3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(10):2382-2397. doi:10.7150/ijms.111775 This issue Cite
Research Paper
Cardiovascular System is Influenced by Skeletal Muscle-derived Extracellular Vesicles, Myokines and MicroRNAs Based on Interorgan Communication: A Systematic Review
1. Key Laboratory of Chinese Internal Medicine of Ministry of Education and Beijing, Dongzhimen Hospital Beijing University of Chinese Medicine, Beijing, China.
2. Department of Cardiology, Dongzhimen Hospital Beijing University of Chinese Medicine, Beijing, China.
Received 2025-2-8; Accepted 2025-4-1; Published 2025-4-28
Abstract
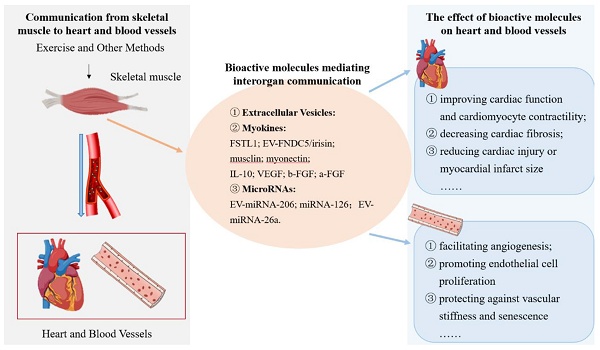
Background/Aims: To illustrate the types and roles of skeletal muscle-derived bioactive molecules in mediating the communication from skeletal muscle to the heart and blood vessels.
Methods: A systematic literature search was performed in four different databases. Eligible articles were screened using the inclusion and exclusion criteria. Two researchers independently performed literature screening and selection, data extraction and literature quality analysis.
Results: This study included 29 articles (2 clinical studies and 27 basic studies). Data analysis of the included studies revealed that skeletal muscle synthesizes and releases abundant extracellular vesicles (EVs), myokines (FSTL1, FNDC5/irisin and others) and microRNAs (miRNA-126 and others) to mediate the communication from skeletal muscle to the heart and blood vessels. Certain skeletal muscle-derived EVs, myokines and miRNAs were found to enhance cardiac function, reduce cardiac fibrosis and inhibit cardiac injury, and improve apoptosis and inflammation. In the blood vessels, these bioactive molecules stimulated angiogenesis, improved endothelial cell function, protected against vascular stiffness, and attenuated atherosclerosis and neointimal hyperplasia. Notably, IL-10, FSTL1, b-FGF, VEGF, irisin, musclin, myonectin, exo-miRNA26a, and miRNA-126 definitely played protective roles in the heart and blood vessels through interorgan communication.
Conclusion: Skeletal muscle synthesizes and releases EVs, myokines and miRNAs, which mediate the communication from skeletal muscle to the heart and blood vessels. The majority of these bioactive molecules are associated with cardiovascular protective effects. And they may provide new targets for more in-depth mechanism and clinical researches of communication from skeletal muscle to the heart and blood vessels.
Keywords: skeletal muscle, heart, blood vessels, interorgan communication, bioactive molecules, myokines
Introduction
Globally, cardiovascular diseases (CVDs) are the leading cause of death in humans. Furthermore, the end-stage of CVDs often manifests as multi-organ and multi-systemic disorder, including skeletal muscle [1, 2]. The majority of CVDs patients are accompanied by skeletal muscle wasting and exercise intolerance. Similarly, muscle wasting is associated with higher cardiovascular mortality [3, 4], but the mechanism remains unclear. As is well known, exercise training attenuates muscle wasting [5, 6] and promotes cardiac rehabilitation in CVDs patients [7, 8]. The beneficial effects of exercise training may be related to the synthesis and release of bioactive molecules during rapid contraction of the muscle fibers. Skeletal muscle is the largest organ in the human body. Previously, it was regarded merely as a locomotor organ. However, after 2000, skeletal muscle was gradually considered as an endocrine organ because A. Steensberg discovered that skeletal muscle secreted abundant interleukin (IL)-6 after exercise in human [9]. Later, the concept of myokines was proposed.
Myokines are cytokines and other peptides produced, expressed, and released by muscle fibers. They exert autocrine, paracrine and endocrine effects [10]. Skeletal muscle secretes abundant extracellular vesicles (EVs), myokines and microRNAs (miRNAs) induced by exercise to exert on the heart and blood vessels [11, 12]. This is a novel mechanism by which exercise protects cardiovascular system. Though a lot of researches related to myokines has not focused on EVs, emerging evidence demonstrates that the majority of them are transported via EVs. In other words, EVs are a crucial vehicle in mediating interorgan communication [13, 14]. Therefore, skeletal muscle cells can be regarded as donor cells that alter the physiological and pathological conditions in the recipient cells through bioactive molecules with EVs. Although there is no direct physical connection between the heart and the skeletal muscle, skeletal muscle-derived EVs, myokines and miRNAs act on the heart and the blood vessels through interorgan communication and paracrine pathways. Thus, it is essential to summarize the mechanisms and roles by which the skeletal muscle communicates with the heart or blood vessels under pathological and physiological conditions.
The sources of myokines are multifaceted and complex. Most of myokines are primarily secreted by the skeletal muscle, but they are also secreted by other tissues or organs. Previous review [12] articles about myokines did not explicitly describe that the source of myokines was skeletal muscle and that the target organs were the heart or blood vessels. Moreover, it did not involve in EVs and miRNAs. This article investigates the mechanisms and roles of bioactive molecules secreted by skeletal muscle on the heart or blood vessels under various cardiovascular disease models. It for the first time systematically summarizes that skeletal muscle-derived EVs, myokines and miRNAs modulate the functional and structural status of the heart and blood vessels through interorgan communication.
Methods
This systematic review was based on the PRISMA 2020 Statement.
Information Sources and Search Strategy
The published articles were searched comprehensively in four electronic databases (PubMed, Embase, Web of Science and Cochrane Library) from inception to June 16, 2024. To collect as much data as possible, we did not restrict the language of the articles. The keywords and Medical Subject Heading (MeSH) terms used in these searches were as follows: ((Cardiovascular Diseases) OR (Cardiovascular System)) AND (Muscle, Skeletal) AND (Extracellular Vesicles, miRNAs, Myokines, musclin/ostn, irisin/FNDC5, IL-6, IL-8, IL-10, IL-15, IL-1β, IL-1α, SPARC, OSM, Fibroblast Growth Factor 21, Fibroblast Growth Factor 2, Decorin, Myostatin, Leukemia Inhibitory Factor, myonectin, Apelin, Follistatin-Related Proteins, Angiopoietin-Like Protein 4, Growth Differentiation Factor 15, Chemokine CX3CL1, Chemokine CXCL10, Chemokine CXCL12, Osteoglycin, Chitinase-3-Like Protein 1, erythroferrone, Ciliary Neurotrophic Factor, Metrn, BRINP3, Ctsb, Ketoglutaric Acids, MG53). The searches were performed without any filtering. The specific study retrieval strategy is described in the Supplement 1. Subsequently, the references of the extracted studies and reviews were further searched to identify more relevant studies.
Inclusion and Exclusion Criteria
The inclusion criteria for the clinical studies and the basic studies were as follows: (1) Articles regarding EVs, myokines or miRNAs secreted by the skeletal muscle, including using skeletal muscle-specific gene knockout or overexpression, intramuscular injection of recombinant protein or plasmid and changes of expression level of bioactive molecules in skeletal muscle through other stimulation. (2) Articles regarding bioactive molecules targeting the heart or blood vessels; (3) Clinical trials, animal studies, and cell studies; (4) Articles without language restriction; studies published in the official journals.
The exclusion criteria were as follows: (1) Articles with incomplete information; (2) Experimental data with detection of bioactive molecules only in blood and absence of data from the skeletal muscle tissue and cells.
Study Selection and Data Extraction
Titles and abstracts of all the articles were assessed independently by two researchers (JX.C. and JX.Z.) based on the inclusion and exclusion criteria. Firstly, the initial results were analyzed and duplicate articles from different databases were removed. Secondly, according to inclusion and exclusion criteria, irrelevant articles were eliminated after reading the title and the abstract. Finally, the full texts of the remaining articles were evaluated and the relevant studies were included.
Two authors (JX.C. and JX.Z.) individually extracted data from the included literature using a standardized sheet prepared for this review. The extracted data included author, year, journal, country, sources of bioactive molecules, ways of producing bioactive molecules, objects of study, models/diseases, targets of the bioactive molecules, whether carried by EVs or not, and the effect of bioactive molecules on the heart and blood vessels. Any disagreement between the two authors was resolved by discussion and consultation with the corresponding authors (MJ.Z. and XW.H.).
Assessment of Bias Risk and Study Quality
Quality assessment of the included animal studies was systematically performed using SYRCLE's Risk of Bias Tool [15] a validated instrument specifically designed for preclinical animal research. This comprehensive tool evaluates methodological rigor across ten critical domains: selection bias (random sequence generation and baseline characteristics), performance bias (random housing and blinding), detection bias (random outcome assessment and blinding), attrition bias (incomplete outcome data), reporting bias (selective outcome reporting), and other potential sources of bias. Each criterion was rigorously appraised and categorized as "low risk," "high risk," or "unclear risk" based on the reported study methodologies, with particular attention to randomization procedures, blinding protocols, outcome assessment, and data completeness.
Quality assessment of included clinical trials was systematically evaluated using the Joanna Briggs Institute (JBI) Critical Appraisal Tool [16], a rigorously validated instrument specifically designed to assess risk of bias in clinical research, with particular applicability to correlational study designs. The evaluation criteria included ten items. Each item was evaluated as “Yes”, No”, “Unclear” or “Not Applicable”.
Results
Literature Search Results
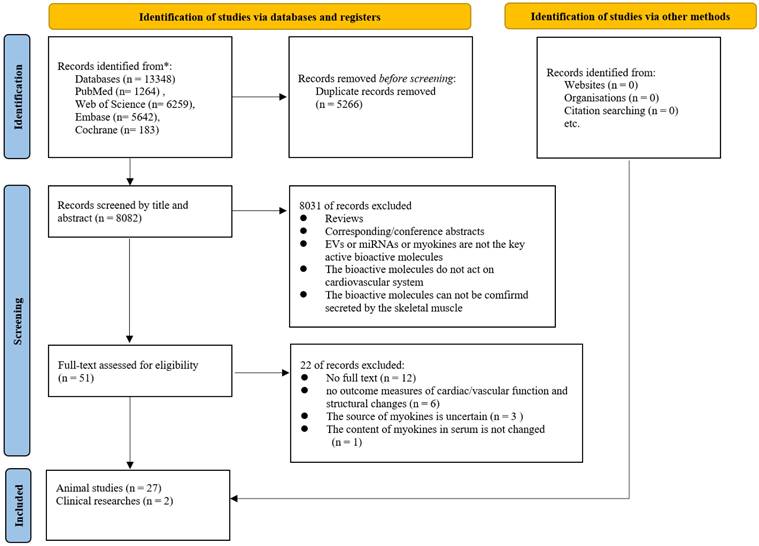
The flowchart of the literature search and study selection process was presented in Figure 1. Based on the initial search criteria, we identified 13,348 records from four electronic databases. After removing 5,266 duplicate articles, we screened the remaining 8,082 records by title and abstract, excluding 8,081 articles. Following full-text evaluation of the remaining 51 articles, we excluded 22 studies. Finally, 29 articles [17-45] (2 clinical studies and 27 basic studies) were included in this study.
Quality Assessment of Included Studies
The risk of bias assessment of the included animal experimental studies was presented in Supplement 2. According to SYRCLE's ROB tool evaluation, several methodological characteristics - including sequence generation, random outcome assessment, blinding procedures, and other potential bias sources - remained unclear. However, most studies demonstrated low risk concerning baseline characteristics, random housing, incomplete outcome data, and selective outcome reporting.
The JBI critical appraisal quality assessment results for the risk of bias and the method quality of the two included clinical studies were shown in Supplement 2. Both investigations followed standardized protocols for all the participants and were deemed methodologically reliable. These studies provided comprehensive reporting of participant demographics and clinical characteristics, clearly documented outcomes and follow-up results, and employed appropriate statistical analyses.
Basic Characteristic of Selected Research
Two clinical studies and twenty-seven basic studies were included. The details of them were summarized in Table 1. The highest number of published articles were authored by researchers from Japan, followed by those from the United States and China. The selected studies primarily investigated the mechanisms and effects of skeletal muscle-derived bioactive molecules on using various animal models and cell cultures. These bioactive molecules originated from skeletal muscle tissues, C2C12 mouse myoblasts or recombinant proteins. The production methods of these molecules were categorized as: exogenous administration (n=19), endogenous stimulation (n=6), or both approaches (n=4). The exogenous methods involved stimulation/injection of recombinant proteins, transgenic animal models, adeno-associated virus (AAV)- mediated gene delivery and molecular mimics. The specific methods of endogenous stimulation included exercise, remote limb ischemia, or muscle wasting models. The main animal models employed comprised: five studies using ischemia-reperfusion (I/R) models, four studies with heart failure (HF) models, and one study each for vascular injury and atherosclerosis (AS) models. Among the twenty-seven basic studies, cellular experiments were performed in eleven studies and animal experiments were performed in twenty-four studies. Additionally, nine articles specifically investigated EVs.
Flowchart of Literature Search and Screening.
The Effects of Skeletal Muscle-derived EVs on the Heart and Blood Vessels
Nine articles investigating EVs were included. From a cargo composition perspective, three studies EV-containing proteins including vascular endothelial growth factor (VEGF), fibronectin type III domain-containing protein 5/irisin (FNDC5/irisin), and musclin; four studies reported EV-associated microRNAs including miR-206, miR-16-5p, and miR-126; while two studies did not provide clear descriptions of EV contents. Given the pivotal role of EV cargos in mediating cardiovascular effects, their biological functions were systematically categorized into myokine-related and miRNA-related mechanisms based on respective cargo compositions, as detailed in subsequent sections. Notably, two studies did not investigate EV cargo components. One study demonstrated that oxidative muscle-derived EVs exhibited superior pro-angiogenic effects compared to glycolytic muscle-derived EVs, manifested through significant enhancing of human umbilical vein endothelial cell (HUVEC) viability, proliferation, migration, tube formation and activation of the protein kinase B (Akt) /endothelial nitric oxide synthase (eNOS) signaling pathway. Another key study revealed that exercise-induced muscle EVs attenuated atherosclerotic plaque size and number in apolipoprotein E-deficient (ApoE-/-) mice, while using GW4869 (an EV secretion inhibitor) counteracted these cardioprotective effects.
The Effects of Skeletal muscle-derived myokines on the Heart and Blood Vessels
Twenty-one studies investigated the effects of myokines on cardiac and vascular systems, with detailed findings presented in Table 2 and Supplement 3. The target organs or cells of myokines included the heart, cardiomyocytes, blood vessels and endothelial cells. Among them, there were fourteen studies targeting the heart or cardiomyocytes, and the main effects included improving cardiac function, alleviating myocardial fibrosis, alleviating cardiac injury and infarct size, inhibiting myocardium apoptosis and inflammatory response. There were thirteen articles targeting blood vessels or endothelial cells, some of which acted on both the heart and blood vessels. The primary effects on blood vessels included promoting angiogenesis and endothelial cell function.
The Basic Characteristics Information of All Studies
| Author, year | Journal | Country | Sources of bioactive molecules | Ways of producing bioactive molecules | Objects of study | Models/Diseases | Carried by EVs |
|---|---|---|---|---|---|---|---|
| Kargl, 2023 [44] | scientific reports | America | oxidative and glycolytic muscle tissue in mice | exogenous (bioactive molecules stimulation) | cells | N/A | Yes |
| Wang, 2023 [43] | Aging and Disease | China | muscle | endogenous (exercise) | animals | atherosclerosis | Yes |
| Li, 2023 [18] | American journal of physiology. Cell physiology | China | recombination proteins | exogenous (bioactive molecules stimulation) | cells | N/A | No |
| Xi, 2022 [21] | Journal Sport and Health Science | China | skeletal muscle | endogenous and exogenous (exercise and AAV) | animals | MI | No |
| recombination proteins | exogenous (bioactive molecules stimulation) | cells | N/A | ||||
| Malgorzata, 2022 [19] | Nature Communication | Germany | skeletal muscle | exogenous (AAV) | animals | HF | No |
| Hiroya, 2022 [17] | circulation journal | Japan | serum of Akt1 transgenic mice | exogenous (transgenic mice) | animals | skeletal muscle growth model | Yes |
| C2C12 mouse myoblasts | exogenous (bioactive molecules stimulation) | cells | Akt overexpression | ||||
| Chi, 2022 [25] | European Heart Journal | China | skeletal muscle | endogenous and exogenous (AAV and exercise) | animals | vascular ageing | Yes |
| recombination proteins | exogenous (bioactive molecules stimulation) | cells | vascular ageing | ||||
| Rubens, 2021 [22] | Frontiers in Physiology | America | skeletal muscle | endogenous (remote hind-limb ischemia) | animals | CHF model | Yes |
| Taiki, 2020 [23] | European Heart Journal | Japan | the atrophic limbs sarcopenic mice | endogenous and exogenous (TS and miRNA-16-5p mimics) | animals | MIRI mouse model | Yes |
| miRNA‑16‑5p mimics | cells | N/A | No | ||||
| Hiroko, 2020 [24] | Stem Cell Research Therapy | Japan | skeletal muscle | exogenous (cell transplantation) | animals | MI | No |
| Chen, 2020 [25] | Aging | China | temporal muscle | exogenous (agonist stimulation) | animals | CCI model | No |
| microRNA-126-5p mimics | cells | N/A | No | ||||
| Nie, 2019 [27] | Experimental Physiology | America | C2C12 myotube | exogenous (exogenous molecules injection) | cells | hypoxia | Yes |
| Wang, 2019 [26] | Theranostics | China | muscle satellite cells | exogenous (bioactive molecules injection) | animals | chronic kidney disease | Yes |
| Naoya, 2018 [28] | Circulation Research | Japan | skeletal muscle (soleus) | endogenous (exercise) | animals | MIRI | No |
| recombinant proteins | exogenous (bioactive molecules stimulation) | cells | hypoxia/reoxygenation | ||||
| João Lucas, 2017 [29] | Oxidative Medicine and Cellular Longevity | Brazil | skeletal muscle | endogenous (exercise) | animals | obesity | No |
| Megumi, 2014 [31] | Cardiovascular Research | Japan | skeletal muscle | exogenous (transgenic mice) | animals | vascular injury | No |
| recombinant proteins | exogenous (bioactive molecules stimulation) | cells | N/A | ||||
| Birgitte, 2013 [32] | Faseb Journal | Denmark | skeletal muscle fibers | endogenous (exercise) | subjects | N/A | Yes |
| Yutaka, 2013 [30] | Cell Medicine | Japan | N/A | exogenous (cells transplantation) | animals | MI | No |
| Cai, 2012 [34] | Basic Research in Cardiology | America | gastrocnemius muscle | endogenous (Remote ischemic preconditioning) | animals | MIRI | No |
| Stewart, 2012 [33] | Circulation-Heart Failure | Boston | skeletal muscle | endogenous (exercise) | subjects | HF | No |
| Yasuhiro, 2012 [45] | Plos one | Japan | skeletal muscle | exogenous (cells transplantation) | animals | MI | No |
| David, 2009 [35] | American Journal of Physiology-Regulatory Integrative and Comparative Physiology | America | recombinant proteins | exogenous (bioactive molecules injection) | animals | HF | No |
| Noriyuki, 2008 [36] | Journal of Biological Chemistry | Boston | gastrocnemius muscle | exogenous (AAV) | animals | hind limb ischemia model | No |
| cells | N/A | ||||||
| Steven, 2008 [37] | circulation | America | N/A | exogenous (cells transplantation) | animals | post-MI CHF | No |
| Efthimiadou, 2006 [39] | British Journal Sports Medicine | Greece | recombinant protein | exogenous (bioactive molecules injection) | animals | tenotomy model | No |
| Carvalho, 2006 [41] | Transplantation Proceedings | Brazil | N/A | exogenous (cells transplantation) | animals | MI | No |
| CD | |||||||
| Reza, 2006 [38] | Journal of Gene Medicine | Switzerland | left tibialis anterior muscle | exogenous (bioactive molecules injection) | animals | heterotopic heart transplantation | No |
| Claudia, 2006 [42] | International Journal of Cardiology | Brazil | skeletal myoblasts transfected withVEGF | exogenous (cells transplantation) | animals | MIRI | No |
| Efthimiadou, 2006 [40] | Journal of Sports Science | Greece | recombinant protein | exogenous (bioactive molecules injection) | animals | N/A | No |
AAV: adeno-associated virus; AKT: protein kinase B; CCI: chronic cerebral ischemia; CD: chagas's disease; CHF: chronic heart failure; EVs: extracellular vesicles; HF: heart failure; HUVECs: human umbilical vein endothelial cells; MI: myocardial infarction; MIRI: myocardial ischemia reperfusion injury; miRNA: microRNA; NRVMs: neonatal rat ventricular myocytes; N/A: Not Applicable; TS: tail suspension; VEGF: vascular endothelial growth factor.
Sixteen studies investigated myokines, including Follistatin-like 1 (FSTL1), musclin, myonectin, FNDC5/irisin, VEGF, IL-10, basic fibroblast growth factor (b-FGF), and acidic fibroblast growth factor (a-FGF), while five additional studies focused on skeletal muscle cell transplantation into the heart. Among them, FSTL1 was investigated in four studies. Three demonstrated its direct effects of HUVECs, promoting endothelial proliferation and migration and angiogenesis via activating vascular endothelial growth factor receptor 2 (VEGFR2)/Akt, Src/vascular endothelial cadherin (VE-Cadherin), phosphoinositide 3-kinase (PI3K)/AKT/eNOS, transforming growth factor beta 1 (TGFβ1)-Smad2/3 signaling pathways and up-regulating disco-interacting protein 2 homolog A (DIP2A) expression. Two of them used AAV to overexpress FSTL1. Another study found that skeletal muscle-specific FSTL1 knockout exacerbated percent luminal narrowing and neointimal thickening in response to vascular injury, while overexpression attenuated neointimal hyperplasia in vascular injury mice model, and suppressed proliferative and migratory activities of human aortic smooth muscle cells (HASMCs) through activation adenosine monophosphate-activated protein kinase (AMPK) signaling pathway. However, one study found that AAV-FSTL1 overexpression in skeletal muscle had no significant effect on promoting vascularization and improving cardiac function and remodeling in myocardial infarction (MI) rat model. Skeletal muscle-specific musclin overexpression in one study demonstrated its inhibition of cardiac fibrosis and dysfunction and improvement of ventricular contractility during chronic pressure overload via competitively bonding to the natriuretic peptide receptors C (NPRC) and increasing the C-type natriuretic peptide (CNP)/natriuretic peptide receptor B (NPR-B) signaling, while musclin knockout aggravated the advancement of HF. There were two studies focused on FNDC5/irisin. One literature showed the skeletal muscle-specific FNDC5/irisin knockout exacerbated vascular stiffness and senescence in aged or AngII-induced mice model by activating the DnaJ/ heat shock protein 40 (Hsp40) chaperone system to stabilize Sirtuin 6 (SIRT6). Another clinical trial found that FNDC5/irisin was positively related to aerobic performance in the HF patients. There were four studies related to VEGF, all of which described that VEGF promoted angiogenesis. Three of them found that it improved cardiac function and alleviated myocardial injury and fibrosis, thereby protecting the heart in cardiac injury models. Two studies respectively employed the methods of injecting VEGF protein into muscle or injecting skeletal muscle cells overexpressing VEGF into the heart. One study revealed that skeletal muscle-specific myonectin knockout aggravated myocardial ischemic injury by suppressing apoptosis and inflammatory reactions, whereas its overexpression attenuated myocyte apoptosis by promoting the cyclic adenosine monophosphate (cAMP)-dependent activation of AKT in myocardial ischemia reperfusion injury (MIRI) mice. There were two studies on the effects of IL-10. One study found that IL-10 reduced myocardial infarction size and improved cardiac function through the PI3K/Akt signaling pathway in MIRI rat. Another study found it prolonged graft survival and reduced histologic rejection by reducing neutrophil content in the allotransplanted heart model rat. Two studies related to b-FGF and a-FGF. Both showed that b-FGF increased cardiac angiogenesis by intramuscular injection. But skeletal muscle derived a-FGF did not significantly increase vascularization in infarcted heart. The remaining five studies evaluated skeletal muscle cell transplantation strategies, including myoblasts or non-myogenic cells sheets, triple-layer skeletal muscle (SkM) sheets, myogenic differentiation factor knockout (MyoD-/-) and wild-type myoblasts, skeletal myoblasts (SMBs) or mononuclear bone marrow cells (BMCs) and skeletal muscle and mesenchymal stem cells. All of them showed that they improved cardiac function in MI animals by inhibiting apoptosis or inflammation response. And three studies demonstrated angiogenesis promotion. One study found that employing cell sheets technology reduced the incidence of arrhythmia after transplantation.
Regarding the source of myokines, among twenty-one articles, individual studies had documented that FSTL1, FNDC5/irisin, musclin and myonectin were confirmed to originate exclusively from skeletal muscle through skeletal muscle-specific AAV interventions or transgenic mouse models, establishing their skeletal muscle origin and subsequent effects on the heart and blood vessels. Fourteen studies employed intramuscular injection of recombinant protein, non-muscle-specific AAV or plasmid, or skeletal muscle cells, including VEGF, b-FGF, a-FGF, FSTL1, IL-10 and various skeletal muscle cells. The remaining three studies employed either exercise or direct cellular interventions.
The Effects of Skeletal muscle-derived Myokines on the Heart and Blood Vessels
| Author, year | Sources of Myokines | Myokines | Models/ Diseases (Methods) | Targeted organs/ cells | Effects on heart/blood vessels |
|---|---|---|---|---|---|
| Li, 2023 [18] | exogenous FSTL1 | FSTL1 | N/A | HUVECs | rhFSTL1 stimulated HUVECs: cell proliferation and migration↑; angiogenesis↑; intercellular adhesion junctions↓ |
| Xi, 2022 [21] | skeletal muscle | FSTL1 | MI rat model (LAD ligation) | heart and blood vessels | AAV-FSTL1 overexpression regulated heart and blood vessels: vascularization of the infarcted myocardium→; cardiac function→; cardiac remodeling→ |
| rhFSTL1 | N/A | HUVECs | RhFSTL1 regulated HUVECs function: ECs proliferation↑; | ||
| Malgorzata, 2022 [19] | skeletal muscle | musclin | HF mice model (TAC) | heart | Skeletal muscle-derived musclin showed the effects in the heart: cardiac fibrosis↓; cardiac function↑; HF progression↓; ventricular contractility↑ |
| Chi, 2022 [20] | skeletal muscle | EV-FNDC5/irisin | vascular ageing mice model (Ang II treatment) | artery | Skeletal muscle-specific FNDC5 overxepression played a role in arteries: vascular stiffness and senescence↓; inflammatory responses↓ |
| exogenous FNDC5/irisin | vascular ageing (Ang II treatment) | VSMCs | Exogenous irisin added in AngII-induced VSMCs: vascular stiffness and senescence↓ | ||
| Rubens, 2021 [22] | skeletal muscle | EV- VEGF | CHF mice model (AV fistula) | heart and blood vessels | Skeletal muscle-derived exosomes exerted the effects in the heart and blood vessels: 1. myocardial fibrosis↓; cardiac function↑ 2. endothelial function↑; angiogenesis↑ |
| Hiroko, 2020 [24] | skeletal muscle | myoblasts or non-myogenic cells sheets | MI rat model | heart | Cell sheets consisting of myoblasts and non-myoblasts transplanted in heart: cardiac function↑; myocardial fibrosis↓; infarct size→; angiogenesis↑ |
| Naoya, 2018 [28] | soleus | myonectin | MIRI murine model | heart | Skeletal muscle-specific myonectin overexpression exerted the effects in the heart: cardiac function↑; ischemic injury↓; apoptosis↓; inflammatory responses↓ |
| recombinant myo nectin protein | H/R | myocytes | Myonectin treatment in myocytes: myocyte apoptosis↓ | ||
| Megumi, 2014 [31] | skeletal muscle | FSTL1 | vascular injury mice model (wire injury operation) | blood vessels | Skeletal muscle-specific FSTL1 overexpression played a role in the blood vessels: percent luminal narrowing↓; neointimal thickening↓; inflammatory responses↓ |
| rhFSTL1 | N/A | HASMCs | Recombinant human FSTL1 played a role in HASMCs: HASMCs proliferation and migratory↓ | ||
| Birgitte, 2013 [32] | endogenous | EV-VEGF | N/A | capillaries | Capillary growth↑ |
| Yutaka, 2013 [30] | N/A | triple-layer SkM sheets | MI porcine model (LAD occlusion) | heart | SkM Sheet transplanted in heart: cardiac function↑; ventricular arrhythmia vulnerability↓; local injury↓; inflammation responses↓ |
| Zheqing, 2012 [34] | gastrocnemius muscle | IL-10 | MIRI mice model | heart | RhIL-10 injected in IL-10 KO and RIPC mice: cardiac function↑; infarct size↓; cardiac contractility↑ |
| Stewart, 2012 [33] | skeletal muscle | FNDC5 | HF patients | heart | Skeletal muscle-derived FNDC5 played roles in HF patients: positively related to aerobic performance |
| Yasuhiro, 2012 [45] | skeletal muscle | MyoD-/- and wild-type myoblasts | MI rat model (LAD ligation) | heart | Wild-type and MyoD-/- myoblasts injected into infarcted mouse heart: cardiac function↑; infarct size↓; apoptosis↓; survival↑; angiogenesis↑ |
| David, 2009 [35] | rhVEGF | VEGF | HF (Bio-TO2 male hamsters) | heart and blood vessels | VEGF intramuscularly injected in the TO2 hamster: ventricular function↑; apoptosis↓; myocardial injury↓; myocardial fibrosis↓; cardiomyogenesis↑; angiogenesis↑ |
| Noriyuki, 2008 [36] | gastrocnemius muscle | FSTL1 | hind limb ischemia murine model | blood vessels | AAV-FSTL1 overexpression played a role in mice: revascularization↑ |
| N/A | N/A | HUVECs | AAV-FSTL1 overexpression played a role in HUVECs: endothelial cell function↑; apoptosis↓; survival↑ | ||
| Steven, 2008 [37] | N/A | SMBs or mononuclear BMCs | post-MI CHF rats (LAD occlusion) | heart | SMBs or mononuclear BMCs transplanted in the heart: cardiac function↑; inflammatory responses↓; gap junctions between grafts and native cardiomyocytes↓ |
| Efthimiadou, 2006 [39] | injected in the right gastrocnemius muscle | b-FGF | tenotomy rat model | blood vessels | B-FGF injection into the gastrocnemius muscle exerted a role in blood vessels: angiogenesis↑ |
| Carvalho,2006 [41] | N/A | skeletal muscle and mesenchymal stem cells | MI rat model (LAD occlusion) | heart and blood vessels | Skeletal muscle and mesenchymal stem cells transplanted in the heart: cardiac function↑; myotube formation↑; angiogenesis↑ |
| CD rat model | Skeletal muscle and mesenchymal stem cells transplanted in the heart: cardiac function↑; angiogenesis↑ | ||||
| Reza, 2006 [38] | injected in lefttibialis anterior muscle | IL-10 | heterotopic heart transplantation rats | heart | Injection of IL-10 plasmid into the tibialis anterior muscle played a role in the heart: graft survival↑; histologic rejection↓; neutrophil content↓ |
| Claudia, 2006 [42] | skeletal myoblasts transfected withVEGF | myoblasts with overexpression VEGF | MIRI rat model (LAD occlusion) | heart and capillary | Injection of skeletal myoblasts with overexpression VEGF into the MIRI rat heart: infarct size↓; cardiac damage↓; angiogenesis↑ |
| Anna, 2006 [40] | injected intramuscularly in the right gastrocnemius | b-FGF, a-FGF | Rats | heart and blood vessels | Intramuscular injection of b-FGF and a-FGF into the gastrocnemius muscle resulted in the effects on cardiac vessels: b-FGF: angiogenesis↑ a-FGF: angiogenesis→ |
AAV: adeno-associated Virus; Ang II: angiotensin I; AV: aorta-vena-cava; a-FGF: acidic- fibroblast growth factor; BMCs: bone marrow cells; b-FGF: basic-fibroblast growth factor; CD: chagas's disease; CHF: chronic heart failure; EV: extracellular vesicle; FNDC5: fibronectin type-III domain-containing protein 5; FSTL1: follistatin-like 1; HASMCs: human aortic smooth muscle cells; HF: heart failure; HUVECs: human umbilical vein endothelial cells; H/R: hypoxia/reoxygenation; IL-10: interleukin-10; KO: knockout; LAD: left anterior descending; MI: myocardial infarction; MIRI: myocardial ischemia reperfusion injury; MyoD-/-: myogenic differentiation factor knockout; N/A: not Applicable; rhVEGF: recombinant human vascular endothelial growth factor; RIPC: remote ischemic preconditioning; SkM: skeletal muscle; SMBs: skeletal myoblasts; TAC: transverse aortic constriction; VEGF: vascular endothelial growth factor; VSMCs: vascular smooth muscle cells;
The Effects of Skeletal Muscle-Derived miRNAs on the Heart and Blood Vessels
Six studies investigated the effects of skeletal muscle-derived miRNAs on cardiovascular systems, as detailed in Table 3 and Supplement 4. Different miRNAs played different roles. There were two studies targeting the heart or cardiomyocytes to protect or injury the heart. There were four articles targeting blood vessels or endothelial cells to promote angiogenesis and endothelial cell function. These skeletal muscle-derived miRNAs mainly included miRNA-206, miRNA-16-5p, miRNA-26a, and miRNA-126. Among them, two studies demonstrated that miRNA-206 improved endothelial cell function and promoted angiogenesis via reactive oxygen species (ROS)/ nuclear factor kappa B (NF-κB) signaling pathway, but not VEGF-dependent signaling pathway. They respectively utilized miRNA-206 mimics and EV-miRNA-206 in HUVECs. One study mainly showed that elevated miRNA-16-5p from atrophic muscle disturbed cardiac repair by modulating the apoptosis and inhibiting autophagy in MIRI mice. A single study highlighted that exo-miRNA-26a, containing muscle surface peptides, attenuated cardiac fibrosis and improved cardiac function through mediating forkhead box O1 (FOXO1) downregulation in chronic kidney disease (CKD) mice. Two studies indicated that miRNA-126 promoted endothelial cells (ECs) proliferation and angiogenesis via the PI3K/Akt signaling pathway. They respectively employed exercise or miRNA-126-5p agomir to increase the expression of miRNA-126-5p. Regarding the source of miRNAs, one study used muscle peptide-labeled exosomes carrying miRNA-26a, three employed miRNA plasmids/mimics (for miRNA-206, -16-5p, and -126), and two utilized exercise or direct cellular interventions.
The Effects of EVs and Bioactive Molecules from Skeletal Muscle on Heart
Seventeen studies investigated the effects of skeletal muscle-derived EVs, myokines, miRNAs, and cells on the heart. These details were shown in Table 4. This article summarized different roles of the bioactive molecules and skeletal muscle cells on the heart. Twelve studies each reported that musclin, VEGF, IL-10, myonectin, myoblasts, skeletal muscle cells and exo-miRNA-26a enhanced cardiac function. Among them, musclin and IL-10 performed improvement of cardiomyocyte contractility. Five studies respectively reported that musclin, VEGF, myoblasts and exo-miRNA-26a decreased cardiac fibrosis. Six studies respectively showed that myonectin, IL-10, VEGF, skeletal muscle cells and myoblasts reduced cardiac injury or myocardial infarct size in animal models of I/R injury. Three studies each demonstrated that myonectin, myoblasts and VEGF protected the heart via suppressing myocardial apoptosis. Three studies respectively showed that myonectin, skeletal muscle cells and myoblasts inhibited inflammatory responses. One clinical study reported that FNDC5/irisin was associated with improving aerobic performance in the HF patients. One article showed that skeletal muscle-derived VEGF attenuated progression of HF by promoting myocardial regeneration and cardiac repair. One study demonstrated that EV-miRNA-16-5p dysregulated the balance between apoptosis and autophagy under oxidative stress; specifically, promoting cardiomyocyte apoptosis and decreasing autophagy.
The Effects of EVs and Bioactive Molecules from Skeletal Muscle on Blood Vessels
Twenty studies reported on the roles of skeletal muscle-derived EVs, bioactive molecules, and cells on the blood vessels. The detailed results presented in Table 5. Seventeen articles each demonstrated that FSTL1, EV-miRNA-206, VEGF, myoblasts, b-FGF, skeletal muscle cells and miRNA-126 promoted angiogenesis and capillary growth. Nine studies confirmed that FSTL1, skeletal muscle-derived EVs and MyoD-/- and wild-type myoblasts, miRNA-126-5p and EV-miRNA-206 improved endothelial cell function, including its proliferation, migration and tube formation. One study demonstrated that FSTL1 alleviated neointimal hyperplasia by inhibiting the proliferation and migration of smooth muscle cells. One study demonstrated that skeletal muscle-derived EVs alleviated atherosclerosis though reducing the plaque size and number in aorta in the ApoE-/- mice, whereas exosomal inhibitor GW4869 counteracted the protective effects. One study showed that EV-irisin protected against vascular stiffness and senescence by increasing the stability of SIRT6.
The Effects of Skeletal muscle-derived MiRNAs on the Heart and Blood Vessels
| Author, year | Sources of MiRNAs | MiRNAs | Models/ Diseases (Methods) | Targeted organs/ cells | Effects on heart/blood vessels |
|---|---|---|---|---|---|
| Hiroya, 2022 [17] | serum of Akt1 transgenic mice | EV-miRNA-206 | mice skeletal muscle growth model (Akt1 transgenic mice) | blood vessels | MiR-206 mimics transfected in HUVECs: angiogenesis↑; endothelial cell function↑ |
| C2C12 mouse myoblasts | Akt overexpression (IGF-1 stimulation) | HUVECs | |||
| Taiki, 2020 [23] | atrophic limbs and heart of sarcopenic mice | EV-miRNA-16-5p | MIRI mouse model | heart | miR-16-5p plasmid intravenously injected in I/R-TS (-) mice: cardiac repair↓ |
| miR-16-5p mimics | N/A | NRVMs | miR‑16‑5‑p mimic added in NRVMs: apoptosis↑; autophagy↓ | ||
| Nie, 2019 [27] | C2C12 myotube | EV- miRNA-206 | hypoxia | HUVECs | Skeletal muscle-derived exosomes stimulated HUVECs: endothelial cell function↑; angiogenesis↑ |
| Chen, 2020 [25] | temporal muscle | miRNA126-5p | 2VO+EMS rat model | vessels | Transfection of temporal muscle with miRNA126-5p agomir played a role in the blood vessels of brain: revascularization↑; perfusion ratio↑ |
| microRNA-126-5p mimics | N/A | HUVECs | MicroRNA-126-5p mimics added in HUVECs: EC function↑; angiogenesis↑ | ||
| Wang, 2019 [26] | muscle satellite cells | Exo-miRNA-26a | CKD model mice (5/6 nephrectomize) | heart | Intramuscular injection of exo-miRNA-26a, containing muscle surface peptides, played the following roles in the heart: cardiac function↑; cardiac fibrosis↓. |
| João Lucas, 2017 [29] | skeletal muscle | miRNA-126 | obesity rat model | capillary | Skeletal muscle-derived miRNA-126 increasing: capillarity angiogenesis↑ |
Akt: protein kinase B; CKD: chronic kidney disease; EC: endothelial cells; EV: extracellular vesicle; HUVECs: human umbilical vein endothelial cells; IGF-1: insulin-like growth factor-1; I/R: ischemia and reperfusion; MIRI: myocardial ischemia reperfusion injury; miRNA: microRNA; NRVMs: neonatal rat ventricular myocytes; TS: tail suspension; 2VO+EMS: two-vessel occlusion plus encephalo-myo-synangiosis.
The Names of Bioactive Molecules from the Skeletal Muscle on the Heart
| Effects of bioactive molecules on heart | Names of bioactive molecules |
|---|---|
| improving cardiac function | EV- VEGF [22]; EV-miRNA-26a [26]; myoblasts or non-myogenic cells [24]; triple-layer SkM sheets [30]; VEGF×2 [35, 42]; skeletal myoblasts and bone marrow cells [37]; MyoD-/- and wild-type myoblasts [45]; IL-10 [34]; skeletal muscle and mesenchymal stem cells [41]; musclin [19]; myonectin [28] |
| decreasing cardiac fibrosis | EV-VEGF[22]; EV-miR-26a [26]; musclin [19]; VEGF [35]; myoblasts or non-myogenic cells [24] |
| reducing cardiac injury or myocardial infarct size | Myonectin [28]; IL-10 [34]; VEGF×2 [35, 42]; triple-layer SkM sheets [30]; MyoD-/- and wild-type myoblasts [45] |
| improving cardiomyocyte contractility | Musclin [19]; IL-10 [34]; |
| improving aerobic performance | FNDC5 [33]; |
| promoting myocardial regeneration | VEGF [35]; |
| Suppressing apoptosis | Myonectin [28]; MyoD-/- and wild-type myoblasts [45]; VEGF [35] |
| inhibiting inflammatory responses | Myonectin [28]; triple-layer SkM sheets [30]; skeletal myoblasts and bone marrow cells [37]; |
| reducing histologic rejection in the model of heart allotransplantation | IL-10 [38]; |
| disturbing cardiac repair, promoting apoptosis and decreasing autophagy | EV-miR-16-5p [23]; |
b-FGF: basic-fibroblast growth factor; BMCs: bone marrow cells; EVs: extracellular vesicles; FNDC5: fibronectin type-III domain-containing protein 5; FSTL1: follistatin-like 1; SkM: Skeletal Muscle; IL-10: interleukin-10; miRNA: microRNA; Skm: skeletal muscle; SMBs: skeletal myoblasts; VEGF: Vascular Endothelial Growth Factor.
The Names of Bioactive Molecules from the Skeletal Muscle on Blood Vessels
| Effects of bioactive molecules on blood vessels | Names of bioactive molecules |
|---|---|
| promoting angiogenesis or capillary growth | EVs×2 [27, 44]; EV-miR-206 [17]; EV-VEGF [22]; EV-VEGF [32]; FSTL1×3 [18, 21, 36]; miRNA-126 [29]; VEGF×2 [35, 42]; MyoD-/- and wild-type myoblasts [45]; b-FGF×2, a-FGF [39, 40]; skeletal muscle and mesenchymal stem cells [41]; myoblasts or non-myogenic cells [24]; miRNA-126-5p [25] |
| improving endothelial cell function | EVs×2 [27, 44]; EV-miR-206 [17]; FSTL1×3 [18, 21, 36]; miRNA-126-5p [25]; skeletal muscle and mesenchymal stem cells [41]; EV-VEGF [22] |
| alleviating neointimal hyperplasia | FSTL1 [31]; |
| alleviating atherosclerosis | EVs [43]; |
| protecting against vascular stiffness and senescence and inflammation | EV-FNDC5/irisin [20]; |
| reducing the plaque size and number in aorta | EVs [43]; |
b-FGF: basic-fibroblast growth factor; EVs: extracellular vesicles; FNDC5: fibronectin type-III domain-containing protein 5; FSTL1: follistatin-like 1; miRNA: microRNA; VEGF: vascular endothelial growth factor.
Discussion
Each organ did not function independently in human body. There existed the information exchange between them, which was called as interorgan communication [46]. It was essential for maintaining normal function and dynamic balance in human body. Interorgan communication was coordinated through a complex regulatory network of secretory factors [47] and adjusted response to environmental changes [46]. The bioactive molecules released by skeletal muscle played a dominant role because it was described as the body's largest endocrine organ. These EVs, myokines and miRNAs mediated the communication with the neighboring cells and distant organs by entering the extracellular fluid and blood circulation.
The systematic review of 2 clinical studies and 27 basic studies demonstrated that the skeletal muscle communicated with the heart and blood vessels by EVs, myokines, and miRNAs. Through summarizing the effects of skeletal muscular-derived myokines on the heart and blood vessels, our studies found that most of them improved cardiac function, reduced cardiac fibrosis and injury, inhibited apoptosis and inflammation, enhanced angiogenesis and endothelial cell function by targeting the various critical signaling pathways. Moreover, in the present studies, some investigated the protective effects of various bioactive molecules on the same animal model, for instance, VEGF, IL-10, myonectin and FSTL1 acting positive effects in the I/R model. However, the same bioactive molecule could elicit similar or distinct effects in different models, such as VEGF. Therefore, it was very valuable to analyze the specific roles of different myokines and miRNAs on the heart and blood vessels in same or different disease models.
The Effects of EVs on the Heart and Blood Vessels
This study concluded that skeletal muscle-derived EVs promoted angiogenesis and alleviated atherosclerosis [43, 44]. EVs were carriers of nucleic acids, proteins, lipids and other molecules from the parent cells. It protected these contents from hydrolysis. They mediated intercellular communication in a paracrine manner, or interorgan communication by entering the blood circulation and reaching the distant organs. Besides, EVs demonstrated exceptional stability, biocompatibility, wide biodistribution, and minimal immunogenicity. Thus, in recent years, EVs have been used to research intercellular and interorgan communication [48, 49], their value as novel clinical biomarkers [50], or as bioengineered vehicles for delivering genetic material or drugs [51, 52]. EVs were the main vehicles of skeletal muscle-derived bioactive molecules, though some studies did not focus on them, such as FSTL1 [53], IL-10 [54], FNDC5/irisin [55], VEGF [56], FGF [57], miRNA-126 [58]. However, these factors were the key to function. Their effects on the heart and blood vessels needed to be further investigated.
The Effects of Myokines on the Heart and Blood Vessels
Myokines were cytokines mainly produced and secreted by the skeletal muscle. They target and exert distant organs in an endocrine manner. Studies had identified hundreds of myokines [13], including inflammatory cytokines, growth factors, metabolic-related factors, and other factors. This study focused on the effects of myokines derived from the skeletal muscle on the heart and blood vessels.
Inflammatory Factors as Myokines
Inflammatory factors, such as IL-6 [10], IL-7 [59], IL-10, IL-15 [60], also acted as myokines. In this study, IL-10 was the main concern. It, as a well-known anti-inflammatory cytokine, suppressed activation and effector functions of the T cells, monocytes, and macrophages [61]. One included study [34] showed that intramuscular injection of IL-10 increased and protected the myocardium, reduced infarct size in the I/R model mice. Studies demonstrated enhancing the levels of IL-10 significantly improved left ventricular function, reduced infarct size, alleviated ventricular wall injury, and increased capillary density by activating signal transducer and activator of transcription 3 (STAT3) in the MI model mice [62-64]. Furthermore, another included study [38] showed that IL-10 suppressed of rejection of heart allograft transplantation by employing intramuscular administration of IL-10 plasmid. This directly substantiated the cardioprotective role of IL-10 through the communication from skeletal muscle to the heart. Generally, chronic rejection significantly affected the long-term survival of heart transplant patients. Studies demonstrated that IL-10 played a key role in the macrophage-mediated post-transplantation immune response. Researchers found that IL-10 alleviated chronic graft rejection by increasing macrophage M2 polarization [65], inhibiting T lymphocyte infiltration and cytotoxicity [66]. These results demonstrated that IL-10 protected against the myocardium injury and suppressed rejection of allogeneic heart transplantation partly through its anti-inflammatory role.
Growth Factors as Myokines
Skeletal muscle also produced and secreted growth factors, such as FGF [67, 68], VEGF[69, 70]. There were many subtypes of FGF, frequently, a-FGF and b-FGF. They activated intracellular signal transduction pathways by binding to the fibroblast growth factor receptor (FGFR) on the target cell surface, thereby promoting cellular proliferation, differentiation, and migration. Two included studies [39, 40] showed that intramuscular injection of b-FGF significantly increased angiogenesis in the rat heart and gastrocnemius muscles. B-FGF was a potent pro-angiogenic factor and acted in synergy with VEGF in various tissues [71, 72]. Therefore, intramuscular injection of b-FGF promoted angiogenesis in the heart through interorgan communication. Four included studies [22, 32, 35, 42] suggested that the main effects of VEGF were promoting angiogenesis and improving cardiac function and attenuating myocardial injury and fibrosis. Two of them [35, 42] respectively employed the methods of injecting VEGF protein into muscle or injecting skeletal muscle cells overexpressing VEGF into the heart. The family of VEGF included several subtypes, mainly involving VEGF-A, VEGF-B, VEGF-C and VEGF-D. Studies showed that VEGF-A/B inhibited cardiomyocyte apoptosis and activated the expression of genes associated with myocardial contraction and metabolism [73]. Designed protein VEGF-CC152S accelerated myocardial lymphangiogenesis, alleviated cardiac inflammation, fibrosis, and dysfunction, and reduced ventricular remodeling after MI [74]. Related studies showed that VEGF-A/C reduced fiber scar area by effectively promoting collateral circulation, reducing edema, and improving cardiac function after MI [75]. Different phenotypes of VEGF might have different effects on myocardium. But in general, they all protected the heart after MI.
Metabolism-Related Myokines
Irisin and musclin mainly derived from skeletal muscle and were important myokines. They were originally found to exerted an important role in metabolism [76, 77]. One included study [20] firstly found that irisin alleviated vascular stiffness and inflammation by stabilizing SIRT6 in the vascular injury models. Studies reported that SIRT6 prevented vascular smooth muscle cells (VSMCs) from aging and maintained vasodilation function by blocking the expression of NF-κB-dependent receptor [78, 79]. And irisin suppressed vascular aging by inhibiting inflammation and oxidative stress [80]. Other studies demonstrated that skeletal muscle-derived irisin played an anti-aging role in multiple target organs [81]. Another included study showed that irisin levels [33] showed positive correlation with the aerobic capacity of patients with HF after exercise. Aerobic capacity was affected by cardiac output, myocardial energy metabolism, as well as the functions of the skeletal muscle and the diaphragm. Irisin increased the cardiac output of zebrafish [82] and protected mitochondria and improved myocardial energy metabolism [83]. Irisin enhanced diaphragm function by increasing the levels of phospho-AMPK (pAMPK) [84]. Furthermore, muscle loss was associated with reduced levels of irisin protein [85]. Therefore, irisin protected vascular function through its anti-aging, anti-inflammation and anti-oxidative stress effects, and improved aerobic capacity by enhancing cardiac output, optimizing myocardial energy metabolism, and improving muscle function. One included study showed that musclin [19] inhibited myocardial fibrosis and increased myocardial contractility and accelerated HF progression via competitively bonding to the NPRC and increasing the CNP/NPRB signaling pathway in the HF model mice induced by transverse aortic constriction (TAC). In this study, using skeletal muscle-specific knockout of musclin and transgenic mice rendered the role of musclin in mediating skeletal muscle-heart communication more credible. Researches have proved that the partial amino acid sequences of musclin and natriuretic peptides (NPs) were homologous and it was an endogenous ligand of NPRC, but it did not demonstrate natriuretic peptide activity. Moreover, it entered blood circulation and acted on distant organs [86]. NPRC was natriuretic peptide clearance receptor. So musclin protected the myocardium by competitively binding to NPRC. It suppressed development of HF by alleviating myocardial fibrosis [87], reducing myocardial infarction size and improving myocardial mitochondrial homeostasis [88]. Musclin was derived mainly from skeletal muscle, and it protected the heart via the natriuretic peptide signaling.
Other Myokines
FSTL1 and myonectin were important myokines. There were four studies about FSTL1 [18, 21, 31, 36] in this study. They reported that the main roles of FSTL1 were promoting endothelial function and angiogenesis in different cell and animal models. Moreover, one of them [31] utilized skeletal muscle-specific FSTL1 transgenic mice model of vascular injury to confirm that FSTL1 reduced intimal hyperplasia. Previous studies have confirmed that FSTL1 promoted synthesis of angiogenic proteins during ischemic stress after MI by activating TGFβ-Smad2/3 [89] and AMPK and mediating the production of nitric oxide (NO) [90]. Epicardial injection of FSTL1 increased myocardial vascularization in the infarct margin area [91]. Therefore, the role of FSTL1 in improving intimal hyperplasia and promoting vascular regeneration was evident and confirmed in vascular injury models. Myonectin, also known as C1q/tumor necrosis factor (TNF)-associated protein 15/erythroferrone, was a cardioprotective factor [92]. According to the included studies, myonectin [28] reduced infarct size and inhibited cardiomyocyte apoptosis and macrophage inflammatory response in transgenic mice with skeletal muscle-specific overexpression of myonectin after MIRI. It proved that skeletal muscle-derived myonectin protected the heart trough circulation. Studies also have found that myonectin alleviated ventricular remodeling, myocardial fibrosis and dysfunction in the TAC mice [93, 94]. At present, myonectin was recognized a novel cardioprotective factor.
However, the results of two included studies were not inconsistent with previous studies. They respectively showed that a-FGF and FSTL1 did not significantly improve damaged heart vascularization. However, several studies had shown that a-FGF also played a role in angiogenesis. They reported that local injections of a-FGF into the heart of MI model pigs promoted angiogenesis in the heart infarcted areas [95, 96]. Possible reasons for this difference could be categorized into three: (1) they did not significantly increase in the myocardium; (2) blood perfusion was reduced in the infarct area; (3) they failed to reach the infarct area or exert their effects through the skeletal muscle-heart. Finally, the content of them in the infarct area was not sufficient to promote angiogenesis. Thus, their function in vascular regeneration following MI awaited further exploration through skeletal muscle-heart communication.
The Effect of MiRNAs on the Heart and Blood Vessels
MiRNAs were small non-coding RNAs that regulated gene expression in cells and tissues. They were usually carried by the EVs and participated in interorgan communication to activating or suppressing downstream signaling pathways by modulating target gene expression of the recipient organs or cells. Therefore, they might be used as potential therapeutic target for various human diseases, including CVDs [97].
In included study, exosomes, containing muscle surface peptides, loaded with miRNA-26a were injected into the muscle. Furtherly, skeletal muscle-derived miRNA-26a [26] improved heart function and reduced myocardial fibrosis in CKD mice. Related studies showed that miRNA-26a not only reduced myocardial fibrosis, regulated autophagy [98] and reduced type I collagen [99], but also inhibited myocardial cell damage and alleviated myocardial fibrosis in the diabetic cardiomyopathy mice [100]. Therefore, miRNA-26a played a positive role in the heart. In two included studies, the expression of miRNA-126 increased following the transfection of miRNA-126 into muscle tissue or exercise. And miRNA-126 [25, 29] promoted endothelial cell proliferation and migration, and increased angiogenesis through the communication from skeletal muscle to the heart. MiRNA-126 was a major physiological and pathological regulator of angiogenesis [101]. It regulated endothelial function by inhibiting endothelial cell apoptosis and promoted endothelium repair after injury [102, 103]. Therefore, miRNA-126 played a protective role in the blood vessels and endothelial cells. Exosomal miRNA-206 [17, 27] promoted angiogenesis by acting on endothelial cells. However, the underlying mechanisms were not clear and required further investigations. In included study, miRNA-16-5p [23] played a negative regulatory role by reducing autophagy, promoting cardiomyocyte apoptosis and inhibiting cardiac repair in the MIRI model mice after muscular atrophy. Related studies showed that reduced expression of miRNA-16-5p promoted hypertrophy of cardiomyocytes in the newborn rats [104] but induced angiogenesis in the hearts of obese rats by increasing VEGF expression [105]. Therefore, the underlying mechanisms of miRNA-16-5p on heart function and heart diseases required further in-depth analysis.
In summary, the degree of confidence in the role of skeletal muscle-derived myokines and miRNAs in the heart or blood vessels was inconsistent. Among them, IL-10, b-FGF, VEGF, irisin, musclin, myonectin and exo-miRNA26a, and miRNA-126 had definite protective effects on the heart or blood vessels. In particular, irisin, musclin and myonectin used skeletal muscle specific overexpression and demonstrated that they were involved in communication from skeletal muscle to the heart or blood vessels and had protective effects on them. FSTL1 derived from skeletal muscle had a definite protective effect on blood vessels. However, whether it and a-FGF mediated communication between skeletal muscle and heart was not clear. Moreover, researches on miRNA-206 and miRNA-16-5p were less and immature and not enough to prove their roles in included studies.
Different Targets and Functions of Various Myokines and miRNAs
Different myokines and miRNAs acted on the same pathological processes of the heart or blood vessels, while the same myokines and miRNAs could also influence different pathological processes. This phenomenon may be related to the broad range of molecular targets affected by myokines and miRNAs [106].
Limitations
This study still had a few limitations. Firstly, the included literature exhibited significant heterogeneity. Different myokines were capable of regulating various pathological processes in the same animal model, while the same myokine could influence similar or different pathological mechanisms across different models. Consequently, it was a challenge to draw consistent conclusions regarding the effects of some bioactive molecules derived from skeletal muscle on the heart and blood vessels. Secondly, a few studies on EVs did not perform tracking experiments to confirm EV transfer from skeletal muscle to the heart or blood vessels. Furthermore, none of the studies used specific fluorescent labeling to trace myocyte-derived myokines or miRNAs for subsequent observation in cardiac or vascular tissues. These issues need to be addressed in future studies.
Conclusion
This article systematically reviewed the included literature and analyzed the mechanisms by which skeletal muscle-derived EVs, myokines and miRNAs regulated cardiac and vascular function and structure, based on 2 clinical studies and 27 basic studies. Three main findings are summarized: First, there exists interorgan communication from skeletal muscle to the heart and blood vessels via myokines and miRNAs, some of which are carried and transported by EVs. Second, most of the myokines and miRNAs demonstrate beneficial effects on the heart and blood vessels. In the heart, they enhance cardiac function, reduce cardiac fibrosis and injury, and inhibit cardiomyocyte apoptosis and inflammation. In the blood vessels, they promote angiogenesis and capillary growth, improve endothelial cell function, protect against vascular stiffness and attenuate atherosclerosis and neointimal hyperplasia. Third, research on the role of skeletal muscle-derived myokines and miRNAs in the heart or blood vessels is uneven. Among these factors, IL-10, b-FGF, VEGF, irisin, musclin, myonectin, exo-miRNA26a, and miRNA-126 have been confirmed to exert protective effects on the heart or blood vessels through various physiological and pathological processes. Specifically, studies using skeletal muscle-specific overexpression demonstrate that irisin, musclin, and myonectin mediated communication from skeletal muscle to the heart or blood vessels, contributing to their protection. Additionally, skeletal muscle-derived FSTL1 has a clear protective effect on blood vessels; however, it remains unclear whether FSTL1 and a-FGF are involved in skeletal muscle-heart crosstalk. Furthermore, research on miRNA-206 and miRNA-16-5p remains limited and inconclusive, with insufficient evidence to support their roles in the included studies.
Overall, these results elucidate the mechanisms by which skeletal muscle-derived EVs, myokines and miRNAs improve cardiac and vascular function and structure through interorgan communication, as well as how exercise may prevent and ameliorate CVDs by bioactive molecules released by skeletal muscle. IL-10, FSTL1, b-FGF, VEGF, irisin, musclin, myonectin, exo-miRNA26a, and miRNA-126 provide new targets for further mechanistic and clinical research on communication from skeletal muscle to the heart and blood vessels. Meanwhile, these findings also provide a possibility for developing skeletal muscle-based therapies for CVDs.
Future Perspectives
The roles of myokines and miRNAs are complex, and more in-depth and comprehensive mechanism studies are needed in the future.
Acknowledgements
Throughout the writing of this article, I have received a great deal of support and assistance.
I would first like to thank my supervisor, Professor Zhao, whose expertise was invaluable in formulating the research questions and methodology. Your insightful feedback pushed me to sharpen my thinking. I would particularly like to acknowledge my team members, Xiaowan Han, Lei Wang, Xiang Ji and Sha Su, for working together to complete this manuscript.
Funding
This work was supported by the Young Scientists Fund of the National Natural Science Foundation of China (Grant Nos. 82204856 and 82104653), the Central High-level Hospital of Traditional Chinese Medicine, and the Wu Jieping Medical Foundation (Grant No. 320.6750.2022-25-3).
Author contributions
JC and XH: theme and design of the research. SS, XJ and JZ: verification of data. JC and JZ: writing of the manuscript. LW, XH and MZ: critical revision of the manuscript for intellectual content and obtaining funding. All authors have approved the final manuscript for submission.
Declarations
The authors declare that the work described has not been published previously, that it is not under consideration for publication elsewhere, that its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder. The authors declare that no generative AI and AI-assisted technologies has been used in the writing process.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Philippou A, Xanthis D, Chryssanthopοulos C. et al. Heart Failure-Induced Skeletal Muscle Wasting. Curr Heart Fail Rep. 2020;17:299-308
2. Damluji AA, Alfaraidhy M, AlHajri N. et al. Sarcopenia and Cardiovascular Diseases. Circulation. 2023;147:1534-1553
3. Chuang SY, Chang HY, Lee MS. et al. Skeletal muscle mass and risk of death in an elderly population. Nutr Metab Cardiovasc Dis. 2014;24:784-91
4. Attaway A, Bellar A, Dieye F. et al. Clinical impact of compound sarcopenia in hospitalized older adult patients with heart failure. J Am Geriatr Soc. 2021;69:1815-1825
5. Gallagher H, Hendrickse PW, Pereira MG. et al. Skeletal muscle atrophy, regeneration, and dysfunction in heart failure: Impact of exercise training. J Sport Health Sci. 2023;12:557-567
6. Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14:58-74
7. Valenzuela PL, Ruilope LM, Santos-Lozano A. et al. Exercise benefits in cardiovascular diseases: from mechanisms to clinical implementation. Eur Heart J. 2023;44:1874-1889
8. Chen H, Chen C, Spanos M. et al. Exercise training maintains cardiovascular health: signaling pathways involved and potential therapeutics. Signal Transduct Target Ther. 2022;7:306
9. Steensberg A, van Hall G, Osada T. et al. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000 529 Pt 1: 237-42
10. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379-406
11. Chow LS, Gerszten RE, Taylor JM. et al. Exerkines in health, resilience and disease. Nat Rev Endocrinol. 2022;18:273-289
12. Severinsen MCK, Pedersen BK. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr Rev. 2020;41:594-609
13. Whitham M, Parker BL, Friedrichsen M. et al. Extracellular Vesicles Provide a Means for Tissue Crosstalk during Exercise. Cell Metab. 2018;27:237-251.e4
14. Safdar A, Tarnopolsky MA. Exosomes as Mediators of the Systemic Adaptations to Endurance Exercise. Cold Spring Harb Perspect Med. 2018;8:a029827
15. Hooijmans CR, Rovers MM, de Vries RB. et al. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43
16. Munn Z, Barker TH, Moola S. et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18:2127-2133
17. Hayashi H, Izumiya Y, Ishida T. et al. Exosomal miR206 Secreted From Growing Muscle Promotes Angiogenic Response in Endothelial Cells. Circ J. 2024;88:425-433
18. Li MY, Gao RP, Zhu Q. et al. Skeletal muscle-derived FSTL1 starting up angiogenesis by regulating endothelial junction via activating Src pathway can be upregulated by hydrogen sulfide. Am J Physiol Cell Physiol. 2023;325:C1252-c1266
19. Szaroszyk M, Kattih B, Martin-Garrido A. et al. Skeletal muscle derived Musclin protects the heart during pathological overload. Nat Commun. 2022;13:149
20. Chi C, Fu H, Li YH. et al. Exerkine fibronectin type-III domain-containing protein 5/irisin-enriched extracellular vesicles delay vascular ageing by increasing SIRT6 stability. Eur Heart J. 2022;43:4579-4595
21. Xi Y, Hao M, Liang Q. et al. Dynamic resistance exercise increases skeletal muscle-derived FSTL1 inducing cardiac angiogenesis via DIP2A-Smad2/3 in rats following myocardial infarction. J Sport Health Sci. 2021;10:594-603
22. Homme RP, Zheng Y, Smolenkova I. et al. Remote Hind-Limb Ischemia Mechanism of Preserved Ejection Fraction During Heart Failure. Front Physiol. 2021;12:745328
23. Hayasaka T, Takehara N, Aonuma T. et al. Sarcopenia-derived exosomal micro-RNA 16-5p disturbs cardio-repair via a pro-apoptotic mechanism in myocardial infarction in mice. Sci Rep. 2021;11:19163
24. Iseoka H, Miyagawa S, Saito A. et al. Role and therapeutic effects of skeletal muscle-derived non-myogenic cells in a rat myocardial infarction model. Stem Cell Res Ther. 2020;11:69
25. Chen C, Ling C, Gong J. et al. Increasing the expression of microRNA-126-5p in the temporal muscle can promote angiogenesis in the chronically ischemic brains of rats subjected to two-vessel occlusion plus encephalo-myo-synangiosis. Aging (Albany NY). 2020;12:13234-13254
26. Wang B, Zhang A, Wang H. et al. miR-26a Limits Muscle Wasting and Cardiac Fibrosis through Exosome-Mediated microRNA Transfer in Chronic Kidney Disease. Theranostics. 2019;9:1864-1877
27. Nie Y, Sato Y, Garner RT. et al. Skeletal muscle-derived exosomes regulate endothelial cell functions via reactive oxygen species-activated nuclear factor-κB signalling. Exp Physiol. 2019;104:1262-1273
28. Otaka N, Shibata R, Ohashi K. et al. Myonectin Is an Exercise-Induced Myokine That Protects the Heart From Ischemia-Reperfusion Injury. Circ Res. 2018;123:1326-1338
29. Gomes JL, Fernandes T, Soci UP. et al. Obesity Downregulates MicroRNA-126 Inducing Capillary Rarefaction in Skeletal Muscle: Effects of Aerobic Exercise Training. Oxid Med Cell Longev. 2017;2017:2415246
30. Terajima Y, Shimizu T, Tsuruyama S. et al. Autologous Skeletal Myoblast Sheet Therapy for Porcine Myocardial Infarction Without Increasing Risk of Arrhythmia. Cell Med. 2014;6:99-109
31. Miyabe M, Ohashi K, Shibata R. et al. Muscle-derived follistatin-like 1 functions to reduce neointimal formation after vascular injury. Cardiovasc Res. 2014;103:111-20
32. Hoier B, Prats C, Qvortrup K. et al. Subcellular localization and mechanism of secretion of vascular endothelial growth factor in human skeletal muscle. Faseb j. 2013;27:3496-504
33. Lecker SH, Zavin A, Cao P. et al. Expression of the irisin precursor FNDC5 in skeletal muscle correlates with aerobic exercise performance in patients with heart failure. Circ Heart Fail. 2012;5:812-8
34. Cai ZP, Parajuli N, Zheng X. et al. Remote ischemic preconditioning confers late protection against myocardial ischemia-reperfusion injury in mice by upregulating interleukin-10. Basic Res Cardiol. 2012;107:277
35. Zisa D, Shabbir A, Mastri M. et al. Intramuscular VEGF repairs the failing heart: role of host-derived growth factors and mobilization of progenitor cells. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1503-15
36. Ouchi N, Oshima Y, Ohashi K. et al. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem. 2008;283:32802-11
37. Coppen SR, Fukushima S, Shintani Y. et al. A factor underlying late-phase arrhythmogenicity after cell therapy to the heart: global downregulation of connexin43 in the host myocardium after skeletal myoblast transplantation. Circulation. 2008;118:S138-44
38. Tavakoli R, Gazdhar A, Pierog J. et al. Electroporation-mediated interleukin-10 overexpression in skeletal muscle reduces acute rejection in rat cardiac allografts. J Gene Med. 2006;8:242-8
39. Efthimiadou A, Nikolettos NK, Lambropoulou M. et al. Angiogenic effect of intramuscular administration of basic fibroblast growth factor in atrophied muscles: an experimental study in the rat. Br J Sports Med. 2006;40:355-8 discussion 358
40. Efthimiadou A, Asimakopoulos B, Nikolettos N. et al. The angiogenetic effect of intramuscular administration of b-FGF and a-FGF on cardiac muscle: the influence of exercise on muscle angiogenesis. J Sports Sci. 2006;24:849-54
41. Carvalho KA, Guarita-Souza LC, Hansen P. et al. Cell transplantation after the coculture of skeletal myoblasts and mesenchymal stem cells in the regeneration of the myocardium scar: an experimental study in rats. Transplant Proc. 2006;38:1596-602
42. Becker C, Lacchini S, Muotri AR. et al. Skeletal muscle cells expressing VEGF induce capillary formation and reduce cardiac injury in rats. Int J Cardiol. 2006;113:348-54
43. Wang Y, Liu Y, Zhang S. et al. Exercise Improves Metabolism and Alleviates Atherosclerosis via Muscle-Derived Extracellular Vesicles. Aging Dis. 2023;14:952-965
44. Kargl CK, Jia Z, Shera DA. et al. Angiogenic potential of skeletal muscle derived extracellular vesicles differs between oxidative and glycolytic muscle tissue in mice. Sci Rep. 2023;13:18943
45. Nakamura Y, Asakura Y, Piras BA. et al. Increased angiogenesis and improved left ventricular function after transplantation of myoblasts lacking the MyoD gene into infarcted myocardium. PLoS One. 2012;7:e41736
46. Katagiri H. Inter-organ communication involved in metabolic regulation at the whole-body level. Inflamm Regen. 2023;43:60
47. Castillo-Armengol J, Fajas L, Lopez-Mejia IC. Inter-organ communication: a gatekeeper for metabolic health. EMBO Rep. 2019;20:e47903
48. Chen T, Ellman DG, Fang S. et al. Transfer of cardiomyocyte-derived extracellular vesicles to neighboring cardiac cells requires tunneling nanotubes during heart development. Theranostics. 2024;14:3843-3858
49. Xourafa G, Korbmacher M, Roden M. Inter-organ crosstalk during development and progression of type 2 diabetes mellitus. Nat Rev Endocrinol. 2024;20:27-49
50. Han C, Yang J, Sun J. et al. Extracellular vesicles in cardiovascular disease: Biological functions and therapeutic implications. Pharmacol Ther. 2022;233:108025
51. Zou A, Xiao T, Chi B. et al. Engineered Exosomes with Growth Differentiation Factor-15 Overexpression Enhance Cardiac Repair After Myocardial Injury. Int J Nanomedicine. 2024;19:3295-3314
52. Zhou P, Du X, Jia W. et al. Engineered extracellular vesicles for targeted reprogramming of cancer-associated fibroblasts to potentiate therapy of pancreatic cancer. Signal Transduct Target Ther. 2024;9:151
53. Yuan HX, Chen YT, Li YQ. et al. Endothelial extracellular vesicles induce acute lung injury via follistatin-like protein 1. Sci China Life Sci. 2024;67:475-487
54. Meier TB, Guedes VA, Smith EG. et al. Extracellular vesicle-associated cytokines in sport-related concussion. Brain Behav Immun. 2022;100:83-87
55. Tao L, Wang J, Wang K. et al. Exerkine FNDC5/irisin-enriched exosomes promote proliferation and inhibit ferroptosis of osteoblasts through interaction with Caveolin-1. Aging Cell. 2024;23:e14181
56. Wang CA, Chang IH, Hou PC. et al. DUSP2 regulates extracellular vesicle-VEGF-C secretion and pancreatic cancer early dissemination. J Extracell Vesicles. 2020;9:1746529
57. Olave NC, Halloran B, Ambalavanan N. FGF2 is Secreted in Extracellular Vesicles from Lung Cells. Am J Physiol Lung Cell Mol Physiol. 2024;327:L359-L370
58. Cui J, Liu N, Chang Z. et al. Exosomal MicroRNA-126 from RIPC Serum Is Involved in Hypoxia Tolerance in SH-SY5Y Cells by Downregulating DNMT3B. Mol Ther Nucleic Acids. 2020;20:649-660
59. Li B, Shaikh F, Zamzam A. et al. The Identification and Evaluation of Interleukin-7 as a Myokine Biomarker for Peripheral Artery Disease Prognosis. J Clin Med. 2024;13:3583
60. Nieman DC, Davis JM, Henson DA. et al. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J Appl Physiol (1985). 2003;94:1917-25
61. Moore KW, de Waal Malefyt R, Coffman RL. et al. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683-765
62. Jung M, Ma Y, Iyer RP. et al. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res Cardiol. 2017;112:33
63. Krishnamurthy P, Rajasingh J, Lambers E. et al. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ Res. 2009;104:e9-18
64. Holladay CA, Duffy AM, Chen X. et al. Recovery of cardiac function mediated by MSC and interleukin-10 plasmid functionalised scaffold. Biomaterials. 2012;33:1303-14
65. Kong G, Chen Y, Liu Z. et al. Adenovirus-IL-10 relieves chronic rejection after mouse heart transplantation by inhibiting miR-155 and activating SOCS5. Int J Med Sci. 2023;20:172-185
66. Furukawa H, Oshima K, Tung T. et al. Liposome-mediated combinatorial cytokine gene therapy induces localized synergistic immunosuppression and promotes long-term survival of cardiac allografts. J Immunol. 2005;174:6983-92
67. Zhou J, Chen X, Chen Q. et al. Novel Muscle-Homing Peptide FGF1 Conjugate Based on AlphaFold for Type 2 Diabetes Mellitus. ACS Appl Mater Interfaces. 2023;15:6397-6410
68. Gries KJ, Zysik VS, Jobe TK. et al. Muscle-derived factors influencing bone metabolism. Semin Cell Dev Biol. 2022;123:57-63
69. Kwon JH, Moon KM, Min KW. Exercise-Induced Myokines can Explain the Importance of Physical Activity in the Elderly: An Overview. Healthcare (Basel). 2020;8:378
70. Bortoluzzi S, Scannapieco P, Cestaro A. et al. Computational reconstruction of the human skeletal muscle secretome. Proteins. 2006;62:776-92
71. Sun P, Zhang K, Hassan SH. et al. Endothelium-Targeted Deletion of microRNA-15a/16-1 Promotes Poststroke Angiogenesis and Improves Long-Term Neurological Recovery. Circ Res. 2020;126:1040-1057
72. Przybylski M. A review of the current research on the role of bFGF and VEGF in angiogenesis. J Wound Care. 2009;18:516-9
73. Zentilin L, Puligadda U, Lionetti V. et al. Cardiomyocyte VEGFR-1 activation by VEGF-B induces compensatory hypertrophy and preserves cardiac function after myocardial infarction. Faseb j. 2010;24:1467-78
74. Henri O, Pouehe C, Houssari M. et al. Selective Stimulation of Cardiac Lymphangiogenesis Reduces Myocardial Edema and Fibrosis Leading to Improved Cardiac Function Following Myocardial Infarction. Circulation. 2016;133:1484-97 discussion 1497
75. Qiao B, Nie JJ, Shao Y. et al. Functional Nanocomplexes with Vascular Endothelial Growth Factor A/C Isoforms Improve Collateral Circulation and Cardiac Function. Small. 2020;16:e1905925
76. Kim H, Wrann CD, Jedrychowski M. et al. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell. 2018;175:1756-1768.e17
77. Zhang Y, He X, Wang K. et al. Irisin alleviates obesity-induced bone loss by inhibiting interleukin 6 expression via TLR4/MyD88/NF-κB axis in adipocytes. J Adv Res. 2025;69:343-359
78. Grootaert MOJ, Finigan A, Figg NL. et al. SIRT6 Protects Smooth Muscle Cells From Senescence and Reduces Atherosclerosis. Circ Res. 2021;128:474-491
79. Cardus A, Uryga AK, Walters G. et al. SIRT6 protects human endothelial cells from DNA damage, telomere dysfunction, and senescence. Cardiovasc Res. 2013;97:571-9
80. Wang Y, Wang M, Wang Y Irisin. A Potentially Fresh Insight into the Molecular Mechanisms Underlying Vascular Aging. Aging Dis. 2023;15:2491-2506
81. Sánchez B, Muñoz-Pinto MF, Cano M. Irisin enhances longevity by boosting SIRT1, AMPK, autophagy and telomerase. Expert Rev Mol Med. 2022;25:e4
82. Sundarrajan L, Yeung C, Hahn L. et al. Irisin regulates cardiac physiology in zebrafish. PLoS One. 2017;12:e0181461
83. Chen K, Xu Z, Liu Y. et al. Irisin protects mitochondria function during pulmonary ischemia/reperfusion injury. Sci Transl Med. 2017;9:eaao6298
84. Zhang J, Tu R, Guan F. et al. Irisin attenuates ventilator-induced diaphragmatic dysfunction by inhibiting endoplasmic reticulum stress through activation of AMPK. J Cell Mol Med. 2024;28:e18259
85. Giannini M, Charles AL, Evrard C. et al. Title: Sarcopenia assessed by DXA and hand-grip dynamometer: a potential marker of damage, disability and myokines imbalance in inflammatory myopathies. Rheumatology (Oxford). 2024;63:2503-2514
86. Nam JS, Cho ES, Kwon YR. et al. Dynamic Response of Musclin, a Myokine, to Aerobic Exercise and Its Interplay with Natriuretic Peptides and Receptor C. J Clin Endocrinol Metab. 2025;110:1305-1314
87. Miyazaki T, Otani K, Chiba A. et al. A New Secretory Peptide of Natriuretic Peptide Family, Osteocrin, Suppresses the Progression of Congestive Heart Failure After Myocardial Infarction. Circ Res. 2018;122:742-751
88. Harris MP, Zeng S, Zhu Z. et al. Myokine Musclin Is Critical for Exercise-Induced Cardiac Conditioning. Int J Mol Sci. 2023;24:6525
89. Xi Y, Gong DW, Tian Z. FSTL1 as a Potential Mediator of Exercise-Induced Cardioprotection in Post-Myocardial Infarction Rats. Sci Rep. 2016;6:32424
90. Shimano M, Ouchi N, Nakamura K. et al. Cardiac myocyte follistatin-like 1 functions to attenuate hypertrophy following pressure overload. Proc Natl Acad Sci U S A. 2011;108:E899-906
91. Wei K, Serpooshan V, Hurtado C. et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature. 2015;525:479-85
92. Seldin MM, Peterson JM, Byerly MS. et al. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem. 2012;287:11968-80
93. Pourranjbar M, Arabnejad N, Naderipour K. et al. Effects of Aerobic Exercises on Serum Levels of Myonectin and Insulin Resistance in Obese and Overweight Women. J Med Life. 2018;11:381-386
94. Zhao Q, Zhang CL, Xiang RL. et al. CTRP15 derived from cardiac myocytes attenuates TGFβ1-induced fibrotic response in cardiac fibroblasts. Cardiovasc Drugs Ther. 2020;34:591-604
95. Lopez JJ, Edelman ER, Stamler A. et al. Angiogenic potential of perivascularly delivered aFGF in a porcine model of chronic myocardial ischemia. Am J Physiol. 1998;274:H930-6
96. Zhao YZ, Lu CT, Li XK. et al. Improving the cardio protective effect of aFGF in ischemic myocardium with ultrasound-mediated cavitation of heparin modified microbubbles: preliminary experiment. J Drug Target. 2012;20:623-31
97. Barwari T, Joshi A, Mayr M. MicroRNAs in Cardiovascular Disease. J Am Coll Cardiol. 2016;68:2577-2584
98. Zheng L, Lin S, Lv C. MiR-26a-5p regulates cardiac fibroblasts collagen expression by targeting ULK1. Sci Rep. 2018;8:2104
99. Chiang MH, Liang CJ, Lin LC. et al. miR-26a attenuates cardiac apoptosis and fibrosis by targeting ataxia-telangiectasia mutated in myocardial infarction. J Cell Physiol. 2020;235:6085-6102
100. Zhu C, Zhang H, Wei D. et al. Silencing lncRNA GAS5 alleviates apoptosis and fibrosis in diabetic cardiomyopathy by targeting miR-26a/b-5p. Acta Diabetol. 2021;58:1491-1501
101. Chistiakov DA, Orekhov AN, Bobryshev YV. The role of miR-126 in embryonic angiogenesis, adult vascular homeostasis, and vascular repair and its alterations in atherosclerotic disease. J Mol Cell Cardiol. 2016;97:47-55
102. Mierzejewski B, Ciemerych MA, Streminska W. et al. miRNA-126a plays important role in myoblast and endothelial cell interaction. Sci Rep. 2023;13:15046
103. Zhou F, Jia X, Yang Y. et al. Nanofiber-mediated microRNA-126 delivery to vascular endothelial cells for blood vessel regeneration. Acta Biomater. 2016;43:303-313
104. Pelozin BRA, Soci UPR, Gomes JLP. et al. mTOR signaling-related microRNAs as cardiac hypertrophy modulators in high-volume endurance training. J Appl Physiol (1985). 2022;132:126-139
105. Fernandes T, Casaes L, Soci Ú. et al. Exercise Training Restores the Cardiac Microrna-16 Levels Preventing Microvascular Rarefaction in Obese Zucker Rats. Obes Facts. 2018;11:15-24
106. Cai Y, Yu X, Hu S. et al. A brief review on the mechanisms of miRNA regulation. Genomics Proteomics Bioinformatics. 2009;7:147-54
Author contact
![]() Corresponding authors: Mingjing Zhao, Email: mjgx2004com; Xiaowan Han, Email: 965396781com
Corresponding authors: Mingjing Zhao, Email: mjgx2004com; Xiaowan Han, Email: 965396781com

 Global reach, higher impact
Global reach, higher impact