes in the MI and control groups together to strengthen the statistical power and the biological plausibility of our findings. We can infer that the elevated endoglin concentrations observed in patients with MI highlight its significance as a marker of increased cardiovascular metabolic risk.
Conclusion
For the first time in patients, we demonstrate that sENG may serve as a biomarker of endothelial dysfunction in MI patients who have not received prior treatment for cardiovascular risk factors. Prospective studies are needed to confirm the value of monitoring sENG levels in the prevention and management of endothelial dysfunction-related diseases.
Acknowledgements
Funding
This study has been funded with the project New Technologies for Translational Research in Pharmaceutical Sciences /NETPHARM, project ID CZ.02.01.01/00/22_008/0004607, is co-funded by the European Union. Furthermore, by a grant by the Ministry of Health of the Czech Republic, grant no. 17-29241A. Moreover, by the project National Institute for Research of Metabolic and Cardiovascular Diseases [Programme EXCELES, ID Project No. LX22NPO5104] - Funded by the European Union and conceptual development of research organization „Institute for Clinical and Experimental Medicine - IKEM, IN 00023001”. The funding body had no role in the design of the study and collection, analysis, and interpretation of data nor in writing the manuscript.
Availability of data and materials
Data will be made available upon reasonable request to the corresponding author. The authors do not intend to share individual de-identified participant data.
Author contributions
Jana Urbankova Rathouska: Organization and coordination of work, laboratory analyses, data analysis, and conclusions, writing the manuscript., Jana Mrazkova: Cooperation on the clinical part of the project, handling patients´ samples., Ctirad Andrýs: Laboratory analyses., Karolina Janeckova: Laboratory analyses., Katarina Tripska: Writing the manuscript., Petra Fikrova: Writing the manuscript., Ivana Nemeckova: Writing the manuscript., Samira Eissazadeh: Writing the manuscript., Peter Wohlfahrt: Specialized cardiologist, clinical monitoring., Jan Pitha: Specialized cardiologist, clinical monitoring, methodological guidance, writing the manuscript., Petr Nachtigal: Data evaluation, writing the manuscript, supervision.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Mitsis A, Myrianthefs M, Sokratous S, Karmioti G, Kyriakou M, Drakomathioulakis M. et al. Emerging Therapeutic Targets for Acute Coronary Syndromes: Novel Advancements and Future Directions. Biomedicines. 2024;12:1670
2. Katsioupa M, Kourampi I, Oikonomou E, Tsigkou V, Theofilis P, Charalambous G. et al. Novel Biomarkers and Their Role in the Diagnosis and Prognosis of Acute Coronary Syndrome. Life (Basel). 2023;13:13 1992
3. Medina-Leyte DJ, Zepeda-Garcia O, Dominguez-Perez M, Gonzalez-Garrido A, Villarreal-Molina T, Jacobo-Albavera L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int J Mol Sci. 2021;22:3850
4. Leite AR, Borges-Canha M, Cardoso R, Neves JS, Castro-Ferreira R, Leite-Moreira A. Novel Biomarkers for Evaluation of Endothelial Dysfunction. Angiology. 2020;71:397-410
5. Paryani M, Gupta N, Jain SK, Butani S. Lowering LDL cholesterol by PCSK9 inhibition: a new era of gene silencing, RNA, and alternative therapies. Naunyn Schmiedebergs Arch Pharmacol. 2025;398:6597-6615
6. Ragusa R, Basta G, Neglia D, De Caterina R, Del Turco S, Caselli C. PCSK9 and atherosclerosis: Looking beyond LDL regulation. Eur J Clin Invest. 2021;51:e13459
7. Helbing T, Arnold L, Wiltgen G, Hirschbihl E, Gabelmann V, Hornstein A. et al. Endothelial BMP4 Regulates Leukocyte Diapedesis and Promotes Inflammation. Inflammation. 2017;40:1862-74
8. Gallardo-Vara E, Gamella-Pozuelo L, Perez-Roque L, Bartha JL, Garcia-Palmero I, Casal JI. et al. Potential Role of Circulating Endoglin in Hypertension via the Upregulated Expression of BMP4. Cells. 2020;9:988
9. Schoonderwoerd MJA, Goumans MTH, Hawinkels L. Endoglin: Beyond the Endothelium. Biomolecules. 2020;10:289
10. Oujo B, Perez-Barriocanal F, Bernabeu C, Lopez-Novoa JM. Membrane and soluble forms of endoglin in preeclampsia. Curr Mol Med. 2013;13:1345-57
11. Aristorena M, Gallardo-Vara E, Vicen M, de Las Casas-Engel M, Ojeda-Fernandez L, Nieto C. et al. MMP-12, Secreted by Pro-Inflammatory Macrophages, Targets Endoglin in Human Macrophages and Endothelial Cells. Int J Mol Sci. 2019;20:3107
12. Hawinkels LJ, Kuiper P, Wiercinska E, Verspaget HW, Liu Z, Pardali E. et al. Matrix metalloproteinase-14 (MT1-MMP)-mediated endoglin shedding inhibits tumor angiogenesis. Cancer Res. 2010;70:4141-50
13. Valbuena-Diez AC, Blanco FJ, Oujo B, Langa C, Gonzalez-Nunez M, Llano E. et al. Oxysterol-induced soluble endoglin release and its involvement in hypertension. Circulation. 2012;126:2612-24
14. El Hamaoui D, Marchelli A, Gandrille S, Reboul E, Stepanian A, Palmier B. et al. Thrombin cleaves membrane-bound endoglin potentially contributing to the heterogeneity of circulating endoglin in preeclampsia. Commun Biol. 2025;8:316
15. Leanos-Miranda A, Navarro-Romero CS, Sillas-Pardo LJ, Ramirez-Valenzuela KL, Isordia-Salas I, Jimenez-Trejo LM. Soluble Endoglin as a Marker for Preeclampsia, Its Severity, and the Occurrence of Adverse Outcomes. Hypertension. 2019;74:991-7
16. Blann AD, Wang JM, Wilson PB, Kumar S. Serum levels of the TGF-beta receptor are increased in atherosclerosis. Atherosclerosis. 1996;120:221-6
17. Visek J, Blaha M, Blaha V, Lasticova M, Lanska M, Andrys C. et al. Monitoring of up to 15 years effects of lipoprotein apheresis on lipids, biomarkers of inflammation, and soluble endoglin in familial hypercholesterolemia patients. Orphanet J Rare Dis. 2021;16:110
18. Rathouska J, Vecerova L, Strasky Z, Slanarova M, Brcakova E, Mullerova Z. et al. Endoglin as a possible marker of atorvastatin treatment benefit in atherosclerosis. Pharmacol Res. 2011;64:53-9
19. Malhotra R, Paskin-Flerlage S, Zamanian RT, Zimmerman P, Schmidt JW, Deng DY. et al. Circulating angiogenic modulatory factors predict survival and functional class in pulmonary arterial hypertension. Pulm Circ. 2013;3:369-80
20. Tomaskova V, Mytnikova A, Hortova Kohoutkova M, Mrkva O, Skotakova M, Sitina M. et al. Prognostic value of soluble endoglin in patients with septic shock and severe COVID-19. Front Med (Lausanne). 2022;9:972040
21. Eissazadeh S, Mohammadi S, Faradonbeh FA, Rathouska JU, Nemeckova I, Tripska K. et al. Endoglin and soluble endoglin in liver sinusoidal endothelial dysfunction in vivo. Biochim Biophys Acta Mol Basis Dis. 2024;1870:166990
22. Vicen M, Vitverova B, Havelek R, Blazickova K, Machacek M, Rathouska J. et al. Regulation and role of endoglin in cholesterol-induced endothelial and vascular dysfunction in vivo and in vitro. FASEB J. 2019;33:6099-114
23. Rathouska J, Jezkova K, Nemeckova I, Nachtigal P. Soluble endoglin, hypercholesterolemia and endothelial dysfunction. Atherosclerosis. 2015;243:383-8
24. Vitverova B, Blazickova K, Najmanova I, Vicen M, Hyspler R, Dolezelova E. et al. Soluble endoglin and hypercholesterolemia aggravate endothelial and vessel wall dysfunction in mouse aorta. Atherosclerosis. 2018;271:15-25
25. Varejckova M, Gallardo-Vara E, Vicen M, Vitverova B, Fikrova P, Dolezelova E. et al. Soluble endoglin modulates the pro-inflammatory mediators NF-kappaB and IL-6 in cultured human endothelial cells. Life Sci. 2017;175:52-60
26. Cruz-Gonzalez I, Pabon P, Rodriguez-Barbero A, Martin-Moreiras J, Pericacho M, Sanchez PL. et al. Identification of serum endoglin as a novel prognostic marker after acute myocardial infarction. J Cell Mol Med. 2008;12:955-61
27. Cui S, Lu SZ, Chen YD, He GX, Meng LJ, Liu JP. et al. Relationship among soluble CD105, hypersensitive C-reactive protein and coronary plaque morphology: an intravascular ultrasound study. Chin Med J (Engl). 2008;121:128-32
28. Chen J, Jiang L, Yu XH, Hu M, Zhang YK, Liu X. et al. Endocan: A Key Player of Cardiovascular Disease. Front Cardiovasc Med. 2021;8:798699
29. Qiu CR, Fu Q, Sui J, Zhang Q, Wei P, Wu Y. et al. Endocan: Endothelial Dysfunction, Inflammation, or Both? Angiology. 2017;68:80
30. Gok M, Kundi H, Kiziltunc E, Topcuoglu C, Ornek E. Endocan Levels and Coronary Collateral Circulation in Stable Angina Pectoris: A Pilot Study. Angiology. 2018;69:43-8
31. Behnoush AH, Khalaji A, Bahiraie P, Alehossein P, Shobeiri P, Peisepar M. et al. Endocan as a marker of endothelial dysfunction in hypertension: a systematic review and meta-analysis. Hypertens Res. 2023;46:2388-99
32. Kose M, Emet S, Akpinar TS, Kocaaga M, Cakmak R, Akarsu M. et al. Serum Endocan Level and the Severity of Coronary Artery Disease: A Pilot Study. Angiology. 2015;66:727-31
33. Bessa J, Albino-Teixeira A, Reina-Couto M, Sousa T. Endocan: A novel biomarker for risk stratification, prognosis and therapeutic monitoring in human cardiovascular and renal diseases. Clin Chim Acta. 2020;509:310-35
34. Cristina Igreja Sa I, Tripska K, Alaei Faradonbeh F, Hroch M, Lastuvkova H, Schreiberova J. et al. Labetalol and soluble endoglin aggravate bile acid retention in mice with ethinylestradiol-induced cholestasis. Front Pharmacol. 2023;14:1116422
35. Wohlfahrt P, Jenca D, Melenovsky V, Sramko M, Kotrc M, Zelizko M. et al. Trajectories and determinants of left ventricular ejection fraction after the first myocardial infarction in the current era of primary coronary interventions. Front Cardiovasc Med. 2022;9:1051995
36. Wohlfahrt P, Jenca D, Stehlik J, Melenovsky V, Mrazkova J, Stanek V. et al. Heart failure-related quality-of-life impairment after myocardial infarction. Clin Res Cardiol. 2023;112:39-48
37. Varjabedian L, Bourji M, Pourafkari L, Nader ND. Cardioprotection by Metformin: Beneficial Effects Beyond Glucose Reduction. Am J Cardiovasc Drugs. 2018;18:181-93
38. Pedersen TR. Pleiotropic effects of statins: evidence against benefits beyond LDL-cholesterol lowering. Am J Cardiovasc Drugs. 2010;10(Suppl 1):10-7
39. Saita E, Miura K, Suzuki-Sugihara N, Miyata K, Ikemura N, Ohmori R. et al. Plasma Soluble Endoglin Levels Are Inversely Associated With the Severity of Coronary Atherosclerosis-Brief Report. Arterioscler Thromb Vasc Biol. 2017;37:49-52
40. Rathouska J, Fikrova P, Mrkvicova A, Blazickova K, Varejckova M, Dolezelova E. et al. High soluble endoglin levels do not induce changes in structural parameters of mouse heart. Heart Vessels. 2017;32:1013-24
41. Heffernan KS, Kuvin JT, Patel AR, Karas RH, Kapur NK. Endothelial function and soluble endoglin in smokers with heart failure. Clin Cardiol. 2011;34:729-33
42. Kapur NK, Heffernan KS, Yunis AA, Parpos P, Kiernan MS, Sahasrabudhe NA. et al. Usefulness of soluble endoglin as a noninvasive measure of left ventricular filling pressure in heart failure. Am J Cardiol. 2010;106:1770-6
43. Vicen M, Igreja Sa IC, Tripska K, Vitverova B, Najmanova I, Eissazadeh S. et al. Membrane and soluble endoglin role in cardiovascular and metabolic disorders related to metabolic syndrome. Cell Mol Life Sci. 2021;78:2405-2418
44. Gallardo-Vara E, Tual-Chalot S, Botella LM, Arthur HM, Bernabeu C. Soluble endoglin regulates expression of angiogenesis-related proteins and induction of arteriovenous malformations in a mouse model of hereditary hemorrhagic telangiectasia. Dis Model Mech. 2018;11:dmm034397
45. Chen Y, Hao Q, Kim H, Su H, Letarte M, Karumanchi SA. et al. Soluble endoglin modulates aberrant cerebral vascular remodeling. Ann Neurol. 2009;66:19-27
46. Yu J, Li Y, Hu J, Wang Y. Interleukin-33 induces angiogenesis after myocardial infarction via AKT/eNOS signaling pathway. Int Immunopharmacol. 2024;143:113433
47. Zhao T, Kecheng Y, Zhao X, Hu X, Zhu J, Wang Y. et al. The higher serum endocan levels may be a risk factor for the onset of cardiovascular disease: A meta-analysis. Medicine (Baltimore). 2018;97:e13407
48. Aminuddin A, Cheong SS, Roos NAC, Ugusman A. Smoking and Unstable Plaque in Acute Coronary Syndrome: A Systematic Review of The Role of Matrix Metalloproteinases. Int J Med Sci. 2023;20:482-92
49. Osenkowski P, Toth M, Fridman R. Processing, shedding, and endocytosis of membrane type 1-matrix metalloproteinase (MT1-MMP). J Cell Physiol. 2004;200:2-10
50. Ikemoto T, Hojo Y, Kondo H, Takahashi N, Hirose M, Nishimura Y. et al. Plasma endoglin as a marker to predict cardiovascular events in patients with chronic coronary artery diseases. Heart Vessels. 2012;27:344-51
51. Kjeldsen EW, Thomassen JQ, Frikke-Schmidt R. HDL cholesterol concentrations and risk of atherosclerotic cardiovascular disease - Insights from randomized clinical trials and human genetics. Biochim Biophys Acta Mol Cell Biol Lipids. 2022;1867:159063
Author contact
![]() Corresponding author: Nachtigal Petr, PhD, Department of Biological and Medical Sciences, Faculty of Pharmacy in Hradec Kralove, Charles University, Heyrovskeho 1203, Hradec Kralove 500 05, Czech Republic, E-mail address: petr.nachtigalcuni.cz.
Corresponding author: Nachtigal Petr, PhD, Department of Biological and Medical Sciences, Faculty of Pharmacy in Hradec Kralove, Charles University, Heyrovskeho 1203, Hradec Kralove 500 05, Czech Republic, E-mail address: petr.nachtigalcuni.cz.

 Global reach, higher impact
Global reach, higher impact