3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(13):2494-2501. doi:10.7150/ijms.101020 This issue Cite
Research Paper
Genetic associations of visfatin polymorphisms with clinicopathologic characteristics of prostate cancer in Taiwanese males
1. School of Medicine, China Medical University, Taichung, Taiwan.
2. Department of Family Medicine, China Medical University Hsinchu Hospital, Hsinchu, Taiwan.
3. Institute of Biomedical Sciences, Mackay Medical College, New Taipei City, Taiwan.
4. Division of Urology, Department of Surgery, Taichung Veterans General Hospital, Taichung, Taiwan.
5. School of Medicine, Chung Shan Medical University, Taichung, Taiwan.
6. School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan.
7. Department of Sports Medicine, College of Health Care, China Medical University, Taichung, Taiwan.
8. Department of Orthopedic Surgery, China Medical University Hospital, Taichung, Taiwan.
9. Department of Orthopedic Surgery, China Medical University Beigang Hospital, Yunlin, Taiwan.
10. Department of Applied Chemistry, National Chi Nan University, Nantou, Taiwan.
11. Department of Pharmacy and Master Program, Collage of Pharmacy and Health Care, Tajen University, Pingtung County, Taiwan.
12. Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan.
13. Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan.
14. Department of Pharmacology, School of Medicine, China Medical University, Taichung, Taiwan.
15. Department of Medical Laboratory Science and Biotechnology, Asia University, Taichung, Taiwan.
16. Chinese Medicine Research Center, China Medical University, Taichung, Taiwan.
17. Department of Medical Research, China Medical University Hsinchu Hospital, Hsinchu, Taiwan.
# These authors contributed equally.
Received 2024-7-16; Accepted 2024-9-3; Published 2024-9-23
Abstract
The most general cancer in men is prostate cancer (PCa), with its risk increasing due to age and obesity. Visfatin, a member of adipokines, is related to cancer progression and metastasis, but its relationship in PCa remains undetermined. In addition, no knowledge is available regarding relations between visfatin polymorphisms and clinicopathological characteristics in PCa. We sought to investigate the functions of four visfatin gene polymorphisms and clinicopathological characteristics on the hazard of developing PCa in 695 Taiwanese males with PCa. Carriers of the GA+AA heterozygote of SNP rs61330082 were at a markedly higher risk of biochemical recurrence than those with the GG genotype. Visfatin rs61330082 and rs11977021 were related with a high risk of perineural invasion, lymphovascular invasion, and biochemical recurrence in prostate-specific antigen (PSA) > 10 PCa patients. The Cancer Genome Atlas database noted that visfatin mRNA level did not prominently differ with pathological T/N stage and overall survival. This finding is the first to document a connection between visfatin polymorphisms and clinicopathological characteristics of PCa in Taiwanese males.
Keywords: Prostate cancer, Single nucleotide polymorphism, Visfatin, Taiwanese males
Introduction
The most general cancer in men is prostate cancer (PCa), with its risk increasing due to age and obesity [1-3]. Projections estimate 300,000 new patients of PCa in the US by 2050. While many initially present with localized disease, over half of PCa patients eventually progress bone metastases, particularly in advanced stages [2, 4, 5]. Despite a 98% five-year associate survival rate for combined diagnosed cases of PCa, numerous patients with metastatic cancer face treatment-resistant disease. PCa metastases can significantly reduce patients' quality of life and are a prominent cause of death [6, 7]. A solid comprehension of the mechanisms driving malignant development and distant metastasis in PCa would facilitate the evolution of early diagnosis and prevention managements.
An expanding body of research suggests a connection between cancer and obesity [8]. This link is particularly evident in cancers such as esophageal, pancreatic, and colorectal cancers [8, 9]. Furthermore, obesity can alter the cancer's microenvironment, accelerating its progression [10]. Visceral fat contains substantial amounts of visfatin, an adipokine that promotes inflammation [11-13]. Visfatin is known to regulate several cellular functions in mammalian cells, including cellular proliferation, migration, differentiation, and apoptosis [11]. It is not surprising that individuals with various malignancies exhibit significantly higher visfatin levels compared to people without cancer [14, 15]. Visfatin expression is recognized as vital in various tumor-associated activities, including survival, angiogenesis, metastasis, and treatment resistance [16]. Reports have demonstrated that visfatin associates PCa growth and survival [17, 18].
The visfatin gene's promoter, 5'- and 3'-untranslated regions (UTRs), have more than 150 single nucleotide polymorphisms (SNPs), and it is thought that this significant variability controls visfatin function and output [19]. Visfatin gene polymorphisms have been linked to hepatocellular and esophageal squamous cell carcinoma [20, 21]. Our lab's earlier research detailed the relationship between several visfatin polymorphisms and the likelihood of developing oral and lung cancer in Taiwanese individuals [22, 23]. Our goal was to ascertain the relationships between four visfatin gene SNPs and clinicopathological characteristics associated with the risk of PCa in Taiwanese males. We believe that this is the first report in a Taiwanese population to demonstrate a substantial correlation between visfatin polymorphisms and PCa.
Materials and Methods
Participants
From 2012 to 2018, Taichung Veteran General Hospital treated 695 patients with adenocarcinoma of the prostate who underwent robot-assisted radical prostatectomy with bilateral standard pelvic lymph node dissection. Prior to the start of the trial, each participant provided informed written consent, and the Taichung Veteran General Hospital's Institutional Review Board (IRB) accepted the protocol (IRB No. CE19062A). The initial prostate-specific antigen (PSA) at diagnosis, clinical and pathological tumor-node-metastasis (TNM) staging, Gleason score of the initial biopsy, D'Amico classification [24], Gleason grade group, and other pathological features from the permanent pathological document [25] were obtained from the medical records of the patients. The eighth edition of the American Joint Committee on Cancer Staging Manual's TNM staging system was used to stage PCa patients. Our patient grouping consisted of 331 individuals with PSA level of less than 10 ng/ml (PSA ≤ 10 group) and 364 individuals with PSA level greater than 10 ng/ml (PSA > 10 group).
Genomic DNA extraction and PCR genotyping
Genomic DNA was isolated from peripheral blood using a QIAamp DNA Blood Kit (Qiagen, CA, USA) according the manufacturer's procedures [26]. Allelic discrimination for visfatin SNPs was analyzed following the manufacturer's procedures, as described in our previous studies [22, 23]. The promoter region contains rs11977021, rs61330082, and rs2110385, three of the four visfatin SNPs that were analyzed. Between exons six and seven, in the intron region, is where visfatin rs4730153 is found. The TaqMan SNP Genotyping Assay and the ABI StepOnePlus Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) were used to perform allele discrimination of four visfatin SNPs: C/T alleles of rs11977021, G/A alleles of rs61330082, G/T alleles of rs2110385, and G/A alleles of rs4730153. The procedure was followed as previously reported [22, 23]. The visfatin SNP probes rs11977021 (C_11405260_10), rs61330082 (C_88870749_10), rs2110385 (C_16125685_10) and rs4730153 (C_2673294_10) was purchased from Applied Biosystems (Foster City, CA, USA).
Analysis of clinical dataset
Levels of visfatin in PCa samples collected from The Cancer Genome Atlas (TCGA) via the UALCAN website (https://ualcan.path.uab.edu/) were analyzed [3], identifying 482 patients whose visfatin gene expression was measured in each tumor sample.
Statistical analysis
Data were analyzed using the statistical software program Statistical Analytic System version 9.1 (SAS Institute Inc., Cary, NC, USA). Chi-square and Student's t-test were employed to compare demographic characteristics between the PSA ≤ 10 and > 10 groups. Odds ratios (ORs) and adjusted ORs (AORs) with 95% confidence intervals (CIs) were estimated using multiple logistic regression models to determine the association between genotypic frequencies and the two PSA groups, as well as the risk of different clinicopathological characteristics. Between-group differences were considered significant when p-values were <0.05.
Results
The blood level of PSA is often elevated in individuals with PCa, serving as a marker to monitor disease progression [27]. This study recruited 695 Taiwanese males (Table 1), compared with the PSA ≤ 10 group, the PSA > 10 group had markedly more patients aged > 65 years, pathological Gleason grade group 4 + 5, clinical and pathologic T3 + 4, and a higher incidence of clinical M1 disease, pathologic N1, seminal vesicle invasion, perineural invasion, and lymphovascular invasion. Pathological examination revealed lymph node metastasis rates of 4.5% and 12.4% in the PSA ≤ 10 and PSA > 10 groups. The percentages of high-risk PCa were 27.8% (92) in the PSA ≤ 10 group and 70.1% (255) in the PSA > 10 group, according to the D'Amico classification (Table 1).
Next, we examined the role of visfatin gene polymorphisms on clinicopathologic characteristics of PCa patients. Compared with having the GG genotype at rs61330082, having the GA+AA heterozygote markedly evaluated the risk of biochemical recurrence (OR 1.462; 95% CI, 1.013-2.110; p<0.05) (Table 3). We then examined the relation of clinicopathological characteristics in the PSA > 10 group, as shown in Tables 4 and 5. Compared with individuals carrying the GG genotype, those carrying the GA+AA genotype showed a higher incidence of pathologic N1 stage (OR 2.589; 95% CI, 1.118-5.994; p<0.05), perineural invasion (OR 1.948; 95% CI, 1.139-3.331; p<0.05), lymphovascular invasion (OR 1.960; 95% CI, 1.089-3.527; p<0.05), and biochemical recurrence (OR 1.755; 95% CI, 1.099-2.805; p<0.05) (Table 4).
Furthermore, among PSA > 10 group PCa patients with the SNP rs11977021, having the CT+TT heterozygote increased the risk of perineural invasion (OR 1.777; 95% CI, 1.038-3.041; p<0.05), lymphovascular invasion (OR 1.824; 95% CI, 1.024-3.249; p<0.05), and biochemical recurrence (OR 1.601; 95% CI, 1.007-2.545; p<0.05) compared with having the CC wild-type (Table 5).
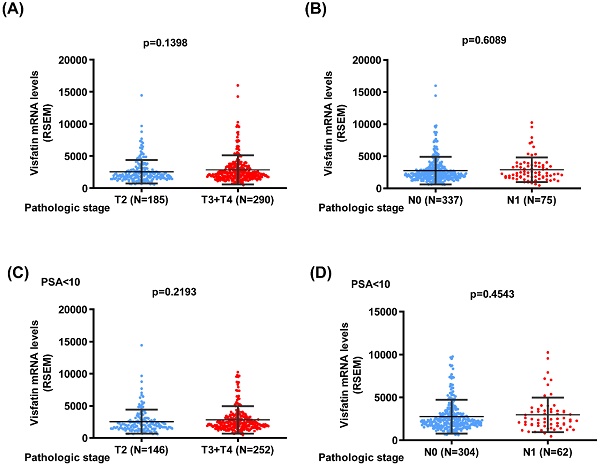
We next used the TCGA database to analyze the visfatin mRNA level, PSA level at diagnosis, pathological T/N stage, and overall survival. No noteworthy difference was found between the T2 and T3 + 4 groups or pathological N stages of PCa in terms of visfatin level (Fig. 1A&B). Similar results were also observed in PCa patients with PSA levels ≤10 ng/ml (Fig. 1C&D). Additionally, no difference was observed in the overall survival between high and low visfatin level in the whole population group (Fig. 2A&B) and PSA levels ≤10 ng/ml group (Fig. 2C&D).
Discussion
Adipose tissue plays a critical effect in PCa progression [28]. PCa is believed to engage in direct or indirect interactions with adipocytes, influencing tumor cell growth, invasion, and metastasis. Notably, bone constitutes the most prevalent metastatic site in PCa [29, 30]. Multiple reports have suggested bone marrow adipocytes as significant contributors to the development and aggravation of these bone metastases [31]. Visfatin is strongly related to oncogenesis, and in many types of cancer, upregulated visfatin production are related with a worse prognosis [32-34]. However, the TCGA database results for the entire population group showed no difference in overall survival between the groups with high and low visfatin mRNA expression. This lack of distinction might be attributed to the brief follow-up period and relatively small sample size. Further research is needed, necessitating an increase in both sample size and follow-up time.
The distributions of demographical characteristics in 695 patients with prostate cancer.
| Variable | PSA at diagnosis (ng/mL) | ||
|---|---|---|---|
| ≤ 10 (n=331) | > 10 (n=364) | p value | |
| Age at diagnosis (years) | |||
| ≤ 65 | 159 (48.0 %) | 136 (36.6 %) | p=0.004* |
| > 65 | 172 (52.0 %) | 228 (63.4 %) | |
| Pathologic Gleason grade group | |||
| 1+2+3 | 301 (90.9 %) | 276 (75.8 %) | p<0.001* |
| 4+5 | 30 (9.1 %) | 88 (24.2 %) | |
| Clinical T stage | |||
| 1+2 | 311 (94.0 %) | 286 (78.6 %) | p<0.001* |
| 3+4 | 20 (6.0 %) | 78 (21.4 %) | |
| Clinical N stage | |||
| N0 | 328 (99.1 %) | 353 (97.0 %) | p=0.047* |
| N1 | 3 (0.9 %) | 11 (3.0 %) | |
| Clinical M stage | |||
| M0 | 331 (100.0 %) | 353 (97.0 %) | p=0.001* |
| M1 | 0 (0.0 %) | 11 (3.0 %) | |
| Pathologic T stage | |||
| 2 | 227 (68.6 %) | 139 (38.2 %) | p<0.001* |
| 3+4 | 104 (31.4 %) | 225 (61.8 %) | |
| Pathologic N stage | |||
| N0 | 316 (95.5 %) | 319 (87.6 %) | p<0.001* |
| N1 | 15 (4.5 %) | 45 (12.4 %) | |
| Seminal vesicle invasion | |||
| No | 300 (90.6 %) | 247 (67.9 %) | p<0.001* |
| Yes | 31 (9.4 %) | 117 (32.1 %) | |
| Perineural invasion | |||
| No | 113 (34.1%) | 71 (19.5 %) | p<0.001* |
| Yes | 218 (65.9 %) | 293 (80.5 %) | |
| Lymphovascular invasion | |||
| No | 305 (92.1 %) | 280 (76.9 %) | p<0.001* |
| Yes | 26 (7.9 %) | 84 (23.1 %) | |
| Biochemical recurrence | |||
| No | 264 (79.8 %) | 211 (58.0 %) | p<0.001* |
| Yes | 67 (20.2 %) | 153 (42.0 %) | |
| D'Amico classification | |||
| Low risk/ Intermediate risk | 239 (72.2 %) | 109 (29.9 %) | p<0.001* |
| High risk | 92 (27.8 %) | 255 (70.1 %) | |
Distribution frequency of visfatin genotypes in 695 patients with prostate cancer.
| Variable | PSA at diagnosis (ng/mL) | |||
|---|---|---|---|---|
| ≤ 10 | > 10 | OR (95% CI) | AOR (95% CI) | |
| rs11977021 | ||||
| CC | 90 (27.2%) | 111 (30.5%) | 1.000 (reference) | 1.000 (reference) |
| CT | 174 (52.6%) | 179 (49.2%) | 0.834 (0.589-1.181) | 0.777 (0.521-1.159) |
| TT | 67 (20.2%) | 74 (20.3%) | 0.896 (0.581-1.379) | 0.701 (0.425-1.155) |
| CT+TT | 241 (72.8%) | 253 (69.5%) | 0.851 (0.612-1.183) | 0.755 (0.517-1.102) |
| rs61330082 | ||||
| GG | 90 (27.2%) | 110 (30.2%) | 1.000 (reference) | 1.000 (reference) |
| GA | 173 (52.3%) | 178 (48.9%) | 0.842 (0.594-1.193) | 0.767 (0.514-1.144) |
| AA | 68 (20.5%) | 76 (20.9%) | 0.914 (0.595-1.405) | 0.709 (0.432-1.165) |
| GA+AA | 241 (72.8%) | 254 (69.8%) | 0.862 (0.620-1.199) | 0.750 (0.513-1.095) |
| rs2110385 | ||||
| GG | 260 (78.5%) | 297 (81.6%) | 1.000 (reference) | 1.000 (reference) |
| GT | 64 (19.3%) | 61 (16.8%) | 0.834 (0.566-1.230) | 0.847 (0.543-1.323) |
| TT | 7 (2.1%) | 6 (1.6%) | 0.750 (0.249-2.261) | 0.839 (0.248-2.844) |
| GT+TT | 71 (21.5%) | 67(18.4%) | 0.826 (0.569-1.200) | 0.847 (0.552-1.298) |
| rs4730153 | ||||
| GG | 262 (79.2%) | 300 (82.4%) | 1.000 (reference) | 1.000 (reference) |
| GA | 64 (19.3%) | 59 (16.2%) | 0.805 (0.545-1.190) | 0.827 (0.529-1.294) |
| AA | 5 (1.5%) | 5 (1.4%) | 0.873 (0.250-3.050) | 0.854 (0.218-3.347) |
| GA+AA | 69 (20.8%) | 64 (17.6%) | 0.810 (0.555-1.183) | 0.829 (0.538-1.278) |
The odds ratios (ORs) and with their 95% confidence intervals (CIs) were estimated by logistic regression models. The adjusted odds ratios (AORs) with their 95% confidence intervals (CIs) were estimated by multiple logistic regression models after controlling for Age at diagnosis, pathologic Gleason grade group, clinical T stage, clinical N stage, clinical M stage, pathologic T stage, pathologic N stage, seminal vesicle invasion, perineural invasion, lymphovascular invasion, biochemical recurrence and D'Amico classification.
Odds ratio (OR) and 95% confidence interval (CI) of clinical status and visfatin rs61330082 genotypic frequencies in 695 patients with prostate cancer.
| Variable | Genotypic frequencies | |||
|---|---|---|---|---|
| rs61330082 | GG (N=200) | GA+AA (N=495) | OR (95% CI) | p value |
| Pathologic Gleason grade group | ||||
| 1+2+3 | 169 (84.5%) | 408 (82.4%) | 1.00 | p=0.509 |
| 4+5 | 31 (15.5%) | 87 (17.6%) | 1.162 (0.743-1.818) | |
| Clinical T stage | ||||
| 1+2 | 169 (84.5%) | 428 (86.5%) | 1.00 | p=0.500 |
| 3+4 | 31 (15.5%) | 67 (13.5%) | 0.853 (0.538-1.354) | |
| Pathologic T stage | ||||
| 2 | 113 (56.5%) | 253 (51.1%) | 1.00 | p=0.198 |
| 3+4 | 87 (43.5%) | 242 (48.9%) | 1.242 (0.893-1.729) | |
| Pathologic N stage | ||||
| N0 | 188 (94.0%) | 447 (90.3%) | 1.00 | p=0.116 |
| N1 | 12 (6.0%) | 48 (9.7%) | 1.682 (0.874-3.239) | |
| Seminal vesicle invasion | ||||
| No | 163 (81.5%) | 384 (77.6%) | 1.00 | p=0.253 |
| Yes | 37 (18.5%) | 111 (22.4%) | 1.273 (0.841-1.928) | |
| Perineural invasion | ||||
| No | 63 (31.5%) | 121 (24.4%) | 1.00 | p=0.056 |
| Yes | 137 (68.5%) | 374 (75.6%) | 1.421 (0.990-2.041) | |
| Lymphovascular invasion | ||||
| No | 173 (86.5%) | 412 (83.2%) | 1.00 | p=0.285 |
| Yes | 27 (13.5%) | 83 (16.8%) | 1.291 (0.807-2.064) | |
| Biochemical recurrence | ||||
| No | 148 (74.0%) | 327 (66.1%) | 1.00 | p=0.042* |
| Yes | 52 (26.0%) | 168 (33.9%) | 1.462 (1.013-2.110) | |
| D''Amico classification | ||||
| Intermediate risk | 103 (51.5%) | 245 (49.5%) | 1.00 | p=0.632 |
| High risk | 97 (48.5%) | 250 (50.5%) | 1.084 (0.780-1.505) | |
The ORs with analyzed by their 95% CIs were estimated by logistic regression models. * p value < 0.05 as statistically significant.
In addition to the aforementioned adipocytokines, visfatin, an adipokine found in visceral fat, has been documented to regulate cancer development and metastasis in studies involving various cancers such as liver, endometrial, esophageal and osteosarcoma [35-38]. However, its impact on the clinicopathologic characteristics of PCa patients has not yet been established. The TCGA database results for the current human PCa investigation showed that there was no statistically significant difference in visfatin mRNA levels between pathological T/N stage in PCa patients. However, compared with having the GG genotype at rs61330082, having the GA+AA heterozygote significantly increased the risk of biochemical recurrence, while no such association was observed with other clinicopathological characteristics. This evidence implicates crucial roles for visfatin polymorphisms in PCa biochemical recurrence.
Over the past ten years, PSA testing has gained popularity worldwide, and a single PSA screening intervention alone can increase the examination of low-risk PCa [39]. Numerous genomic-based assays have been developed to predict metastasis, cancer-related mortality, and recurrence following surgery [40, 41]. To ascertain predictive variables for active surveillance, several alleles were evaluated [42]. This is the first time to assess the relation between visfatin SNPs and the clinicopathological features of PCa with iPSA levels > 10 ng/ml. The data indicated that individuals with the GA+AA genotype at visfatin rs61330082 had a high risk of pathologic N1 stage, perineural invasion, lymphovascular invasion, and biochemical recurrence in the PSA > 10 group. The visfatin SNP rs11977021, having the CT+TT heterozygote, increased the risk of perineural invasion, lymphovascular invasion, and biochemical recurrence in PSA > 10 group PCa patients.
The term 'tumor metastasis' refers to the process through which the primary tumor disseminates to diverse tissues or organs via the blood or lymphatic system. Given that restraining lymphangiogenesis can effectively impede both tumor progression and metastasis [43-45], comprehending the intricate nature of metastasis is of paramount importance. The link between lymphangiogenesis and tumor growth as well as metastasis has been substantiated through various scholarly publications [46, 47]. Our results indicated that visfatin SNPs, rs61330082 and rs11977021, are associated with lymphovascular invasion in PSA > 10 group PCa patients. Therefore, visfatin SNPs in PSA > 10 group PCa patients can be used as prognostic factors for lymphangiogenesis.
In conclusion, our examination demonstrates associations between visfatin gene variants and biochemical recurrence for PCa, particularly among Taiwanese males carrying the visfatin rs61330082 polymorphism. Additionally, visfatin SNPs, rs61330082 and rs11977021, are associated with perineural invasion, lymphovascular invasion, and biochemical recurrence in PSA > 10 group PCa patients.
Odds ratio (OR) and 95% confidence interval (CI) of clinical status and visfatin rs61330082 genotypic frequencies in 364 patients with prostate cancer with PSA concentration > 10 ng/mL.
| Variable | Genotypic frequencies | |||
|---|---|---|---|---|
| rs61330082 | GG (N=110) | GA+AA (N=254) | OR (95% CI) | p value |
| Pathologic Gleason grade group | ||||
| 1+2+3 | 88 (80.0%) | 188 (74.0%) | 1.00 | p=0.221 |
| 4+5 | 22 (20.0%) | 66 (26.0%) | 1.404 (0.814-2.422) | |
| Clinical T stage | ||||
| 1+2 | 87 (79.1%) | 199 (79.3%) | 1.00 | p=0.874 |
| 3+4 | 23 (20.9%) | 55 (21.7%) | 1.045 (0.604-1.808) | |
| Pathologic T stage | ||||
| 2 | 50 (45.5%) | 89 (35.0%) | 1.00 | p=0.060 |
| 3+4 | 60 (54.5%) | 165 (65.0%) | 1.545 (0.980-2.436) | |
| Pathologic N stage | ||||
| N0 | 103 (93.6%) | 216 (85.0%) | 1.00 | p=0.022* |
| N1 | 7 (6.4%) | 38 (15.0%) | 2.589 (1.118-5.994) | |
| Seminal vesicle invasion | ||||
| No | 81 (73.6%) | 166 (65.4%) | 1.00 | p=0.120 |
| Yes | 29 (26.4%) | 88 (34.6%) | 1.481 (0.901-2.433) | |
| Perineural invasion | ||||
| No | 30 (27.3%) | 41 (16.1%) | 1.00 | p=0.014* |
| Yes | 80 (72.7%) | 213 (83.9%) | 1.948 (1.139-3.331) | |
| Lymphovascular invasion | ||||
| No | 93 (84.5%) | 187 (73.6%) | 1.00 | p=0.023* |
| Yes | 17 (15.5%) | 67 (26.4%) | 1.960 (1.089-3.527) | |
| Biochemical recurrence | ||||
| No | 74 (67.3%) | 137 (53.9%) | 1.00 | p=0.018* |
| Yes | 36 (32.7%) | 117 (46.1%) | 1.755 (1.099-2.805) | |
| D'Amico classification | ||||
| Intermediate risk | 40 (36.4%) | 69 (27.2%) | 1.00 | p=0.078 |
| High risk | 70 (63.6%) | 185 (72.8%) | 1.532 (0.951-2.468) | |
The ORs with analyzed by their 95% CIs were estimated by logistic regression models. * p value < 0.05 as statistically significant.
Odds ratio (OR) and 95% confidence interval (CI) of clinical status and visfatin rs11977021 genotypic frequencies in 364 patients with prostate cancer with PSA concentration > 10 ng/mL.
| Variable | Genotypic frequencies | |||
|---|---|---|---|---|
| rs11977021 | CC (N=111) | CT+TT (N=253) | OR (95% CI) | p value |
| Pathologic Gleason grade group | ||||
| 1+2+3 | 88 (79.3%) | 188 (74.3%) | 1.00 | p=0.308 |
| 4+5 | 23 (20.7%) | 65 (25.7%) | 1.323 (0.772-2.267) | |
| Clinical T stage | ||||
| 1+2 | 88 (79.3%) | 198 (78.3%) | 1.00 | p=0.827 |
| 3+4 | 23 (20.7%) | 55 (21.7%) | 1.063 (0.615-1.838) | |
| Pathologic T stage | ||||
| 2 | 48 (43.2%) | 91 (36.0%) | 1.00 | p=0.188 |
| 3+4 | 63 (56.8%) | 162 (64.0%) | 1.356 (0.861-2.138) | |
| Pathologic N stage | ||||
| N0 | 103 (92.8%) | 216 (85.4%) | 1.00 | p=0.057 |
| N1 | 8 (7.2%) | 37 (14.6%) | 2.205 (0.992-4.905) | |
| Seminal vesicle invasion | ||||
| No | 80 (72.1%) | 167 (66.0%) | 1.00 | p=0.254 |
| Yes | 31 (27.9%) | 86 (34.0%) | 1.329 (0.815-2.168) | |
| Perineural invasion | ||||
| No | 29 (26.1%) | 42 (16.6%) | 1.00 | p=0.035* |
| Yes | 82 (73.9%) | 211 (83.4%) | 1.777 (1.038-3.041) | |
| Lymphovascular invasion | ||||
| No | 93 (83.8%) | 187 (73.9%) | 1.00 | p=0.040* |
| Yes | 18 (16.2%) | 66 (26.1%) | 1.824 (1.024-3.249) | |
| Biochemical recurrence | ||||
| No | 73 (65.8%) | 138 (54.5%) | 1.00 | p=0.046* |
| Yes | 38 (34.2%) | 115 (45.5%) | 1.601 (1.007-2.545) | |
| D'Amico classification | ||||
| Intermediate risk | 39 (35.1%) | 70 (27.7%) | 1.00 | p=0.152 |
| High risk | 72 (64.9%) | 183 (72.3%) | 1.416 (0.879-2.282) | |
The ORs with analyzed by their 95% CIs were estimated by logistic regression models. * p value < 0.05 as statistically significant.
The visfatin mRNA level of PCa patients from TCGA database. Levels of visfatin mRNA expression in whole population (A&B) and PSA < 10 group (C&D) PCa patients retrieved from TCGA dataset records.
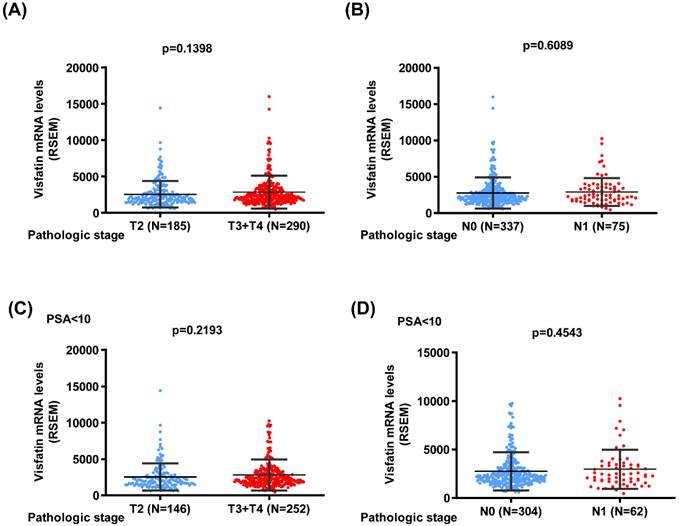
The visfatin mRNA level and overall survival of PCa from the TCGA database. Kaplan-Meier analysis examined levels of visfatin expression and overall survival rates of whole population (A&B) and PSA < 10 group (C&D) PCa patients.
Acknowledgements
This study was supported by grants from the Ministry of Science and Technology of Taiwan (MOST 111-2314-B-039-048-MY3; NSTC 113-2320-B-039-049-MY3), China Medical University Hsinchu Hospital (CMUHCH-DMR-113-021), China Medical University Beigang Hospital (CMUBHR109-007) and China Medical University Hospital (DMR-113-070; DMR-113-200).
Author contributions
SFY and CHT conceived and designed the experiments. SLH, SCL, CYL, YCF, SSW and LCC performed the experiments. SLH and SCL contributed to analyzing experiment data. SFY and CHT contributed to the manuscript's writing. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Force USPST, Grossman DC, Curry SJ, Owens DK, Bibbins-Domingo K, Caughey AB. et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901-13
2. Uehara H, Kobayashi T, Matsumoto M, Watanabe S, Yoneda A, Bando Y. Adipose tissue:Critical contributor to the development of prostate cancer. J Med Invest. 2018;65:9-17
3. Lin TH, Chang SL, Khanh PM, Trang NTN, Liu SC, Tsai HC. et al. Apelin Promotes Prostate Cancer Metastasis by Downregulating TIMP2 via Increases in miR-106a-5p Expression. Cells. 2022;11:3285
4. Furesi G, Rauner M, Hofbauer LC. Emerging Players in Prostate Cancer-Bone Niche Communication. Trends Cancer. 2021;7:112-21
5. Tai HC, Wang SW, Swain S, Lin LW, Tsai HC, Liu SC. et al. Melatonin suppresses the metastatic potential of osteoblastic prostate cancers by inhibiting integrin alpha(2) beta(1) expression. J Pineal Res. 2022;72:e12793
6. DiNatale A, Fatatis A. The Bone Microenvironment in Prostate Cancer Metastasis. Adv Exp Med Biol. 2019;1210:171-84
7. Chang AC, Chen PC, Lin YF, Su CM, Liu JF, Lin TH. et al. Osteoblast-secreted WISP-1 promotes adherence of prostate cancer cells to bone via the VCAM-1/integrin alpha4beta1 system. Cancer Lett. 2018;426:47-56
8. Iacobini C, Pugliese G, Blasetti Fantauzzi C, Federici M, Menini S. Metabolically healthy versus metabolically unhealthy obesity. Metabolism. 2019;92:51-60
9. Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. 2019;92:121-35
10. Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol. 2019;15:139-54
11. Abdalla MMI. Role of visfatin in obesity-induced insulin resistance. World J Clin Cases. 2022;10:10840-51
12. Law YY, Lin YM, Liu SC, Wu MH, Chung WH, Tsai CH. et al. Visfatin increases ICAM-1 expression and monocyte adhesion in human osteoarthritis synovial fibroblasts by reducing miR-320a expression. Aging (Albany NY). 2020;12:18635-48
13. Wu MH, Tsai CH, Huang YL, Fong YC, Tang CH. Visfatin Promotes IL-6 and TNF-alpha Production in Human Synovial Fibroblasts by Repressing miR-199a-5p through ERK, p38 and JNK Signaling Pathways. International journal of molecular sciences. 2018;19:190
14. Lin TC. The role of visfatin in cancer proliferation, angiogenesis, metastasis, drug resistance and clinical prognosis. Cancer management and research. 2019;11:3481-91
15. Song CY, Wu CY, Lin CY, Tsai CH, Chen HT, Fong YC. et al. The stimulation of exosome generation by visfatin polarizes M2 macrophages and enhances the motility of chondrosarcoma. Environ Toxicol. 2024;39:3790-8
16. Song CY, Chang SL, Lin CY, Tsai CH, Yang SY, Fong YC. et al. Visfatin-Induced Inhibition of miR-1264 Facilitates PDGF-C Synthesis in Chondrosarcoma Cells and Enhances Endothelial Progenitor Cell Angiogenesis. Cells. 2022;11:3470
17. Wang B, Hasan MK, Alvarado E, Yuan H, Wu H, Chen WY. NAMPT overexpression in prostate cancer and its contribution to tumor cell survival and stress response. Oncogene. 2011;30:907-21
18. Patel ST, Mistry T, Brown JE, Digby JE, Adya R, Desai KM. et al. A novel role for the adipokine visfatin/pre-B cell colony-enhancing factor 1 in prostate carcinogenesis. Peptides. 2010;31:51-7
19. Jin LW, Zheng SB, Zhou ZH, Pan SF, Zheng Y. Correlation between polymorphisms in the visfatin gene and its expression in the serum and coronary artery calcification. Genetics and molecular research: GMR. 2016 15
20. Wu Z, Sun Y, Huang Y, Zhu S, Feng Y, Ye H. et al. Genetic variant in visfatin gene promoter contributes to reduced risk of hepatocellular carcinoma in a Chinese population. Oncotarget. 2016;7:77968-77
21. Zhang C, Yan D, Wang S, Xu C, Du W, Ning T. et al. Genetic polymorphisms of NAMPT related with susceptibility to esophageal squamous cell carcinoma. BMC gastroenterology. 2015;15:49
22. Chang SL, Yang PJ, Lin YY, Jiang YJ, Liu PI, Huang CL. et al. Genetic Associations of Visfatin Polymorphisms with EGFR Status and Clinicopathologic Characteristics in Lung Adenocarcinoma. International journal of environmental research and public health. 2022;19:15172
23. Chen KJ, Hsieh MH, Lin YY, Chen MY, Lien MY, Yang SF. et al. Visfatin Polymorphisms, Lifestyle Risk Factors and Risk of Oral Squamous Cell Carcinoma in a Cohort of Taiwanese Males. International journal of medical sciences. 2022;19:762-8
24. D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA. et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. Jama. 1998;280:969-74
25. Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA. et al. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. The American journal of surgical pathology. 2016;40:244-52
26. Yang MD, Lin KC, Lu MC, Jeng LB, Hsiao CL, Yueh TC. et al. Contribution of matrix metalloproteinases-1 genotypes to gastric cancer susceptibility in Taiwan. Biomedicine (Taipei). 2017;7:10
27. Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: data from SEER-Medicare. The Journal of urology. 2011;186:1830-4
28. Ku HC, Cheng CF. Role of adipocyte browning in prostate and breast tumor microenvironment. Tzu Chi Med J. 2022;34:359-66
29. Gandaglia G, Abdollah F, Schiffmann J, Trudeau V, Shariat SF, Kim SP. et al. Distribution of metastatic sites in patients with prostate cancer: A population-based analysis. Prostate. 2014;74:210-6
30. Chang AC, Lin LW, Chen YC, Chen PC, Liu SC, Tai HC. et al. The ADAM9/WISP-1 axis cooperates with osteoblasts to stimulate primary prostate tumor growth and metastasis. International journal of biological sciences. 2023;19:760-71
31. Otley MOC, Sinal CJ. Adipocyte-Cancer Cell Interactions in the Bone Microenvironment. Front Endocrinol (Lausanne). 2022;13:903925
32. Stepien S, Olczyk P, Gola J, Komosinska-Vassev K, Mielczarek-Palacz A. The Role of Selected Adipocytokines in Ovarian Cancer and Endometrial Cancer. Cells. 2023;12:1118
33. Chinapayan SM, Kuppusamy S, Yap NY, Perumal K, Gobe G, Rajandram R. Potential Value of Visfatin, Omentin-1, Nesfatin-1 and Apelin in Renal Cell Carcinoma (RCC): A Systematic Review and Meta-Analysis. Diagnostics. 2022;12:3069
34. Rajput PK, Sharma JR, Yadav UCS. Cellular and molecular insights into the roles of visfatin in breast cancer cells plasticity programs. Life sciences. 2022;304:120706
35. Wang GJ, Shen NJ, Cheng L, Yehan F, Huang H, Li KH. Visfatin triggers the in vitro migration of osteosarcoma cells via activation of NF-kappaB/IL-6 signals. Eur J Pharmacol. 2016;791:322-30
36. Cymbaluk-Ploska A, Chudecka-Glaz A, Pius-Sadowska E, Sompolska-Rzechula A, Machalinski B, Menkiszak J. Circulating Serum Level of Visfatin in Patients with Endometrial Cancer. Biomed Res Int. 2018;2018:8576179
37. Liang N, Chen Y, Yang L, He S, Liu T. Visfatin increases miR-21 to promote migration in HCC. Cell Mol Biol (Noisy-le-grand). 2018;64:48-52
38. Huang CL, Achudhan D, Liu PI, Lin YY, Liu SC, Guo JH. et al. Visfatin upregulates VEGF-C expression and lymphangiogenesis in esophageal cancer by activating MEK1/2-ERK and NF-kappaB signaling. Aging. 2023;15:4774-93
39. Martin RM, Donovan JL, Turner EL, Metcalfe C, Young GJ, Walsh EI. et al. Effect of a Low-Intensity PSA-Based Screening Intervention on Prostate Cancer Mortality: The CAP Randomized Clinical Trial. Jama. 2018;319:883-95
40. Klein EA, Cooperberg MR, Magi-Galluzzi C, Simko JP, Falzarano SM, Maddala T. et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. European urology. 2014;66:550-60
41. Alshalalfa M, Crisan A, Vergara IA, Ghadessi M, Buerki C, Erho N. et al. Clinical and genomic analysis of metastatic prostate cancer progression with a background of postoperative biochemical recurrence. BJU international. 2015;116:556-67
42. McGuire BB, Helfand BT, Kundu S, Hu Q, Banks JA, Cooper P. et al. Association of prostate cancer risk alleles with unfavourable pathological characteristics in potential candidates for active surveillance. BJU international. 2012;110:338-43
43. Dieterich LC, Detmar M. Tumor lymphangiogenesis and new drug development. Adv Drug Deliv Rev. 2016;99:148-60
44. Lin CY, Wang SW, Chen YL, Chou WY, Lin TY, Chen WC. et al. Brain-derived neurotrophic factor promotes VEGF-C-dependent lymphangiogenesis by suppressing miR-624-3p in human chondrosarcoma cells. Cell Death Dis. 2017;8:e2964
45. Lee HP, Wang SW, Wu YC, Lin LW, Tsai FJ, Yang JS. et al. Soya-cerebroside inhibits VEGF-facilitated angiogenesis in endothelial progenitor cells. Food Agr Immunol. 2020;31:193-204
46. Paduch R. The role of lymphangiogenesis and angiogenesis in tumor metastasis. Cell Oncol (Dordr). 2016;39:397-410
47. Su CM, Tang CH, Chi MJ, Lin CY, Fong YC, Liu YC. et al. Resistin facilitates VEGF-C-associated lymphangiogenesis by inhibiting miR-186 in human chondrosarcoma cells. Biochem Pharmacol. 2018;154:234-42
Author contact
![]() Corresponding authors: Chih-Hsin Tang, PhD (E-mail: chtangcmu.edu.tw) and Shun-Fa Yang, PhD (Email: ysfedu.tw).
Corresponding authors: Chih-Hsin Tang, PhD (E-mail: chtangcmu.edu.tw) and Shun-Fa Yang, PhD (Email: ysfedu.tw).

 Global reach, higher impact
Global reach, higher impact