3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(10):2373-2381. doi:10.7150/ijms.110231 This issue Cite
Research Paper
Advantage of Tolerability following Arsenic Trioxide-VTD vs VRD in newly diagnosed multiple myeloma patients: a prospective, open-label study
1. Department of Hematology, Huadong Hospital, Fudan University, Shanghai, China.
2. Department of Hematology, The First Affiliated Hospital of Fujian Medical University, Fuzhou, China.
* Zuo and Yang contributed equally to this work.
Received 2025-1-10; Accepted 2025-4-1; Published 2025-4-28
Abstract
Multiple myeloma is the second most common hematologic malignancy in older patients. The standard front-line VRD regimen (bortezomib/lenalidomide/dexamethasone) achieves high efficacy but is associated with significant toxicity, leading to infections, bone marrow suppression, and treatment discontinuation in approximately 20% of patients. Alternative regimens with reduced toxicity are needed for this demographic. Prior studies suggest adding arsenic trioxide to bortezomib/dexamethasone (BD) enhances remission depth with acceptable safety, while bortezomib/thalidomide/dexamethasone (VTD) offers reduced toxicity, but lower efficacy compared to VRD.
This study evaluates the efficacy, safety, and cost-effectiveness of an arsenic trioxide-VTD regimen (AVTD) versus VRD in newly diagnosed multiple myeloma (NDMM) patients. Among 116 participants, AVTD demonstrated comparable efficacy to VRD but significantly reduced infection rates (14.0% vs. 40.7%, P < 0.001) and severe bone marrow suppression (0% vs. 11.9%, P = 0.013). Subgroup analysis of patients >60 years yielded consistent results. Additionally, AVTD was associated with lower treatment costs.
In conclusion, the AVTD regimen offers a safer, more cost-effective alternative to VRD for NDMM, particularly in older adult patients, without compromising treatment efficacy.
Keywords: multiple myeloma, arsenic trioxide, tolerability, treatment response, cost-effectiveness
Introduction
Multiple myeloma (MM) is a malignancy characterized by clonal plasma cell proliferation. It stands as the second most prevalent malignant tumor within the hematological system [1]. Worldwide the disease sees over 588,000 new diagnoses annually, with nearly 100,000 new deaths [2]. The financial burden of MM is also significant; after commencing anti-myeloma treatments, the average annual adjusted costs surpass $110,000, ranking it among the most costly cancers to manage [3-5]. In 2016, China reported an overall incidence rate of MM at 1.03/100,000 and a mortality rate of 0.67/100,000, both of which have been on a steady rise [6]. Notably, two-thirds of MM patients are 65 years or older [7]. Given the global trend towards an aging population, the number of MM patients is expected to rise, and the physiological changes accompanying aging can diminish a patient's treatment tolerance. Adverse events related to treatments leading to interruptions or discontinuations are significant contributors to unfavorable prognosis [8].
The combination of bortezomib, lenalidomide, and dexamethasone (VRD) is the standard front-line treatment for newly diagnosed multiple myeloma (NDMM). However, its use in older patients is associated with a heightened risk of adverse reactions [9]. Common adverse reactions, such as bone marrow suppression (47%), neurotoxicity (33%), fatigue (16%) and infections (14.5%) have been reported. Consequently, between 17.2% and 22.6% of patients discontinue the medication due to these adverse effects and subsequent relapses [9-12]. Thus, there is a pressing need, especially for older patients, to devise a treatment strategy that strikes a balance between efficacy and safety. Addressing the economic implications of prolonged treatment is also of paramount socio-economic importance [13], it will also be a pivotal research area in myeloma management to minimize treatment suspensions due to adverse reactions.
Arsenic acid first demonstrated its therapeutic potential in China, where it was effectively used to treat acute promyelocytic leukemia [14]. Following this success, the drug was explored in preclinical and early clinical trials for other malignancies, including MM [15]. Both theoretical analyses and clinical trials have indicated that arsenic trioxide exhibits potent anti-myeloma effects, either as a stand-alone treatment or in combination with other anti-cancer agents, like MAC regimen (melphalan/arsenic trioxide/Vitamin C) and ABC regimen (arsenic trioxide/bortezomib/ascorbic acid) [16-19]. Our prior clinical research has established that for NDMM patients, the combination of arsenic trioxide with the bortezomib/dexamethasone regimen offers superior safety and efficacy compared to the bortezomib/dexamethasone (BD) regimen alone [20].
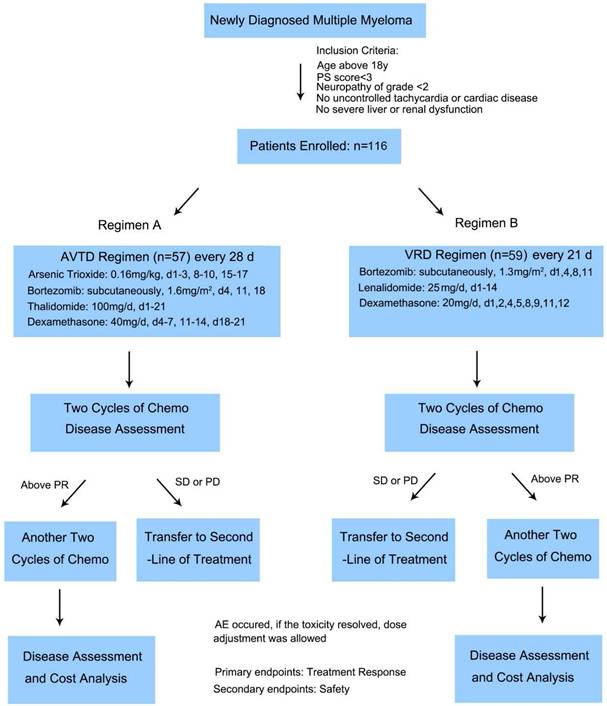
In MM therapy, bortezomib/thalidomide/dexamethasone (VTD) regimen remains a valuable treatment option for older adult patients, while VTD showed weaker efficacy over the first-line VRD regimen [21]. Integrating arsenic acid with the VTD regimen might further amplify VTD's therapeutic benefits without significantly elevating its toxicity profile. Yet, it remains uncertain whether the combination of arsenic trioxide and VTD offers any advantages over the standard first-line MM treatment, VRD. To address this, we compared the arsenic trioxide/bortezomib/thalidomide/dexamethasone (AVTD) regimen with the VRD regimen in treating newly diagnosed MM patients in a prospective study. This clinical trial was conducted across two medical centers: Huadong Hospital Affiliated with Fudan University and the First Hospital Affiliated with Fujian Medical University. We enrolled a total of 116 patients, with median age above 60 years. Our objective was to discern differences in efficacy, safety, and treatment costs between the AVTD and VRD regimens in newly diagnosed myeloma patients, especially the older adult patients.
Methods
Patients
This study was a prospective, open-label trials conducted at 2 centers in China. Between January 2022 and January 2024, 116 patients from Huadong Hospital and The First Hospital Affiliated with Fujian Medical University were enrolled. Eligible participants for this study were newly diagnosed MM patients with measurable serum and/or urine M protein. Key inclusion criteria included: age ≥18 years; a new MM diagnosis; a Zubrod performance status score of <4; no prior treatment with arsenic therapy; a left ventricular ejection fraction >40%; absence of uncontrolled arrhythmia or unstable cardiac conditions; a corrected QT interval <470 ms; no symptomatic pulmonary conditions with satisfactory pulmonary function tests; serum glutamic pyruvic transaminase levels <4 × the upper limit of normal; serum bilirubin levels <2 × the upper limit of normal; and a performance status <3. Major exclusion criteria encompassed peripheral neuropathy grade ≥2, systemic amyloidosis, and a positive serology for HIV (and HIV-1, HIV-2) or hepatitis B or C. The Huadong Hospital's institutional ethics committee approved the study (2022K117), and it adhered to the principles of the Declaration of Helsinki. All participants provided written informed consent. The study was registered in the Chinese Clinical Trials Registry (Number ChiCTR2400083240).
Study Design and Endpoints
Patients enrolled into this study were randomly divided into two groups receiving AVTD regimen or VRD regimen. The AVTD regimen for patients was as follows: arsenic trioxide 0.16 mg/kg on days 1-3, 8-10, and 15-17 over 2 hours; thalidomide 100mg on days 1-21; Bortezomib 1.6 mg/m2 subcutaneously on days 4, 11, and 18; and dexamethasone 40 mg/day IV on days 4-7, 11-14, and 18-21. For patients over 65 years or those with diabetes, dexamethasone dosage was reduced to 20 mg/day. This regimen was repeated every 28 days. The VRD regimen comprised four 21-day cycles Bortezomib was given at 1.3 mg/mg2 intravenously on days 1, 4, 8 and 11, combined with oral lenalidomide 25 mg daily on days 1-14 plus oral dexamethasone 20 mg daily on days 1, 2, 4, 5, 8, 9, 11, and 12 [9]. Supportive care was provided as per departmental guidelines. Patients received granulocyte colony-stimulating factor 5 μg/kg/day if the absolute neutrophil count dropped below 0.5 ×109/L for two consecutive days. Prophylactic oral levofloxacin, acyclovir, and fluconazole were administered during neutropenia. Blood products were given if hemoglobin levels were <6 g/dL or platelet counts were <20 × 109/L. The induction therapy duration was consistent at 16 weeks across all arms. Patients on lenalidomide induction therapy were recommended either low-molecular-weight heparin or aspirin thromboprophylaxis. After two and four cycles of chemotherapy respectively, each patient's disease status was evaluated. Those with stable disease (SD) or progressive disease (PD) after two cycles were transitioned to alternative regimens. The primary endpoint was the treatment response, with safety being the secondary endpoint.
FISH Studies
Bone marrow plasma cells were isolated using anti-CD138-coated magnetic beads via the AutoMACs automated separation system (Miltenyi Biotec). Interphase fluorescence in situ hybridization (FISH) was conducted using specific probes (Abbott Molecular/Vysis) targeting 17p deletions, as well as immunoglobulin heavy chain translocations, including t(4;14) and t(14;16), and 1q21, as previously detailed [22]. All cytogenetic evaluations were centrally conducted at the Huadong Hospital.
Response Criteria
Patient responses were assessed at the onset of each treatment cycle, following standard International Myeloma Working Group (IMWG) response criteria.23 Safety was monitored for 30 days post the final drug dose.
Safety
Adverse events were categorized based on the National Cancer Institute Common Toxicity Criteria (Version 5.0) [24]. Bortezomib or combination chemotherapy was withheld in instances of grade 4 hematologic toxicity or grade ≥3 nonhematologic toxicity until the toxicity subsided to grade ≤2. For bortezomib-related toxicities, once resolved, the drug was reintroduced at a 25% reduced dose. Management of bortezomib-induced peripheral neuropathy or neuropathic pain followed established protocols [25,26]. Thalidomide-related peripheral neuropathy of grade 2 led to a 50% dose reduction, while grade 3 necessitated discontinuation until symptoms reduced to grade ≤ 1, followed by a 50% dose reintroduction. For dexamethasone-related grade 3 or 4 adverse events, the drug was withheld until toxicity subsided to grade 2 or less, followed by a 50% dose reduction. Lenalidomide was discontinued in cases of extensive rash. With dosage adjustments as necessary using slide adjustment scale within the VRD protocol.
Costs
This study considered direct medical costs, encompassing drug expenses. Related medical costs included hospitalization, laboratory tests, diagnostic procedures, concomitant treatments, hospital visits, and treatment costs for severe adverse reactions. The pricing for lenalidomide, thalidomide, arsenic trioxide, and bortezomib was based on the medical insurance payment standards negotiated by the China National Medical Security Administration. Dexamethasone pricing followed the tender prices in Shanghai City and Fujian Province. In our study, all costs were updated to March 2024 US dollars by the United States Consumer Price Index.
Disease Monitoring
Disease assessments, which included skeletal surveys, complete neurologic examinations, Karnofsky performance status evaluations, beta2-microglobulin, C-reactive protein, serum and urine electrophoresis for immunoglobulin quantification, immunofixation, bone marrow aspiration, and biopsy, were conducted within 14 days prior to the first day of the initial treatment cycle. Comprehensive medical histories were collected, and baseline physical and complete neurologic examinations were performed. Additionally, 12-lead electrocardiography and posteroanterior and lateral chest X-rays were taken. Bone marrow aspirates were evaluated, and biopsies were supplemented with flow cytometry, chromosome analysis and FISH. Clinical laboratory tests, including hematology, clinical chemistry, electrolyte and glucose panels, total protein, amylase, albumin tests, urinalysis, and serum pregnancy tests for women of child-bearing potential, were conducted on the first day of each cycle. Treatment assessment staging was initiated at entry, with restaging after two cycle and four cycles. Three authors independently collected patient medical data to minimize bias. Extracted patient information included age, sex, disease stage, blood cell count, treatment response, and survival status. No significant selection bias is anticipated in these clinical data collections and analyses.
Statistical Analyses
Patient characteristics were summarized using means and 95% confidence intervals (CI) for numerical variables and frequencies with percentages for categorical variables. Differences between the two treatment groups were assessed using two-sample t-tests or Mann-Whitney U-test for numerical variables and Chi-squared tests or Fisher's exact test for categorical variables. Logistic regression was employed to analyze factors influencing treatment response. To enhance statistical efficiency and mitigate confounding factors, patients in two treatment regimens were matched in terms of age ± 5 (year) as a group matching at a 1:1 ratio. All statistical analyses were performed using SPSS version 27.0 for Windows (IBM SPSS, Chicago, IL, USA) with statistical significance defined as two-sided and P-values < 0.05.
Results
Clinical Characteristics of the Two Treatment Groups
Of the 116 enrolled and treated patients, 57 received the AVTD regimen and 59 underwent the VRD regimen. The Flowchart of the study was shown in Figure 1, Table 1 displays the pretreatment characteristics of patients based on their treatment arm. Prognostic factors, such as ISS stage and cytogenetic status, were evenly distributed between the two treatment groups. The median age for both groups exceeded 60 years. Factors like gender, isotype, Durie-Salmon Stage at diagnosis, ISS stage at diagnosis, cytogenetic abnormalities, Lactate Dehydrogenase (LDH) level, albumin level, and beta2-microglobulin level at diagnosis exhibited no significant disparities between the two treatment groups. However, the VRD group had a higher incidence of del(17/17p) (11.9% vs 0%, P=0.013), while the AVTD group had a higher incidence of t(4,14) (28.1% vs 8.5%, P=0.008). No significant differences were observed between the two groups regarding high-risk subtypes detected by FISH according to Mayo mSMART risk stratification22 (P=0.460).
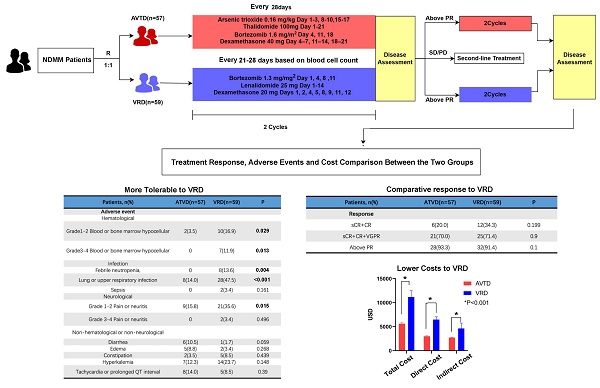
The Flowchart of the study and the protocols of the two regimens
Baseline Clinical Characteristics of Patients Receiving the Two Regimens
| Characteristics | AVTD(n=57) | VRD (n=59) | p value |
|---|---|---|---|
| Age, years | 0.399 | ||
| Median (range) | 60(47-84) | 62(46-81) | |
| Gender, n (%) | 0.922 | ||
| Male | 30(52.6) | 31(52.5) | |
| Myeloma type, n (%) | 0.232 | ||
| Immunoglobulin G | 25(43.9) | 33(58.9) | |
| Immunoglobulin A | 13(22.8) | 13(23.2) | |
| Light chain disease only | 13(22.8) | 8(14.3) | |
| Others | 6(10.5) | 2(3.6) | |
| Durie-Salmon stage at diagnosis, n (%) | 0.088 | ||
| I | 5(8.8) | 9(15.3) | |
| II | 12(21.1) | 5(8.5) | |
| III | 40(70.2) | 45(76.3) | |
| International Staging System stage at diagnosis, n (%) | 0.139 | ||
| I | 10(17.5) | 17(28.8) | |
| II | 20(35.1) | 24(40.7) | |
| III | 27(47.4) | 18(30.5) | |
| Cytogenetic abnormalities determined by FISH, n (%) | |||
| del(17/17p) | 0(0) | 7(11.9) | 0.013 |
| t(4,14) | 16(28.1) | 5(8.5) | 0.008 |
| t(14,16) | 1(1.8) | 1(1.7) | 1.000 |
| 1q21 | 19(33.3) | 18(30.5) | 0.744 |
| High risk [22] | 36(63.2) | 31(52.5) | 0.460 |
| LDH | 0.589 | ||
| Mean, 95% CI | 204.15 (166.68-241.62) | 222.34 (168.23-276.46) | |
| Median beta2-microglobuli, mg/L, n (%) | 0.183 | ||
| <3.5 | 20(35.1) | 25(42.4) | |
| ≥3.5, <5.5 | 14(24.6) | 19(32.2) | |
| ≥5.5 | 23(40.4) | 15(25.4) | |
| Albumin | 0.297 | ||
| Mean, 95% CI | 34.46 (32.41-36.52) | 38.24 (31.83-44.64) |
Treatment Response, Safety, and Tolerability to the Induction Therapy
All patients completed the planned therapy sequence, were evaluable for response, and underwent comprehensive assessment. Table 2 summarizes the response rates. The clinical response between the AVTD and VRD groups did not differ significantly (P=0.268). The percentage of patients with a better response (sCR + CR + VGPR) in the AVTD group was higher than that in the VRD group, but the difference was not statistically significant (75.4% vs 62.7%, P=0.139). Adverse effects and their frequencies are detailed in Table 3. All adverse effects were manageable with appropriate treatment, and no fatalities were attributed to these effects. The AVTD group exhibited a lower incidence of any grade AEs related to hematologic toxicity. The incidence of Grade Ⅰ to Ⅱ blood hypocellular was lower in AVTD group (3.5% vs 17.2%, P=0.015) compared to VRD group, as well as the grade III to IV blood hypocellarity (0% vs 12.5%, P=0.006). Common infection was in respiratory system (14.0% vs 47.5%, P<0.001) and febrile neutropenia (0 vs 13.6%, P=0.004). Any grade AEs (pain or neuritis) in neurological (Grade I to II:15.8% vs 35.6%, P=0.015) is lower compared to the VRD group. Grade III to IV neuritis was scarcely seen in both groups (Table 3).
Treatment Response (After Four Cycles)
| AVTD | VRD | p value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Response | 0.268 | ||||
| sCR | 2 | 3.5 | 5 | 8.5 | |
| CR | 13 | 22.8 | 13 | 22.0 | |
| VGPR | 28 | 49.1 | 19 | 32.2 | |
| PR | 11 | 19.3 | 15 | 25.4 | |
| PD | 3 | 5.3 | 7 | 11.9 | |
| sCR+CR | 15 | 26.3 | 18 | 30.5 | 0.617 |
| sCR+CR+VGPR | 43 | 75.4 | 37 | 62.7 | 0.139 |
| Above PR | 54 | 94.7 | 52 | 88.1 | 0.322 |
Comparison of Adverse Effects Associated with the Two Regimens
| ATVD (n=57) | VRD (n=59) | p value | |
|---|---|---|---|
| Hematological | |||
| Grade1-2 Blood or bone marrow hypocellarity, n (%) | 2(3.5) | 10(16.9) | 0.029 |
| Grade3-4 Blood or bone marrow hypocellarity, n (%) | 0 | 7(11.9) | 0.013 |
| Infection | |||
| Febrile neutropenia, n (%) | 0 | 8(13.6) | 0.004 |
| Lung or upper respiratory infection, n (%) | 8(14.0) | 28(47.5) | <0.001 |
| Sepsis, n (%) | 0 | 2(3.4) | 0.161 |
| Neurological | |||
| Grade 1-2 Pain or neuritis, n (%) | 9(15.8) | 21(35.6) | 0.015 |
| Grade 3-4 Pain or neuritis, n (%) | 0 | 2(3.4) | 0.496 |
| Non-hematological or non-neurological | |||
| Diarrhea, n (%) | 6(10.5) | 1(1.7) | 0.059 |
| Edema, n (%) | 5(8.8) | 2(3.4) | 0.268 |
| Constipation, n (%) | 2(3.5) | 5(8.5) | 0.439 |
| Hyperkalemia, n (%) | 7(12.3) | 14(23.7) | 0.148 |
| Tachycardia or prolonged QT interval, n (%) | 8(14.0) | 5(8.5) | 0.390 |
Treatment Response, Safety, and Tolerability to the Induction Therapy in Patients Above 60 Years Old
To ascertain the efficacy and safety of the AVTD regimen for older adult patients, we segregated the two treatment groups based on age (above or below 60 years). We then compared the clinical characteristics (Table S1), treatment response (Table S2), and adverse effects (Table S3) of the subgroup aged over 60 years. The results revealed no significant statistical differences between the AVTD and VRD groups in terms of clinical characteristics. For older adult patients, the deep response rate (sCR+CR) and treatment response rate (sCR+CR+VGPR) between the AVTD and VRD regimens were comparable (70% vs 71.4%, P=0.9, Table S2). The AVTD group had a lower incidence of infection in lung or upper respiratory, (20.0% vs 57.1%, P=0.002) and febrile neutropenia (0 vs 14.3%, P=0.021) with less hematologic toxicity compared to the VRD group. Lower incidence of Grade I to II neuropathy (16.7% vs 40.0%, P=0.036, Table S3). None patients in the study experienced severe neuropathy leading to treatment interruption or discontinuation. The neuropathy was mild to moderate and tolerable, only 5% patients in VRD group suffered Grade III to IV neuritis.
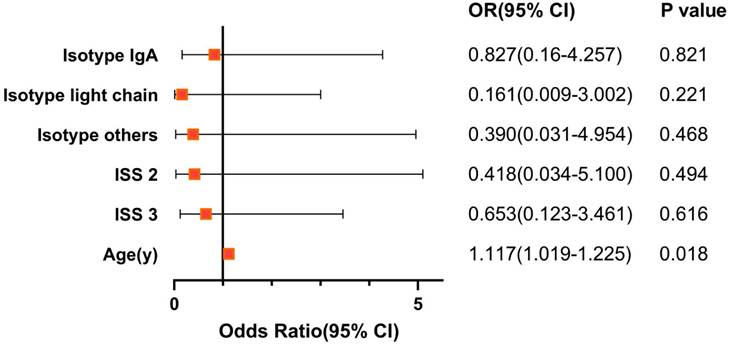
Independent Prognostic Factor of the AVTD Regimen and Age-matched Paired Chi-Square Test of the Two Regimens
To identify independent prognostic factors influencing the efficacy of the AVTD regimen, both univariate and multivariate logistic regression analyses were performed specifically on the AVTD group. Multivariate regression analysis indicated that age might serve as an independent prognostic factor for the AVTD regimen, as depicted in Figure 2. To enhance the precision of the statistical analysis and mitigate the bias introduced by age, we paired patients from the AVTD and VTD regimens in a 1:1 ratio, ensuring an age difference of no more than 5 years between matched pairs. Post-matching, 23 pairs were identified, totaling 46 patients. Subsequent paired chi-square analysis, as presented in Table S4, reaffirmed the earlier conclusion that the efficacy of the AVTD regimen is comparable to that of the VRD regimen (sCR+CR+VGPR: 76.1% vs 58.7%, P=0.134). Regarding adverse reactions, the AVTD regimen exhibited a significantly lower incidence of grade III to IV bone marrow suppression (0% vs 13.0%, P=0.011) and infections in respiratory system (15.2% vs 50.0%, P<0.001), as well as febrile neutropenia (0 vs 15.2%, P=0.006).
Economic Cost Comparison between the AVTD and VRD Regimens
Costs were categorized into three segments: in-hospital expenses (Total cost), direct drug costs (expenses related to in-hospital and outpatient myeloma treatments), and treatment-related costs (expenses associated with complications induced by the disease and its treatment, such as infections). Across all categories, the AVTD regimen was found to be significantly more cost-effective than the VRD regimen (P<0.01, Table 4). This conclusion held true both for the subgroup aged above 60 years and in the age-matched paired analysis. The cost of treatment was reduced either by drugs themselves or by the supportive medical treatment for complications such as infections and pancytopenia.
The Forest Plot of prognostic factor on AVTD regimen
The Cost of Treatment in the Two Treatment Regimens
| AVTD vs VRD | p value | |
|---|---|---|
| All patients | n=57 vs n=59 | |
| Total cost of each treatment cycles, USD (mean, 95% CI) | 5633.42(5366.46-5900.38) vs 11184.89(9856.31-12513.74) | <0.001 |
| Direct drug cost, USD (mean, 95% CI) | 3003.44(2861.79-3145.08) vs 6481.30(5919.85-7042.75) | <0.001 |
| Treatment related cost, USD (mean, 95% CI) | 2735.32(2657.52-2813.12) vs 4629.59(4091.56-5761.61) | <0.001 |
| Subgroup of Age above 60 y | n=30 vs n=35 | |
| Total cost of each treatment cycles, USD (mean, 95% CI) | 5846.29(5513.04-6179.53) vs 11309.54 (9526.12-13079.31) | <0.001 |
| Direct drug cost, USD (mean, 95% CI) | 3089.49(2850.60-3328.37) vs 6590.41 (5871.05-7309.76) | <0.001 |
| Treatment related cost, USD (mean, 95% CI) | 2756.80(2638.64-2874.96) vs 4997.96 (3934.23-6061.68) | <0.001 |
| Age matched analysis | 23 pairs | |
| Total cost of each treatment cycles, USD (mean, 95% CI) | 5759.14(5518.76-5999.52) vs 10374.12(9076.41-11671.84) | <0.001 |
| Direct drug cost, USD (mean, 95% CI) | 3028.29(2858.73-3197.85) vs 6130.26 (5586.82-6673.69) | <0.001 |
| Treatment related cost, USD (mean, 95% CI) | 2727.91(2638.52-2817.30) vs 4571.87(3746.71-5397.02) | <0.001 |
Discussion
Our findings indicate that for newly diagnosed multiple myeloma patients, particularly the older adult patients, the AVTD regimen demonstrates comparable efficacy to the VRD regimen in terms of inducing remission. AVTD regimen mirrors the therapeutic effects of the VRD regimen as well markedly diminishes the risks of bone marrow suppression, infections and neuropathy. Additionally, AVTD regimen substantially alleviates the long-term economic burden on patients. The likelihood of halting treatment is also minimized, ensuring optimal disease management and enhancing patients' outcomes. Given its potent efficacy coupled with reduced toxicity, this regimen may be particularly beneficial for the older adult patients.
With the advent of proteasome inhibitors and immunomodulatory drugs, there have been significant advancements on prognosis in newly diagnosed multiple myeloma, notably in the older adult patients [27]. Daratumumab, a human IgGκ monoclonal antibody that targets CD38, combined with lenalidomide and dexamethasone, reduces disease progression or mortality risk compared to lenalidomide and dexamethasone alone but has higher incidence of neutropenia and pneumonia [28]. Beyond disease control, life quality is of paramount importance. The AVTD regimen, through addition of arsenic trioxide with anti-tumor agents like bortezomib, thalidomide, and dexamethasone, has demonstrated promising outcomes and safety in treating multiple myeloma.
We postulate that the diminished incidence of adverse reactions, such as infections and bone marrow suppression in the AVTD regimen, can be attributed to the following reasons: lenalidomide is known to exhibit greater bone marrow suppressive toxicity compared to thalidomide [29]. Additionally, arsenic trioxide typically induces minimal adverse reactions and is rarely associated with bone marrow suppression and infection in patients [30]. Further, Arsenic acid might booster specific and non-specific immune responses against MM cells by modulating antigen-presenting function of dendritic cells (DC), NK cells [31] and inhibiting the production of IL-6 and VEGF [32,33] to overcome the immune-compromised state in MM [34].
The observed efficacy of the AVTD regimen, comparable to that of the VRD regimen, may be attributed to the synergistic effects of arsenite combined with bortezomib, thalidomide, and dexamethasone. Interleukin-6 (IL-6), a known growth factor for MM [35], promote the tumor progression via activating the JAK-STAT3 pathway [36], facilitating the adhesion of myeloma cells to bone marrow mesenchymal stem cells [37] and obstructing dexamethasone-induced apoptosis [38]. Arsenic acid has been shown to inhibit STAT3 activation in myeloma cells [39], consequently reducing IL-6 release. Arsenite also has synergy with dexamethasone to induce MM cell apoptosis through the Caspase-9 signaling pathway [40]. Arsenic trioxide can diminish key effector proteins in the classical Wnt signaling pathway, reducing β-Catenin accumulation, which results in inhibited myeloma cell proliferation [41], increased apoptotic cell proportions, and heightened sensitivity of myeloma cells to bortezomib [42].
The value of AVTD regimen's value, especially for older adult patients, is evident given its reduced adverse reactions, outstanding tolerability, and potent therapeutic response. Does this regimen make any sense in younger patients? An intriguing observation, which we didn't heavily underscore in our results, emerges from logistic regression analysis. Age appears to be an independent prognostic factor for AVTD, suggesting that younger patients might exhibit a more favorable treatment response. The treatment response rate (sCR+CR+VGPR) of the AVTD regimen surpassed that of the VRD regimen in patients under 60 years old, which might due to arsenite exerts its anti-tumor effects by increased production of reactive oxygen species (ROS). ROS can trigger a cascade of events culminating in cell apoptosis [43]. Studies have indicated that advanced age confers a protective effect on oxidative stress [44]. Consequently, compared to older patients, an elevation in ROS levels may have a more pronounced impact on myeloma cells in younger individuals.
With age, there's a consistent rise in incidence and mortality rates [45]. Hence, for the older adult patients, the AVTD regimen, being safer, more effective, and cost-efficient, warrants deeper investigation and further research to discern the underlying mechanism. And the data we currently have are limited and definitive conclusions remain elusive. Future prospective, multicenter clinical trials may provide greater clarity.
Abbreviations
VRD: bortezomib/lenalidomide/dexamethasone; BD: bortezomib/dexamethasone; VTD: bortezomib/thalidomide/dexamethasone; NDMM: newly diagnosed multiple myeloma; AVTD: arsenic trioxide/bortezomib/thalidomide/dexamethasone; MM: multiple myeloma; HIV: human immunodeficiency virus; SD: stable disease; PD: progressive disease; FISH: Fluorescence in situ hybridization; IMWG: International Myeloma Working Group; CI: confidence intervals; LDH: Lactate Dehydrogenase; ROS: reactive oxygen species.
Supplementary Material
Supplementary tables.
Acknowledgements
This work was supported by Shanghai Municipal Health Commission (grant number 202140518), Science and Technology Commission of Shanghai Municipality (grant number 21Y11909000), the Elite Project of Huadong hospital (HD0103) and Natural Science Foundation of Fujian Province (No. 2020J01120429).
Data Availability
Additional information that supports the findings of this study are available from Jiexian Ma (majiexian@fudan.edu.cn).
Author Contributions
Conceptualization, J. X. Ma and Z.Y. Zeng; Methodology, J. X. Ma; Formal Analysis, X.Y. Zuo; Data curation, X.Y. Zuo, A. P. Yang, and P.P. Chen; Investigation, A. P. Yang, P.P. Chen, Y. H. Xie, Z. Y. Zeng and J. X. Ma. Writing - Original Draft, X.Y. Zuo and J.X. Ma. Writing - Review & Editing, all authors. Funding Acquisition and Supervision, J. X. Ma and Z.Y. Zeng. All authors agreed to submit the manuscript, read and approved the final draft and take full responsibility of its content, including the accuracy of the data and the fidelity of the trial to the registered protocol and its statistical analysis.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Mateos MV, San Miguel JF. Management of multiple myeloma in the newly diagnosed patient. Hematology Am Soc Hematol Educ Program. 2017;2017:498-507
2. Cowan AJ, Green DJ, Kwok M. et al. Diagnosis and Management of Multiple Myeloma: A Review. Jama. 2022;327:464-77
3. Teitelbaum A, Ba-Mancini A, Huang H. et al. Health care costs and resource utilization, including patient burden, associated with novel-agent-based treatment versus other therapies for multiple myeloma: findings using real-world claims data. Oncologist. 2013;18:37-45
4. Chen Y, Lairson DR, Chan W. et al. Cost-Effectiveness of Novel Agents in Medicare Patients with Multiple Myeloma: Findings from a US Payer's Perspective. J Manag Care Spec Pharm. 2017;23:831-43
5. McCabe C, Bergmann L, Bosanquet N. et al. Market and patient access to new oncology products in Europe: a current, multidisciplinary perspective. Ann Oncol. 2009;20:403-12
6. Liu J, Liu W, Mi L. et al. Incidence and mortality of multiple myeloma in China, 2006-2016: an analysis of the Global Burden of Disease Study 2016. J Hematol Oncol. 2019;12:136
7. Palumbo A, Mina R. Management of older adults with multiple myeloma. Blood Rev. 2013;27:133-42
8. Dimopoulos MA, Jakubowiak AJ, McCarthy PL. et al. Developments in continuous therapy and maintenance treatment approaches for patients with newly diagnosed multiple myeloma. Blood Cancer J. 2020;10:17
9. Durie BGM, Hoering A, Abidi MH. et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389:519-27
10. McCaughan GJ, Gandolfi S, Moore JJ. et al. Lenalidomide, bortezomib and dexamethasone induction therapy for the treatment of newly diagnosed multiple myeloma: a practical review. Br J Haematol. 2022;199:190-204
11. Attal M, Lauwers-Cances V, Hulin C. et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N Engl J Med. 2017;376:1311-20
12. Kumar SK, Jacobus SJ, Cohen AD. et al. Carfilzomib or bortezomib in combination with lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma without intention for immediate autologous stem-cell transplantation (ENDURANCE): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21:1317-30
13. Rosiñol L, Oriol A, Rios R. et al. Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood. 2019;134:1337-45
14. Shen ZX, Chen GQ, Ni JH. et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354-60
15. Anderson KC, Boise LH, Louie R. et al. Arsenic trioxide in multiple myeloma: rationale and future directions. Cancer J. 2002;8:12-25
16. Berenson J, Boccia R, Siegel D. et al. A multicenter phase II study of combination treatment with melphalan, arsenic trioxide and vitamin C (MAC) for patients with relapsed or refractory multiple myeloma. Blood. 2005;106:2564-64
17. He X, Yang K, Chen P. et al. Arsenic trioxide-based therapy in relapsed/refractory multiple myeloma patients: a meta-analysis and systematic review. Onco Targets Ther. 2014;7:1593-9
18. Berenson JR, Matous J, Swift RA. et al. A phase I/II study of arsenic trioxide/bortezomib/ascorbic acid combination therapy for the treatment of relapsed or refractory multiple myeloma. Clin Cancer Res. 2007;13:1762-8
19. Sanaat Z, Rezazadeh M, Gharamaleki JV. et al. Arsenic trioxide in patients with refractory multiple myeloma: a prospective, phase II, single-arm study. Acta Med Iran. 2011;49:504-8
20. Qian W, Wang L, Li P. et al. Efficiency and tolerability of induction and consolidation therapy with arsenic trioxide/bortezomib/ascorbic acid/dexamethasone (ABCD) regimen compared to bortezomib/dexamethasone (BD) regimen in newly diagnosed myeloma patients. Cancer Manag Res. 2020;12:431-41
21. Rosiñol L, Oriol A, Teruel AI. et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 2012;120:1589-96
22. Rajkumar SV. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97:1086-107
23. Kumar SK, Dispenzieri A, Lacy MQ. et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122-8
24. Facon T, Kumar S, Plesner T. et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med. 2019;380:2104-15
25. Zweegman S, van der Holt B, Mellqvist UH. et al. Melphalan, prednisone, and lenalidomide versus melphalan, prednisone, and thalidomide in untreated multiple myeloma. Blood. 2016;127:1109-16
26. Lengfelder E, Hofmann WK, Nowak D. Impact of arsenic trioxide in the treatment of acute promyelocytic leukemia. Leukemia. 2012;26:433-42
27. Roboz GJ, Dias S, Lam G. et al. Arsenic trioxide induces dose- and time-dependent apoptosis of endothelium and may exert an antileukemic effect via inhibition of angiogenesis. Blood. 2000;96:1525-30
28. Blimark C, Holmberg E, Mellqvist UH. et al. Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica. 2015;100:107-13
29. Chomarat P, Banchereau J, Davoust J. et al. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1:510-4
30. Gabrilovich DI, Chen HL, Girgis KR. et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096-103
31. Deaglio S, Canella D, Baj G. et al. Evidence of an immunologic mechanism behind the therapeutical effects of arsenic trioxide (As₂O₃) on myeloma cells. Leuk Res. 2001;25:227-35
32. Anderson KC, Lust JA. Role of cytokines in multiple myeloma. Semin Hematol. 1999;36:14-20
33. Ihle JN. STATs: signal transducers and activators of transcription. Cell. 1996;84:331-4
34. Chauhan D, Uchiyama H, Akbarali Y. et al. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996;87:1104-12
35. Chauhan D, Pandey P, Hideshima T. et al. SHP2 mediates the protective effect of interleukin-6 against dexamethasone-induced apoptosis in multiple myeloma cells. J Biol Chem. 2000;275:27845-50
36. Hayashi T, Hideshima T, Akiyama M. et al. Arsenic trioxide inhibits growth of human multiple myeloma cells in the bone marrow microenvironment. Mol Cancer Ther. 2002;1:851-60
37. Chauhan D, Hideshima T, Rosen S. et al. Apaf-1/cytochrome c-independent and Smac-dependent induction of apoptosis in multiple myeloma (MM) cells. J Biol Chem. 2001;276:24453-6
38. Zhou L, Hou J, Fu W. et al. Arsenic trioxide and 2-methoxyestradiol reduce beta-catenin accumulation after proteasome inhibition and enhance the sensitivity of myeloma cells to Bortezomib. Leuk Res. 2008;32:1674-83
39. Zhou LL, Fu WJ, Yuan ZG. et al. [Effect of arsenic trioxide combined with bortezomib on proliferation, apoptosis and beta-catenin level in myeloma cell lines]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008;16:84-8
40. Boerman EM, Segal SS. Depressed perivascular sensory innervation of mouse mesenteric arteries with advanced age. J Physiol. 2016;594:2323-38
41. Jing Y, Dai J, Chalmers-Redman RM. et al. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:2102-11
42. Kumar S, Paiva B, Anderson KC. et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328-e46
43. National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5. US Department of Health and Human Services. 2017
44. Delforge M, Bladé J, Dimopoulos MA. et al. Treatment-related peripheral neuropathy in multiple myeloma: the challenge continues. Lancet Oncol. 2010;11:1086-95
45. Richardson PG, Delforge M, Beksac M. et al. Management of treatment-emergent peripheral neuropathy in multiple myeloma. Leukemia. 2012;26:595-608
Author contact
![]() Corresponding authors: Jiexian Ma, MD, PhD, 221 West Yan'an Road, Shanghai 200040, China, E-mail: majiexianedu.cn, Phone number: +086-021-62483180-561303; Zhiyong Zeng MD, PhD, the First Affiliated Hospital, Fujian Medical University, Fuzhou, 350005, China. E-mail: zengzhiyong049com, Phone number: +086-0591- 87982222-1680.
Corresponding authors: Jiexian Ma, MD, PhD, 221 West Yan'an Road, Shanghai 200040, China, E-mail: majiexianedu.cn, Phone number: +086-021-62483180-561303; Zhiyong Zeng MD, PhD, the First Affiliated Hospital, Fujian Medical University, Fuzhou, 350005, China. E-mail: zengzhiyong049com, Phone number: +086-0591- 87982222-1680.

 Global reach, higher impact
Global reach, higher impact