3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(13):2464-2479. doi:10.7150/ijms.98183 This issue Cite
Research Paper
BuyangHuanwu Decoction alleviates Endothelial Cell Apoptosis and Coronary Microvascular Dysfunction via Regulation of the MAPKK4/p38 Signaling Axis
1. Guang'anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, 100053, China.
2. Division of Vascular Surgery, the First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510800, China; National-Guangdong Joint Engineering Laboratory for Diagnosis and Treatment of Vascular Disease, First Affiliated Hospital, Sun Yat-sen University, Guangzhou, 510080, China.
3. Outpatient Department of the Sixth Medical Center of the PLA General Hospital, China.
4. The First Affliated Hospital of Zhejiang Chinese Medical University, Hangzhou, China.
5. Beijing University of Chinese Medicine, Beijing, 100028, China.
6. School of Pharmaceutical Sciences, Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, 510006, China
7. Senior Department of Cardiology, The Sixth Medical Center of People's Liberation Army General Hospital, Beijing, China.
†These authors contributed equally to this article.
Received 2024-5-7; Accepted 2024-9-3; Published 2024-9-23
Abstract
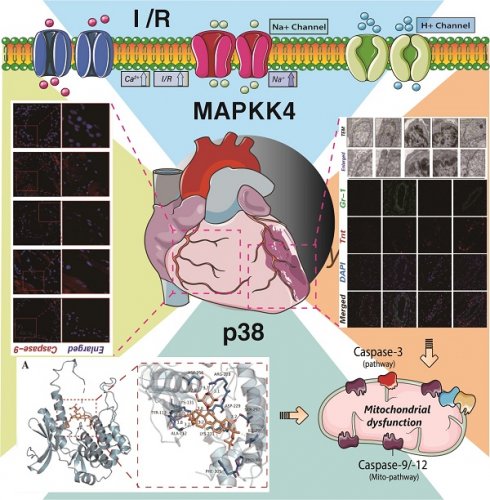
MAPKK4 has been implicated in the pathological mechanisms underlying myocardial and vascular injury, specifically influencing endothelial cell damage and programmed cell death via subcellular pathways. Nevertheless, the regulatory role of MAPKK4 in coronary microvascular injury following myocardial infarction remains unconfirmed, and the exploration of targeted mitochondrial protective therapeutic agents remains unaddressed. In light of this gap, we established a MAPKK4 gene-modified mouse model of ischemia-reperfusion injury and employed Buyang Huanwu decoction (BYHW), a traditional cardiovascular therapeutic formula, to assess its efficacy in treating coronary microvascular injury post-ischemia-reperfusion. The study aimed to elucidate the mechanism by which BYHW mitigates coronary microvascular injury induced by ischemia-reperfusion through the attenuation of endothelial cell apoptosis. Experimental outcomes revealed that high-dose BYHW significantly ameliorated coronary microvascular injury post-ischemia-reperfusion, restoring the structural integrity of the coronary microvasculature and reducing inflammation and oxidative stress. Contrarily, in transgenic mice overexpressing MAPKK4, BYHW intervention failed to attenuate microvascular inflammation and oxidative stress. To further investigate, we simulated hypoxia/reoxygenation injury in vascular endothelial cells using a MAPKK4-related cellular gene modification model. The results indicated that BYHW attenuates inflammatory damage and enhances the viability of vascular endothelial cells following hypoxic stress, inhibiting apoptosis via the mitochondrial pathway. However, overexpression of MAPKK4/p38 negated the therapeutic effects of BYHW, showing no impact on endothelial cell apoptosis and oxidative stress under hypoxic conditions. Molecular interaction studies confirmed that the active components of BYHW, Astragaloside IV and Ligustrazine, interact with the MAPKK4/P38 axis. In vitro experiments further suggested that the interaction between MAPKK4 and P38 play a crucial role in the ability of BYHW to inhibit apoptosis in coronary microvascular endothelial cells. Therapeutically, MAPKK4 may potentiate the apoptotic pathway in microvascular endothelial cells by modulating downstream P38 expression and phosphorylation, thereby exacerbating ischemia-reperfusion-induced coronary microvascular endothelial injury. From an in vivo perspective, the transgenic overexpression of MAPKK4 and P38 inhibited the microvascular protective effects of BYHW. These findings collectively underscore the significance of the MAPKK4-P38 axis in the protection of coronary microvascular endothelial cells.
Keywords: MAPKK4, P38, Coronary microvascular injury, Buyang Huanwu decoction, Cell apoptosis, Inflammatory injury
Introduction
The pathological and physiological foundations of coronary microcirculation injury are intricate, marked by the interplay of multiple processes, including ischemia-induced injury and hypoxia stress response [1, 2]. Early research primarily supported the hypothesis that no-reflow results from microvascular obstruction, with some studies also highlighting irreversible microvascular damage and subsequent myocardial hemorrhage as critical contributors to no-reflow [3]. Currently, various studies have identified key pathophysiological factors underlying coronary microvascular injury, including oxidative stress, mitochondrial dysfunction, inflammatory cascade reactions, programmed cell death, endoplasmic reticulum stress, and calcium overload, among other mechanisms [4]. Substantial evidence has underscored the pivotal role of mitochondrial pathway-induced apoptosis and oxidative stress in maintaining cellular homeostasis.
Our previous research has elucidated the regulatory mechanisms of mitochondrial energy metabolism disorders, programmed cell death via mitochondrial pathways, and mitochondrial oxidative stress in the context of coronary microvascular injury [5, 6]. We have also validated the interaction mechanisms between downstream targets and protein sites within the mitochondrial quality control regulatory pathway [7, 8]. Additionally, further investigations into the pathological changes associated with ischemic myocardial injury before, during, and after coronary microvascular injury revealed a close association between the MAPPK4-P38 signaling pathway and microcirculation injury [7, 9].
Despite numerous studies suggesting the targeted regulatory effects of this pathway on endothelial and myocardial cells, the development of targeted therapeutic drugs and elucidation of myocardial/endothelial protective mechanisms specific to this pathway remain incomplete [10]. Coronary microvascular endothelial cells play a crucial role in endothelial injury post-ischemia-reperfusion, and the P38 mitogen-activated protein kinase (MAPK) is among the key signaling pathways facilitating endothelial cell adaptation to ischemia/hypoxia and nutrient deprivation [11, 12]. While p38-MAPK is known to regulate ATPase synthesis and oxidative phosphorylation metabolism during stress injury, research into its targeted therapeutic applications is ongoing. Multiple studies have affirmed the regulatory role of the mitogen-activated protein kinase (MAPK) signaling cascade, including MAPKKK10, MAPKK4, and p38 MAPK, in myocardial and endothelial stress injury [13]. However, the development of targeted regulatory drugs remains in the research phase. Buyang Huanwu decoction (BYHW) is a traditional formula predominantly composed of Huangqi (Astragalus membranaceus), Danggui (Angelica sinensis), Chishao, Dilong, Chuanxiong, and Honghua, among others, and is a well-established therapeutic agent for cardiovascular diseases [14-16]. Flavonoids present in Astragalus membranaceus, such as Astragalus polysaccharides and Astragaloside IV, exhibit significant anti-inflammatory, antioxidant, and metabolic regulatory properties, contributing to the protection of cardiovascular and myocardial cells [17, 18]. Similarly, the constituents of Angelica sinensis, including ferulic acid and Angelica sinensis polysaccharides, provide antioxidant protection and inhibit myocardial apoptosis, thereby preserving myocardial and vascular endothelial function [19, 20]. The combination of Ligusticum chuanxiong and Angelica sinensis is particularly effective in exerting anti-inflammatory and analgesic effects, primarily by inhibiting inflammatory mediators such as TNF-α and IL-6. Ferulic acid and caffeic acid, common components of both Angelica sinensis and Ligusticum chuanxiong, work synergistically to deliver potent antioxidant effects [21, 22]. Additionally, this combination demonstrates anticoagulant properties, lowers blood lipid levels, and inhibits platelet activation. The multiple active ingredients in BYHW collectively offer robust myocardial and vascular protection, alongside anti-inflammatory, antioxidative, and anti-apoptotic effects. Endothelial cell apoptosis, a form of programmed cell death, is influenced by a range of factors including inflammatory damage, oxidative stress, calcium homeostasis disruption, and various stress stimuli targeting key subcellular organelles such as mitochondria and the endoplasmic reticulum [23, 24]. The apoptotic pathway is tightly regulated by upstream proteins in endothelial cells, particularly in the context of cellular inflammation [25]. The caspase family, as markers of apoptosis, can be activated by inducing factors that destabilize cellular homeostasis [26, 27]. Given the regulatory influence of the MAPKK4-p38 axis on myocardial and vascular endothelial cells and the targeted therapeutic potential of BYHW, we undertook a series of studies to investigate the interaction mechanisms in both animal and cellular models. These studies, conducted through both in vitro and in vivo experiments, validated the protective effects of BYHW and further elucidated the mechanism by which BYHW ameliorates coronary microvascular injury post-ischemia-reperfusion by modulating cell apoptosis through the MAPKK4-p38 pathway.
Materials and Methods
Ethical statement
This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals at the School of Pharmaceutical Sciences, Guangzhou University of Chinese Medicine. Animal procedures were performed according to protocols approved by the Animal Ethics Committee at Guangzhou University of Chinese Medicine (No. 20211207002).
Experimental animals
MAPKK4CKO/TG and p38TG mice were generated based on previous studies [28-30]. This study was approved by the Ethics Center of Guangzhou university of Traditional chinese medicine in accordance with the ARRIVE Guidelines for Animal Research. Adult male C57BL/6 mice, aged 7-8 weeks, were homogenously housed at the Animal Experiment Center of Guangzhou university of Traditional chinese medicine. The laboratory maintained a temperature of 23-25 C, followed a 12h light/dark cycle, sustained a humidity level of 55% ± 5%, and supplied approved standard food and free access to water for a minimum of 7 days.
Animals model
Establish a coronary microvascular injury model through myocardial ischemia-reperfusion model [7, 8]. C57BL/6 mice were anesthetized by intraperitoneal injection of 50mg/kg pentobarbital. After confirming the anesthesia status, use hair removal cream for hair removal. The temperature range of the small constant temperature operating table is 35-37 ℃, and a cardiac monitor is installed. The prepared skin is locally disinfected, the intercostal skin is cut open, the pericardium is opened, the heart is pushed out of the body, and the left anterior descending coronary artery is quickly ligated after exposure. When the bottom heart turns white, quickly close the chest and squeeze to prevent pneumothorax, and suture the muscles and skin layer by layer [31]. The surgery resulted in myocardial ischemia of the left ventricular anterior wall. After 30-45 minutes of ischemia, perform a second thoracotomy to ligate and release the ligature, restoring blood flow to the left outer branch of the coronary artery and myocardial tissue. Close the chest and compress to prevent pneumothorax, and suture the muscles and skin layer by layer. Similarly, the mice in the sham surgery group received anesthesia, thoracotomy, and thread insertion without causing myocardial ischemia [32, 33].
Cell culture
In order to conduct in vitro modeling of coronary microvascular cells, oxygen glucose deprivation was used to simulate ischemia, and oxygen glucose supply was restored to simulate reperfusion [34]. Use a pre saturated simulated ischemic solution of 95% N2+5% CO2 instead of culture medium, and incubate cells in a cell culture box containing 95% N2 followed by incubation with DMED simulated reperfusion solution containing 10% fetal bovine serum instead of simulated ischemic solution for 3 hours [35]. All groups underwent the aforementioned ischemia-reperfusion procedure. Cells were cultured in DMEM supplemented with glutamine, 10% fetal bovine serum, and 100 μ g/mL penicillin/streptomycin. Cells were transfected with MAPKK4/P38 adenovirus to obtain overexpression or knockout gene modification treatment [32, 36].
Enzyme linked immunosorbent assay
The enzyme-linked immunosorbent assay (ELISA) kits for creatine kinase (CK-MB), troponin T, and lactate dehydrogenase (LDH) were purchased from Wuhan Feien Biotechnology Co., Ltd. (Wuhan, China) and measured according to the manufacturer's instructions [37, 38].
Transmission electron microscopy detection
Determine the sampling site of fresh coronary microvascular tissue using transmission electron microscopy to minimize mechanical damage. Prior to sampling, a culture dish containing a fixed solution for electron microscopy was prepared in advance. Immediately place the tissue into a culture dish and cut it into small pieces to prevent it from settling in the fixed solution [39]. Then, add these small woven blocks with fresh electron microscope fixative. Then rinse the tissue three times with 0.1M phosphate buffer (pH 7.4) for 15 minutes each time. Copper mesh slices were stained and dried overnight in copper cages in the room. Analyze images under transmission electron microscopy [40].
Immunofluorescence in paraffin sections
Paraffin sections of myocardial tissue were deparaffinized, and the tissue sections were placed in EDTA antigen repair buffer (pH 8.0) and removed in a microwave oven. After the remaining part is slightly dried, use a chemical pen to draw circles around the tissue (to prevent antibody loss) [41], dry, rinse with phosphate buffered saline (PBS), block in bovine serum albumin, and then seal. Then cultivate the glass slide with primary and secondary antibodies in sequence, and keep it away from light at room temperature for 50 minutes [42]. After retraining with DAPI, the nuclei and sections were dried and sealed with anti-fluorescence quenching slides. Observe under a fluorescence electron microscope [43].
Mitochondrial respiratory chain function
Each group of myocardial cells was used for ELISA detection. Mitochondrial respiratory complex I (ab109721, Abcam, MA, USA), mitochondrial respiratory complex II (ab109908, Abcam), and mitochondrial respiratory complex III (A089-3-1, manufactured by Nanjing Jiancheng) were evaluated. Wash the cells with PBS and mix. Grind or repeatedly freeze thaw after ultrasonic treatment, centrifuge the homogenate at 5000g and stir for 5-10 minutes, and use the supernatant for detection [44].
Real-time quantitative PCR
Extract 10mg of total RNA from heart tissue using TRIzol reagent (Invitrogen), followed by chloroform purification and isopropanol precipitation. Use NanoDrop instrument (Thermo Fisher Scientific) to detect RNA concentration [45]. Reverse transcribe RNA using PrimeScriptTM RT kit and amplify the obtained cDNA using a rapid real-time PCR instrument (ABI-7900-384, Applied Biosystems) equipped with TB Green Premix Ex TaqTM II (RR820A, Takara, Japan). QPCR was used to amplify the cytochrome c oxidase subunit I (CO1) gene and NDUFV1 nDNA gene of mtDNA [46, 47]. The reaction was initiated at 94 ° C for 10 minutes, followed by 40 cycles at 94 ° C for 10 seconds, 60 ° C for 30 seconds, and 94 ° C for 10 seconds. All reactions were repeated. Use SDS 1.9.1 software (Applied Biosystems) to analyze amplification curves and determine the relative ratio of mtDNA: nDNA in each sample based on previous studies using these curves [48, 49].
Statistical analysis
Use GraphPad Prism 9.0 statistical software to analyze the results. The data is displayed as mean ± standard error of mean (SEM). For the comparison between the two groups, parametric Student t-test or nonparametric Mann Whitney test was used. For comparisons between two or more groups, use parametric one-way ANOVA test, followed by Bonferroni test. The histopathological parameters of the tissue were statistically analyzed using chi square test. P<0.05 is considered significant.
Results
Buyang Huanwu decoction improves coronary microvascular injury by regulating inflammation and cell apoptosis
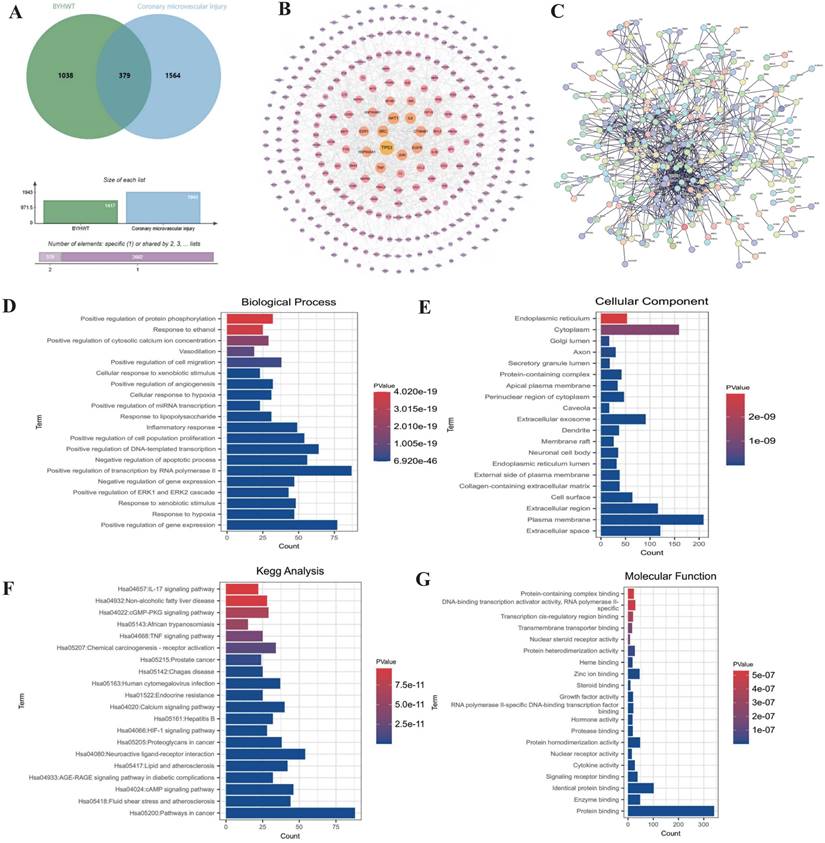
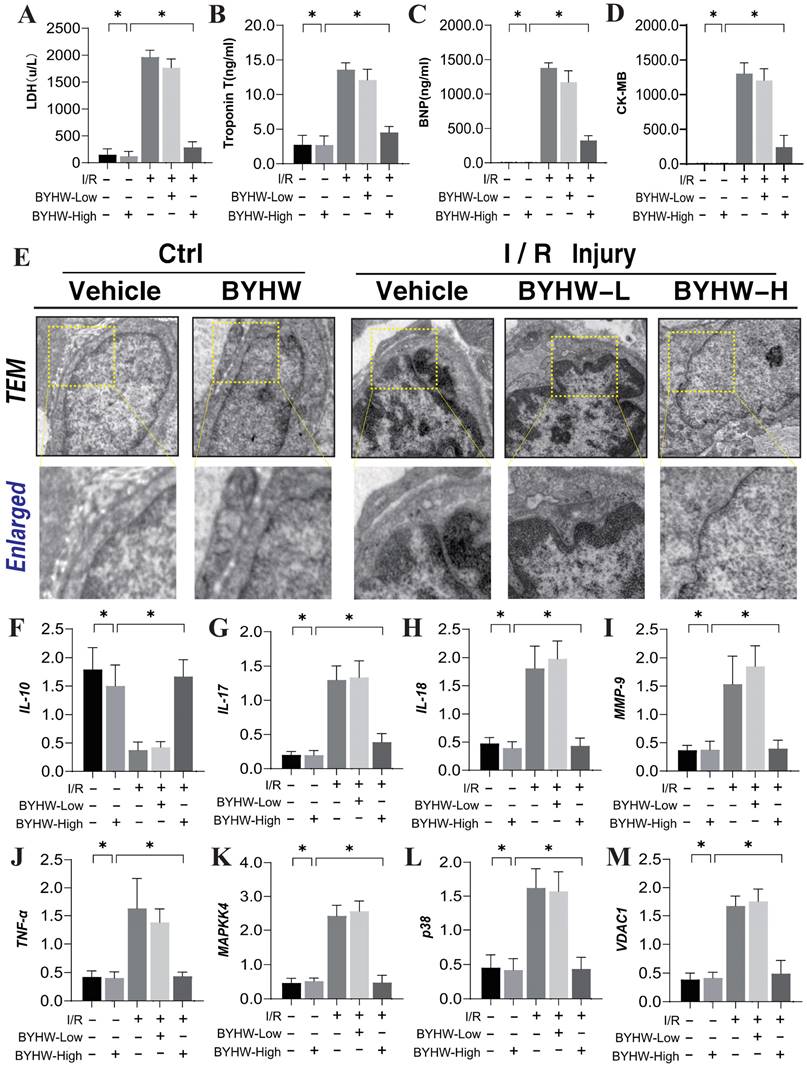
To investigate the mechanisms underlying coronary microvascular injury following ischemia-reperfusion (IR), we assessed the therapeutic effects of Buyang Huanwu decoction (BYHW) on this condition, using network pharmacology. Through this analysis, we identified key drug targets and mechanisms, revealing that protective effects of BYHW on coronary microvascular injury are primarily associated with the mitigation of inflammatory injury, endoplasmic reticulum stress, protein homeostasis disruption, transmembrane transport dysfunction, and impaired energy metabolism (Figure 1A-G). To further validate the mechanisms by which BYHW ameliorates coronary microvascular injury post-IR, we established a mouse model of coronary microvascular injury induced by IR and administered BYHW without a dose gradient for intervention. Transmission electron microscopy results demonstrated classic indicators of microvascular damage in the modeled mice, such as structural compromise, stenosis, and disrupted blood flow (Figure 2E). Importantly, BYHW did not affect the structural integrity or function of coronary microvasculature in control group mice. However, high-dose BYHW markedly improved microvascular damage, preserving the structural integrity of the microvessels (Figure 2E), whereas low-dose BYHW failed to provide substantial improvement in microvascular structural damage (Figure 2E).
Further biochemical assays measuring LDH, Troponin T (Trop T), BNP, and CK-MB indicated significant elevations of these markers following IR modeling, reflective of severe myocardial and microvascular damage (Figure 2A-D). High-dose BYHW significantly reversed these effects, reducing the expression levels of LDH, Trop T, BNP, and CK-MB, thereby mitigating microvascular damage (Figure 2A-D). In contrast, low-dose BYHW did not effectively reverse these elevations (Figure 2A-D). Additionally, post-IR, a marked reduction in the anti-inflammatory factor IL-10 was observed in microvascular tissues, accompanied by significant increases in pro-inflammatory factors IL-17, IL-18, and MMP-9, indicative of an inflammatory storm (Figure 2F-J). High-dose BYHW was able to counteract this inflammatory surge, restoring IL-10 levels and suppressing the upregulation of IL-17, IL-18, and MMP-9, thereby alleviating the microvascular inflammatory response post-IR (Figure 2F-J). Conversely, low-dose BYHW was ineffective in ameliorating the inflammatory damage (Figure 2F-J). Mechanistic studies revealed a significant upregulation of MAPK44/P38 and VDAC1 expression following IR, suggesting that MAPK44/P38-mediated overexpression of VDAC1 may contribute to energy metabolism dysfunction and disruptions in membrane permeability transition pores, further exacerbating microvascular inflammatory storms and structural damage (Figure 2K-L). Notably, high-dose BYHW effectively inhibited the expression of MAPK44/P38 and VDAC1 in microvessels post-IR, whereas low-dose BYHW did not reverse the overexpression of MAPK44/P38 (Figure 2K-L). These findings suggest that the MAPK44/P38 axis is a critical regulatory mechanism in the therapeutic effects of BYHW on microvascular injury post-IR.
Network pharmacology analysis of the mechanism of action of Buyang Huanwu decoction in treating coronary microvascular injury after ischemia-reperfusion injury. (A) Wayne diagram analysis of the intersection targets between BYHW and coronary microvascular injury; (B-C) PPI network analysis of the targets and signaling pathways of BYHW in treating coronary microvascular injury; (D-G) KEGG and GO enrichment analysis of differentially expressed genes and the mechanism of action of BYHW.
Buyang Huanwu decoction improves coronary microvascular injury after IR by regulating Caspase mediated cell apoptosis through MAPK44. (A) LDH expression level; (B) Troponon T expression level; (C) BNP expression level; (D) CK-MB expression level; (E) Representative TEM images of heart tissues from control and SCM mice. White arrows indicate disorganized myofibrils and tortuous Z discs while yellow arrows indicate swollen mitochondria. Scale bar, 2 μm (F) IL-10 expression level; (G) IL-17 expression level; (H) IL-18 expression level; (I) MMP-9 expression level; (J) TNF - α expression level; (K) MAPKK4 expression level; (L) P38 expression level; (M) VDAC1 expression level; Data are shown as mean ± SEM (n=10 mice per group or ten independent cell isolations per group). *p<0.05.
Buyang Huanwu decoction improves coronary microvascular injury after IR by regulating Caspase mediated cell apoptosis through MAPK44
Our previous studies have established that the Caspase-mediated mitochondrial pathway is a critical pathological regulator of apoptosis and necroptosis in coronary microvascular endothelial cells during coronary microvascular injury [50-52]. This study further corroborates the mechanism underlying apoptotic damage, demonstrating a significant increase in Caspase-9 expression within microvascular tissue following ischemia-reperfusion (IR) modeling. Notably, high-dose Buyang Huanwu decoction (BYHW) treatment effectively reversed the elevated fluorescence expression of Caspase-9 (Figure 3A). Interestingly, the introduction of MAPKK4 transgenic treatment attenuated the regulatory effect of BYHW on Caspase-9 expression levels. In contrast, cardiac-specific knockout of MAPKK4 did not diminish the therapeutic efficacy of BYHW (Figure 3A).
Buyang Huanwu decoction improves coronary microvascular injury after IR by regulating energy metabolism and cell apoptosis through MAPK44-P38. (A) Organize immunofluorescence detection of Caspase-9 expression levels; (B) Caspase-3 transcription level; (C) Caspase-9 transcription level; (D) Caspase-12 transcription level; (E) Bax expression level; (F) Bcl-2 expression level; (G) SOD expression level; (H) GPX expression level; (I) MDA expression level; Data are shown as mean ± SEM (n= ten independent cell isolations per group). *p<0.05.
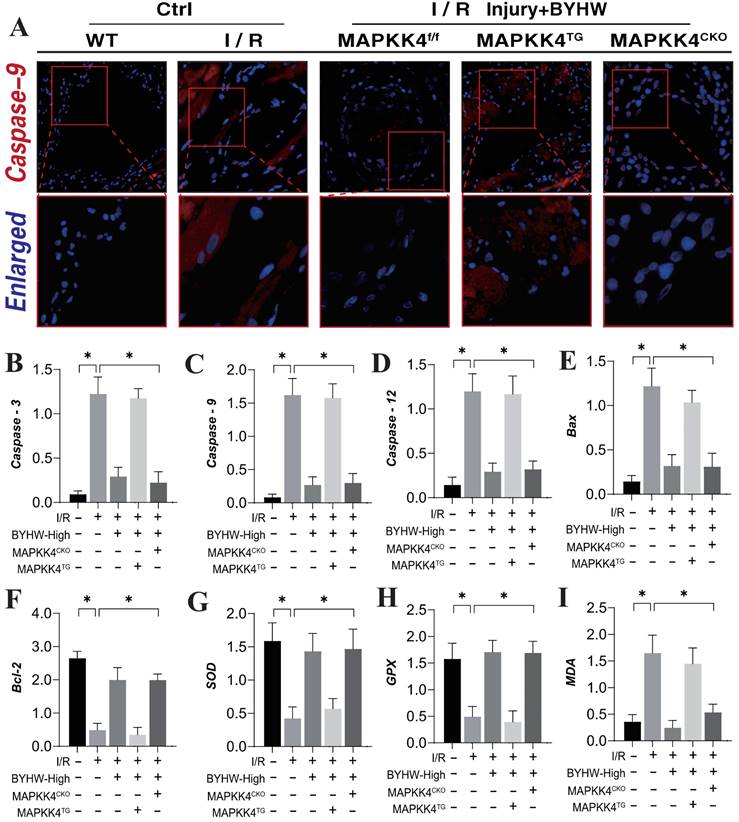
Further analysis of serum levels of Caspase-3, Caspase-9, and Caspase-12 supported these findings, showing consistency with the results observed in microvascular tissue fluorescence detection (Figure 3B-D). Moreover, the assessment of Bax/Bcl-2 ratios and oxidative stress-related markers indicated that IR modeling led to an increase in Bax and the oxidative stress marker MDA, alongside a reduction in Bcl-2 and antioxidant enzymes SOD and GPX (Figure 3E-I). BYHW effectively reversed these changes, inhibiting the activation of apoptotic pathways and reducing oxidative stress damage (Figure 3E-I). These results suggest that BYHW ameliorates microvascular damage post-IR by modulating apoptosis through the mitochondrial pathway and mitigating oxidative stress. It is noteworthy that the therapeutic benefits of BYHW were negated by MAPKK4 gene knockout, while MAPKK4 transgenic treatment did not reverse the protective effects of BYHW.
Buyang Huanwu decoction improves coronary microvascular injury after IR by regulating energy metabolism and cell apoptosis through MAPK44-P38
To further elucidate the mechanism of action of Buyang Huanwu decoction (BYHW), we conducted molecular docking experiments to simulate the interaction between MAPKK4 and Astragaloside IV, one of the active ingredients in BYHW. The results demonstrated a high affinity between MAPKK4 and Astragaloside IV, reinforcing the hypothesis that MAPKK4 is a critical target for the microvascular therapeutic effects of BYHW (Figure 4A). To verify the therapeutic effects of BYHW at a cellular level, we utilized gene modification technology to create models of MAPK44 and P38 knockdown/overexpression in microvascular endothelial cells, followed by hypoxia/reoxygenation experiments to simulate ischemia/reperfusion (IR) injury. The experimental results indicated a marked reduction in coronary microvascular endothelial cell activity post-modeling, which was accompanied by a decrease in the expression levels of antioxidant enzymes (SOD, MDA, GPX) and a suppression of mitochondrial respiratory chain complexes (Complex I/II/III) (Figure 4B-H). BYHW treatment effectively reversed these effects, enhancing the expression of SOD, MDA, and GPX, and upregulating the expression of mitochondrial respiratory chain complexes (Complex I/II/III) (Figure 4B-H).
Further results indicated that BYHW also inhibited the expression levels of apoptosis-related proteins Caspase-3, Caspase-9, Caspase-12, and Bax, while increasing the expression of the anti-apoptotic protein Bcl-2 (Figure 4I-M). Notably, transgenic treatments with MAPKK4 and P38 counteracted these beneficial effects of BYHW, whereas overexpression of MAPKK4/P38 did not diminish the protective effects of BYHW (Figure 4B-M). These experimental results confirm that BYHW enhances mitochondrial energy metabolism, mitigates oxidative stress, and protects against microvascular endothelial cell apoptosis via the MAPKK4/P38 pathway. This, in turn, boosts endothelial cell activity and preserves endothelial function. However, the precise regulatory mechanisms and crosstalk between BYHW and the MAPKK4-P38 signaling axis require further investigation.
Buyang Huanwu decoction improves coronary microvascular injury by inhibiting cell apoptosis through MAPPK4-P38 interaction
We further investigated the interaction between MAPKK4 and the active ingredient ligustrazine in Buyang Huanwu decoction (BYHW) using molecular docking experiments. The results demonstrated a strong correlation between MAPKK4 and ligustrazine, suggesting a potential mechanism through which BYHW mitigates microvascular injury via MAPKK4 (Figure 5A). Additional experimental findings indicate that BYHW enhances mitochondrial energy metabolism, inhibits apoptosis via the mitochondrial pathway, and promotes mitochondrial biosynthesis (Figure 5B-M). However, treatments with MAPKK4 transgenic (MAPKK4TG) and MAPKK4TG/ad-p38 models blocked regulatory effects of BYHW on mitochondrial biosynthesis, mitochondrial pathway-induced apoptosis, and mitochondrial respiratory chain function (Figure 4B-M). In contrast, treatments with MAPKK4 conditional knockout (MAPKK4CKO) and MAPKK4CKO/s-p38 did not negate the protective effects of BYHW (Figure 4B-M).
These results further suggest that protective effects of BYHW on coronary microvascular endothelial cells may be mediated through the MAPKK4-P38 interaction pathway, consistent with our previous findings. Our earlier research also highlighted the crucial role of mitochondrial biosynthesis in the context of ischemia-reperfusion injury [53, 54]. Mitochondrial biosynthesis is essential for constructing and modifying cellular structures, regulating reaction conditions, and optimizing cellular functions [55]. The mitochondrial biosynthesis process is regulated by a variety of factors, including transcription factors, RNA stabilization elements, helicases, and proteases [56, 57]. These factors interact in a complex manner to control mitochondrial biosynthesis, differentiation, and functional development, thereby influencing the activation of mitochondrial pathways involved in programmed cell death. Mitochondrial biosynthesis is also sensitive to the cellular environment, signal transduction, and external stimuli [58]. Malfunctions in this process can lead to nutrient and energy deficiencies within cells, impairing their normal function. Our study confirms that BYHW modulates the programmed cell death network by addressing mitochondrial biosynthesis dysfunction, providing experimental evidence for the MAPKK4-mediated multi-pathway death program in coronary microvascular endothelial cells. However, the in vivo regulatory mechanism of BYHW on p38 has yet to be fully elucidated.
Buyang Huanwu decoction improves coronary microvascular injury by inhibiting cell apoptosis through MAPPK4-P38 interaction. (A) Molecular docking correlation prediction between MAPKK4 and Astragaloside IV; (B) CCK8 detects the activity of coronary microvascular endothelial cells; (C) SOD expression level; (D) MDA expression level; (E) GPX expression level; (F) Complex-I activity; (G) Complex II activity; (H) Complex III activity; (I) Caspase-3 transcription level; (J) Caspase-9 transcription level; (K) Caspase-12 transcription level; (L) Bax expression level; (M) Bcl-2 expression level; Data are shown as mean ± SEM (n= ten independent cell isolations per group). *p<0.05.
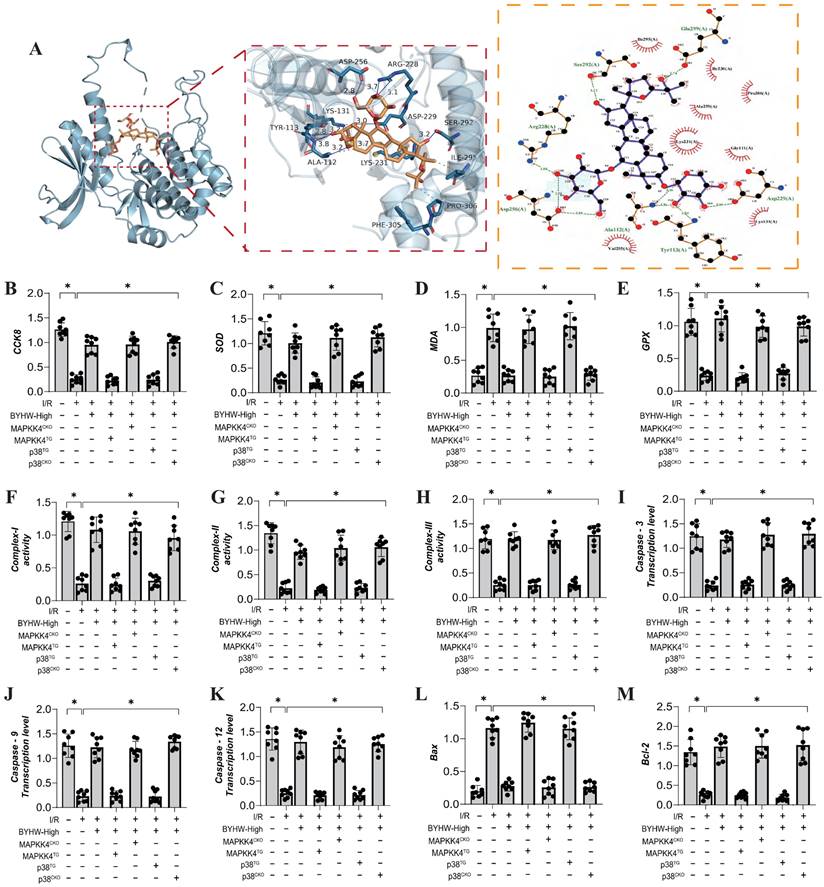
Buyang Huanwu decoction improves coronary microvascular injury by inhibiting cell apoptosis through MAPPK4-P38 interaction. (A) Molecular docking correlation prediction between MAPKK4 and ligustrazine; (B) CCK8 detects the activity of coronary microvascular endothelial cells; (C) Bax expression level; (D) Bcl-2 expression level; (E) Caspase-3 transcription level; (F) Caspase-9 transcription level; (G) Caspase-12 transcription level; (H) Complex-I activity; (I) Complex II activity; (J) Complex III activity; (K) PGC1- α transcription level; (L) Tfam transcription level; (M) Nrf-1 transcription level; Data are shown as mean ± SEM (n= ten independent cell isolations per group). *p<0.05.
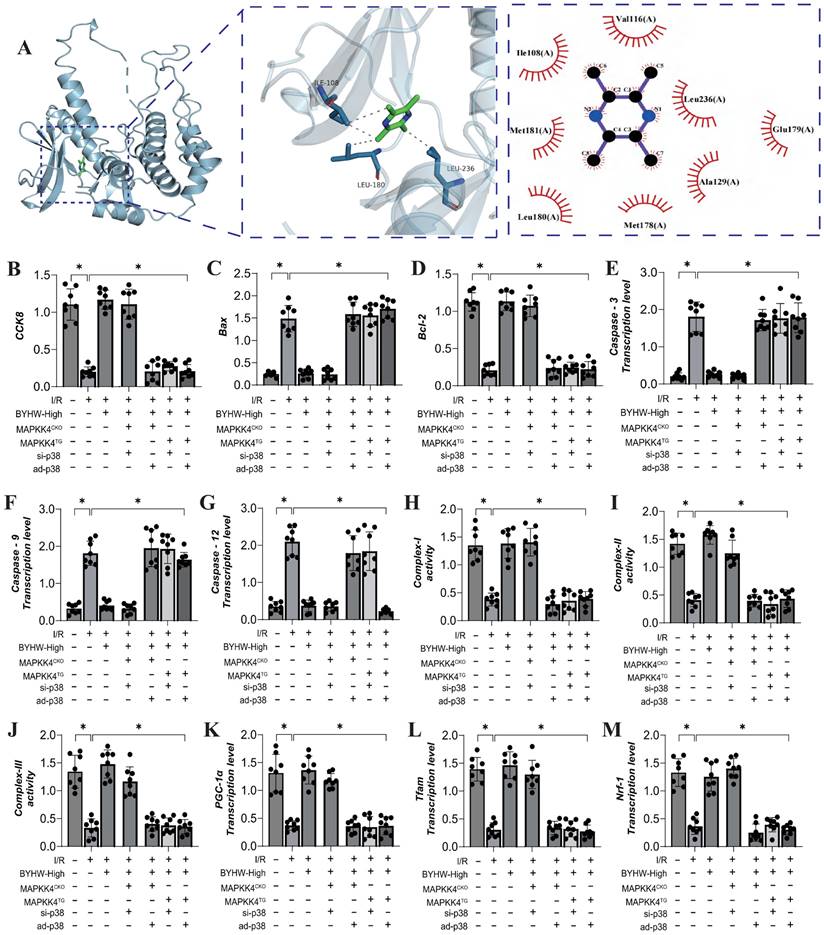
Buyang Huanwu decoction improves coronary microvascular injury after IR by inhibiting P38 mediated inflammatory storm
The p38 mitogen-activated protein kinase (MAPK) is activated by various extracellular stresses and cytokines, and substantial evidence indicates that p38 MAPK is a key mediator of inflammatory responses and endothelial injury [59]. To elucidate the role of the MAPKK4 downstream protein p38 in inflammation-driven coronary microvascular injury, we established a transgenic mouse model expressing p38 in microvascular endothelial cells following ischemia-reperfusion (IR) injury (P38TG). The experimental results demonstrated that p38 transgenic treatment negated the therapeutic effects of Buyang Huanwu decoction (BYHW) on microvascular injury. Furthermore, BYHW intervention significantly attenuated myocardial inflammation following IR, inhibited endothelial cell apoptosis, and enhanced mitochondrial biosynthesis (Figure 6A-P). However, the transgenic overexpression of p38 also abolished these beneficial effects of BYHW.
Buyang Huanwu decoction improves coronary microvascular injury after IR by inhibiting P38 mediated inflammatory storm. (A, K) Tissue immunofluorescence detection of Gr-1/Tnt expression levels; (B) LDH expression level; (C) Troponon T expression level; (D) BNP expression level; (E) CK-MB expression level; (F) Caspase-3 transcription level; (G) Caspase-9 transcription level; (H) Caspase-12 transcription level; (I) MMP-9 expression level; (J) TNF - α expression level; (L) Bax expression level; (M) Bcl-2 expression level; (N) PGC1- α transcription level; (O) Tfam transcription level; (P) Nrf-1 transcription level; Data are shown as mean ± SEM (n= ten independent cell isolations per group). *p<0.05.
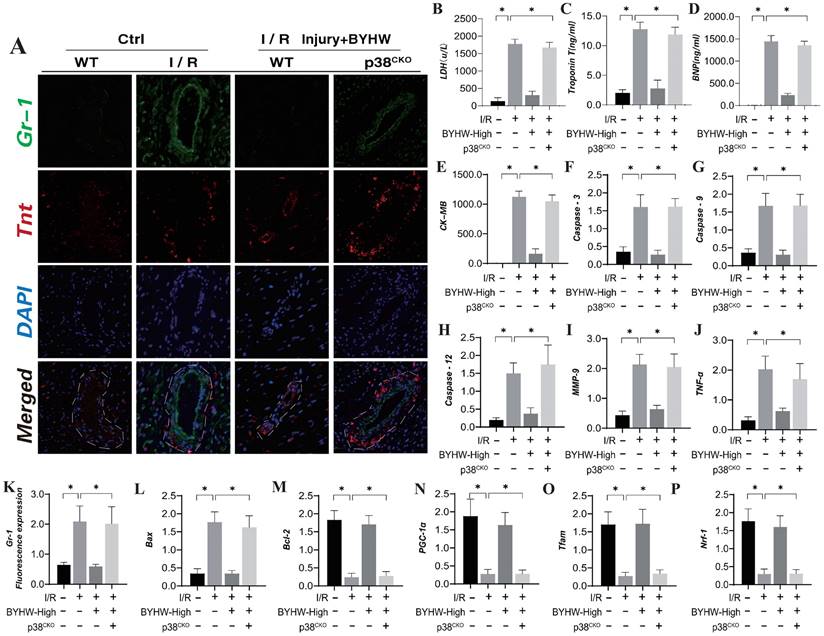
These findings suggest that p38 activation may impede the targeted therapeutic effects of BYHW on cell apoptosis and the inflammatory response. BYHW likely exerts its protective effects on endothelial cells by modulating apoptosis and inflammatory damage through the p38 pathway, thereby enhancing endothelial cell homeostasis and maintaining vascular endothelial stability. This conclusion is consistent with earlier animal studies involving MAPKK4, further supporting the notion that BYHW regulates cell apoptosis and improves coronary microvascular endothelial function via the MAPKK4-p38 axis.
Discussion
Coronary microvascular injury after myocardial infarction has always been a difficult problem of concern in the medical community [60, 61]. Under stress, acute occlusion of coronary arteries can further lead to pathological changes in endothelial cells caused by acute hypoxia/ischemia stimulation and intrinsic shear stress [62]. A series of biochemical and metabolic stress changes occur in endothelial cells and cardiomyocytes, such as a shift in metabolic mode from aerobic glycolysis to anaerobic glycolysis, which leads to disturbances in intracellular acid-base balance and calcium homeostasis [63]. In addition, oxidative stress mediated by ROS and increased production and secretion of inflammatory mediators mediated by MMP-9/IL-17 can further activate the Caspase mediated apoptotic pathway [64]. If the duration of ischemia/hypoxia is prolonged (more than 20 minutes), myocardial cell apoptosis begins from the endocardium and expands towards the epicardium. Although endothelial cells are more tolerant to long-term ischemia than cardiomyocytes, myocardial injury and excessive activation of apoptotic genes after ischemia-reperfusion injury also led to swelling and rupture of microvascular endothelial cells, disruption of cell structural integrity, and changes in microvascular permeability, further resulting in loss of microvascular dilation response, hemodynamic disorders, and contraction of peripheral cells in the heart [65, 66]. More importantly, microvascular injury in the ischemic area of the heart is mainly determined by the duration of myocardial ischemia, but the degree of myocardial ischemic injury may also be an important determining factor [67, 68]. Therefore, synchronous treatment targeting myocardial ischemic injury and microvascular dysfunction is a widely concerned scientific issue in the medical community. In this study, the experimental results suggest that BY can dose-dependently improve ischemia-reperfusion injury after IR, and further inhibit microvascular inflammation storm, oxidative stress damage, and microvascular endothelial cell apoptosis. The P38/MAPKK4 gene modification experiment confirmed that the mechanism of BY on post-IR coronary microvascular injury may be achieved through the MAPKK4 pathway. And it also suggests that MAPKK4 can regulate the activity of downstream P38, thereby affecting coronary microvascular injury and mitochondrial pathway cell apoptosis after IR. BY may regulate the activity of P38 through MAPKK4 to produce a protective effect against microvascular injury after IR. Animal and cell experiments have also confirmed that the transgenic (overexpression treatment) of P38/MAPKK4 blocks the protective effect of BY on microvascular inflammation/oxidative stress damage. In the early stage, we have confirmed through experimental research that the effective active ingredients in BY have an improving effect on myocardial injury [28, 37]. Quercetin alleviates myocardial ischemia-reperfusion injury caused by mitochondrial oxidative stress through DNA PKcs, regulates mitochondrial autophagy and mitochondrial dynamics, and maintains normal levels of mitochondrial energy metabolism [37]. And it was found that quercetin can regulate the stability of DNA PKcs through SIRT5, maintain the synergistic effect of "mitochondrial autophagy unfolded protein response," and inhibit the activation of mitochondrial pathway cell necrotic apoptosis [37]. The active ingredient quercetin in BY can regulate mitochondrial calcium homeostasis and mitochondrial quality control system abnormalities through the TMBIM6-VDAC1 interaction mechanism, which is helpful for the treatment of ischemic myocardial injury [28].
Coronary microvascular injury following myocardial infarction remains a significant challenge in the medical community [3, 69]. Under stress conditions, acute occlusion of coronary arteries can precipitate pathological changes in endothelial cells, triggered by acute hypoxia/ischemia and intrinsic shear stress [70, 71]. This leads to a cascade of biochemical and metabolic stress responses in endothelial cells and cardiomyocytes, including a metabolic shift from aerobic to anaerobic glycolysis, resulting in disturbances in intracellular acid-base balance and calcium homeostasis [72]. Additionally, oxidative stress mediated by reactive oxygen species (ROS) and the increased production and secretion of inflammatory mediators, such as MMP-9 and IL-17, can further activate the Caspase-mediated apoptotic pathway [73]. Prolonged ischemia/hypoxia (exceeding 20 minutes) initiates myocardial cell apoptosis starting from the endocardium and progressing towards the epicardium [74]. Although endothelial cells exhibit greater tolerance to prolonged ischemia compared to cardiomyocytes, myocardial injury and the excessive activation of apoptotic genes following ischemia-reperfusion (IR) injury can lead to swelling and rupture of microvascular endothelial cells [45, 75]. This results in the disruption of cell structural integrity, alterations in microvascular permeability, loss of microvascular dilation response, hemodynamic disorders, and contraction of surrounding cardiac cells. Importantly, the extent of microvascular injury in the ischemic heart region is primarily determined by the duration of myocardial ischemia, though the severity of myocardial ischemic injury is also a crucial factor. Consequently, the synchronous treatment of myocardial ischemic injury and microvascular dysfunction remains a critical area of focus in medical research.
In this study, our experimental results suggest that Buyang Huanwu decoction (BYHW) can dose-dependently improve IR-induced injury, while inhibiting microvascular inflammation, oxidative stress, and endothelial cell apoptosis. The P38/MAPKK4 gene modification experiments confirmed that the therapeutic effects of BYHW on post-IR coronary microvascular injury are likely mediated through the MAPKK4 pathway. This also indicates that MAPKK4 regulates downstream P38 activity, influencing coronary microvascular injury and apoptosis via the mitochondrial pathway following IR. BYHW may exert its protective effects against microvascular injury after IR by modulating P38 activity through MAPKK4. Both animal and cell experiments further confirmed that transgenic overexpression of P38/MAPKK4 negates the protective effects of BYHW on microvascular inflammation and oxidative stress. Previous studies have demonstrated that the active ingredients in BYHW significantly improve myocardial injury. Specifically, quercetin has been shown to alleviate myocardial ischemia-reperfusion injury by mitigating mitochondrial oxidative stress via DNA-PKcs, regulating mitochondrial autophagy and dynamics, and maintaining normal mitochondrial energy metabolism. Moreover, quercetin has been found to stabilize DNA-PKcs through SIRT5, ensuring the synergistic function of the "mitochondrial autophagy unfolded protein response," and inhibiting mitochondrial pathway-mediated necrotic apoptosis [37]. Additionally, quercetin in BYHW regulates mitochondrial calcium homeostasis and corrects mitochondrial quality control system abnormalities through the TMBIM6-VDAC1 interaction mechanism, which is beneficial for treating ischemic myocardial injury [28].
While myocardial reperfusion is essential for maximizing myocardial recovery, it also exacerbates damage to the myocardium and its microvascular system. Historically, the focus has predominantly been on myocardial injury, often neglecting microvascular injury [76]. This dichotomy, resulting from myocardial ischemia-reperfusion, is a complex process that intensifies microvascular injury initially caused by ischemia. Research indicates that reperfusion injury accounts for 50% of the total irreversible myocardial damage and is a significant factor contributing to severe microcirculatory dysfunction. Our preliminary studies revealed that myocardial reperfusion not only induces intracellular calcium overload but also triggers VDAC1-mediated opening of the mitochondrial permeability transition pore (mPTP) and the activation of inflammatory cascades [77]. However, the active ingredients in Buyang Huanwu decoction (BYHW) have been shown to effectively mitigate myocarditis injury via the TMBIM6-VDAC1 pathway, consistent with our findings. This suggests that BYHW is a potential therapeutic agent for treating microvascular dysfunction following ischemia-reperfusion injury, with its targeted regulatory effects closely tied to the apoptosis of coronary microvascular endothelial cells.
Cell apoptosis is intricately regulated by genes that either inhibit (e.g., CrmA, Mcl-1, Bcl-2, Bcl-xL, Bcl-w) or promote (e.g., p53, Myc, Fas, Bax, Bad, Bak) apoptosis, as well as by bidirectionally regulated genes (e.g., Bcl-x, C-Myc) [43, 44, 78]. Various inducing factors lead to different apoptotic pathways: internal pathways regulated by Bcl-2 family proteins, external pathways triggered by caspase activation mediated by tumor necrosis factor, and pathways mediated by endoplasmic reticulum stress-related factors [47, 79]. Under stress conditions such as abnormal sympathetic activity, disrupted cardiac electrical activity, myocardial injury, vascular endothelial injury, and energy metabolism dysfunction, the apoptosis of myocardial and vascular endothelial cells can be accelerated[80, 81]. Additionally, factors such as inflammation, high glucose, high-fat environments, endotoxins, free radicals, oxidatively modified low-density lipoprotein (oxLDL), and aberrant platelet activation can also induce apoptosis in vascular endothelial cells. Previous studies have demonstrated that quercetin, an active ingredient in BYHW, ameliorates myocardial cell apoptosis following ischemia-reperfusion injury via the DNA-PKcs-SIRT5 pathway. Quercetin further regulates calcium homeostasis and mPTP dysfunction through the TMBIM6-VDAC1 interaction, thereby mitigating necrotic apoptosis via the mitochondrial pathway. Additionally, quercetin improves myocardial injury and cell apoptosis post-myocardial infarction by modulating the "mitochondrial autophagy unfolded protein response" through the SIRT5-β-tubulin axis. This study further elucidates the anti-apoptotic mechanism of BYHW targeting coronary microvascular endothelial cells and confirms its regulatory effect on inflammation and oxidative stress via the MAPKK4-p38 interaction mechanism at the molecular level.
Mitogen-activated protein kinases (MAPKs) are serine/threonine protein kinases widely present in eukaryotic cells, responsible for transmitting various extracellular stimuli to intracellular signaling pathways and mediating diverse cellular responses [82]. The MAPK family of signaling pathways identified in current research primarily includes extracellular signal-regulated kinase 1/2 (ERK1/2), p38 mitogen-activated protein kinase (p38 MAPK), c-Jun N-terminal kinase (JNK), and extracellular signal-regulated kinase 5 (ERK5). Although each MAPK pathway has distinct characteristics, they share common mechanisms [83]. All MAPK pathways transmit extracellular signals to cells via a cascade of tertiary kinases, regulating various physiological and pathological processes, including growth, differentiation, and apoptosis of myocardial and endothelial cells [84]. Among these, the p38 pathway is the most extensively studied. It can be activated by various stimuli, has relatively independent functions, and exhibits broad interactions with other MAPK pathways, with either synergistic or inhibitory effects. Previous studies have shown that H2O2-induced oxidative stress significantly activates cAMP response element-binding protein (CREB), enhances transcription and expression of IP3R and VDAC, and activates mitochondrial-dependent death pathways. Melatonin can protect cardiac microvascular endothelial cells (CMECs) from oxidative stress by stimulating the MAPK/ERK pathway [85, 86]. This study confirms that MAPKK4 mediates energy metabolism dysfunction in endothelial cells and the activation of apoptosis-regulatory genes in the mitochondrial pathway after ischemia-reperfusion (IR) by regulating p38 activity. In vitro and in vivo experiments further confirm that Buyang Huanwu decoction (BYHW) ameliorates coronary microvascular injury through the MAPKK4-p38 axis. These findings provide new avenues for potential drug development and clinical translation in treating coronary microvascular injury.
BYHW is a well-established traditional formula for the clinical treatment of cardiovascular and cerebrovascular diseases, with significant therapeutic effects demonstrated over many years. Previous studies have shown that this formula can reduce inflammatory responses and exert therapeutic effects on chronic cerebral ischemic injury by targeting AKT1, ALB, TP53, IL-1β, and IL-6[14]. Further research indicates that BYHW can improve ischemic injury in the brain after IR by targeting Aprt, Pde1b, Gpd1 and HEXB, as well as pathways involved in glycerophospholipid metabolism [87]. Our research aligns with these previous findings, further confirming BYHW's mechanisms of action on microcirculatory disorders and identifying relevant therapeutic targets for this condition. Specifically, our study confirms the pathway through which BYHW improves microvascular injury after myocardial infarction by regulating p38 via MAPKK4, offering a preliminary basis and reference for MAPK-targeted drug therapy research.
Despite the comprehensive exploration and confirmation of BYHW's targeted therapeutic mechanisms, several issues remain unresolved in our in vitro and in vivo experiments. Firstly, we did not explore the impact of subcellular phenotypes, such as mitochondria or the endoplasmic reticulum, on the apoptosis of coronary microvascular endothelial cells. Future research will focus on confirming BYHW's targeted regulatory effects on organelles through in vitro and subcellular interaction experiments to elucidate its upstream regulatory influence on apoptosis. Secondly, we have not yet validated the effects of other endothelial cell programmed cell death pathways, such as necroptosis, pyroptosis, and ferroptosis, on the pathological mechanisms of post-IR coronary microvascular injury and BYHW's treatment mechanisms. Future experiments will investigate BYHW's targeted effects on various programmed cell death regulatory mechanisms in endothelial cells. Finally, we have not validated the interaction mechanisms between MAPKK4 and p38, nor the effects of BYHW on cell apoptosis, mitochondrial respiratory chain complexes, or mitochondrial biosynthesis, through immunoprecipitation and immunoblotting experiments. Future studies will aim to confirm these interactions through more advanced experimental methods.
Acknowledgements
This study is supported by the NSFC (NO. 82170241, 82270279).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Abe J, Vujic A, Prag HA, Murphy MP, Krieg T. Malonate given at reperfusion prevents post-myocardial infarction heart failure by decreasing ischemia/reperfusion injury. Basic research in cardiology. 2024;119:691-697
2. Chen X, Wang M, Yu K, Xu S, Qiu P, Lyu Z. et al. Chronic stress-induced immune dysregulation in breast cancer: Implications of psychosocial factors. J Transl Int Med. 2023;11:226-33
3. Vora KP, Kumar A, Krishnam MS, Prato FS, Raman SV, Dharmakumar R. Microvascular Obstruction and Intramyocardial Hemorrhage in Reperfused Myocardial Infarctions: Pathophysiology and Clinical Insights From Imaging. JACC Cardiovascular imaging. 2024;17:795-810
4. Jufar AH, May CN, Booth LC, Evans RG, Cochrane AD, Marino B. et al. Effects of dexmedetomidine on kidney and brain tissue microcirculation and histology in ovine cardiopulmonary bypass: a randomised controlled trial. Anaesthesia. 2023;78:1481-92
5. Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox biology. 2018;15:335-46
6. Zhou H, Wang J, Zhu P, Hu S, Ren J. Ripk3 regulates cardiac microvascular reperfusion injury: The role of IP3R-dependent calcium overload, XO-mediated oxidative stress and F-action/filopodia-based cellular migration. Cellular signalling. 2018;45:12-22
7. Zhu H, Jin Q, Li Y, Ma Q, Wang J, Li D. et al. Melatonin protected cardiac microvascular endothelial cells against oxidative stress injury via suppression of IP3R-[Ca(2+)]c/VDAC-[Ca(2+)]m axis by activation of MAPK/ERK signaling pathway. Cell Stress Chaperones. 2018;23:101-13
8. Zhou H, Hu S, Jin Q, Shi C, Zhang Y, Zhu P. et al. Mff-Dependent Mitochondrial Fission Contributes to the Pathogenesis of Cardiac Microvasculature Ischemia/Reperfusion Injury via Induction of mROS-Mediated Cardiolipin Oxidation and HK2/VDAC1 Disassociation-Involved mPTP Opening. J Am Heart Assoc. 2017;6:e005328
9. Diaz-Juarez J, Suarez J, Cividini F, Scott BT, Diemer T, Dai A. et al. Expression of the mitochondrial calcium uniporter in cardiac myocytes improves impaired mitochondrial calcium handling and metabolism in simulated hyperglycemia. American journal of physiology Cell physiology. 2016;311:C1005-c13
10. Zhou Z, Arroum T, Luo X, Kang R, Lee YJ, Tang D. et al. Diverse functions of cytochrome c in cell death and disease. Cell death and differentiation. 2024;31:387-404
11. Sakano Y, Sakano K, Hurrell BP, Shafiei-Jahani P, Kazemi MH, Li X. et al. SIRPα engagement regulates ILC2 effector function and alleviates airway hyperreactivity via modulating energy metabolism. Cell Mol Immunol. 2024
12. Puy C, Moellmer SA, Pang J, Vu H, Melrose A, Lorentz CU. et al. Coagulation factor XI regulates endothelial cell permeability and barrier function in vitro and in vivo. Blood. 2024
13. Zhang H, Zhang G, Xiao M, Cui S, Jin C, Yang J. et al. Two-polarized roles of transcription factor FOSB in lung cancer progression and prognosis: dependent on p53 status. J Exp Clin Cancer Res. 2024;43:237
14. Cao Y, Yao W, Yang T, Yang M, Liu Z, Luo H. et al. Elucidating the mechanisms of Buyang Huanwu Decoction in treating chronic cerebral ischemia: A combined approach using network pharmacology, molecular docking, and in vivo validation. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2024;132:155820
15. Chen B, Xu Y, Tian F, Liu Y, Yi J, Ouyang Y. et al. Buyang Huanwu decoction promotes angiogenesis after cerebral ischemia through modulating caveolin-1-mediated exosome MALAT1/YAP1/HIF-1α axis. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2024;129:155609
16. She Y, Shao L, Jiao K, Sun R, Lang T, Long H. et al. Glycosides of Buyang Huanwu decoction inhibits pyroptosis associated with cerebral ischemia-reperfusion through Nrf2-mediated antioxidant signaling pathway both in vivo and in vitro. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2023;120:155001
17. Li L, Zou J, Zhou M, Li H, Zhou T, Liu X. et al. Phenylsulfate-induced oxidative stress and mitochondrial dysfunction in podocytes are ameliorated by Astragaloside IV activation of the SIRT1/PGC1α /Nrf1 signaling pathway. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2024;177:117008
18. Liu X, Ding Y, Jiang C, Xin Y, Ma X, Xu M. et al. Astragaloside IV mediates radiation-induced neuronal damage through activation of BDNF-TrkB signaling. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2024;132:155803
19. Shan Y, Yu M, Dai H, Zhu X, Wang F, You Y. et al. The role of macrophage-derived Exosomes in reversing peritoneal fibrosis: Insights from Astragaloside IV. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2024;129:155683
20. Hao J, Zhang X, Hu R, Lu X, Wang H, Li Y. et al. Metabolomics combined with network pharmacology reveals a role for astragaloside IV in inhibiting enterovirus 71 replication via PI3K-AKT signaling. J Transl Med. 2024;22:555
21. Wen LP, Gao SW, Chen HX, Liu Q, Xiao GZ, Lin HC. et al. Astragaloside IV Ameliorates Colonic Adenomatous Polyps Development by Orchestrating Gut Bifidobacterium and Serum Metabolome. The American journal of Chinese medicine. 2024:1-28
22. Nie B, Chen X, Hou Z, Guo M, Li C, Sun W. et al. Haplotype-phased genome unveils the butylphthalide biosynthesis and homoploid hybrid origin of Ligusticum chuanxiong. Sci Adv. 2024;10:eadj6547
23. He J, Chen Y, Ding H, Zhou JA, Xing Z, Yang X. et al. Autocrine VEGF-B signaling maintains lipid synthesis and mitochondrial fitness to support T cell immune responses. The Journal of clinical investigation. 2024;134:e176586
24. Wang WG, Jiang XF, Zhang C, Zhan XP, Cheng JG, Tao LM. et al. Avermectin induced vascular damage in zebrafish larvae: association with mitochondria-mediated apoptosis and VEGF/Notch signaling pathway. J Hazard Mater. 2024;477:135376
25. Qu Y, Wang Z, Dong L, Zhang D, Shang F, Li A. et al. Natural small molecules synergize mesenchymal stem cells for injury repair in vital organs: a comprehensive review. Stem Cell Res Ther. 2024;15:243
26. Glover HL, Schreiner A, Dewson G, Tait SWG. Mitochondria and cell death. Nature cell biology. 2024;26:1434-1446
27. Ai Y, Meng Y, Yan B, Zhou Q, Wang X. The biochemical pathways of apoptotic, necroptotic, pyroptotic, and ferroptotic cell death. Molecular cell. 2024;84:170-9
28. Chang X, Zhou S, Liu J, Wang Y, Guan X, Wu Q. et al. Zishenhuoxue decoction-induced myocardial protection against ischemic injury through TMBIM6-VDAC1-mediated regulation of calcium homeostasis and mitochondrial quality surveillance. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2024;132:155331
29. Ma L, Chang X, Gao J, Zhang Y, Chen Y, Zhou H. et al. METTL3 boosts mitochondrial fission and induces cardiac fibrosis after ischemia/reperfusion injury. International journal of biological sciences. 2024;20:433-45
30. Jin Q, Li R, Hu N, Xin T, Zhu P, Hu S. et al. DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol. 2018;14:576-87
31. Xu Z, Mo X, Kong Y, Wen Q, Han T, Lyu M. et al. Mini-dose methotrexate combined with methylprednisolone as a first-line treatment for acute graft-versus-host disease: A phase 2 trial. J Transl Int Med. 2023;11:255-64
32. Zhou H, Li D, Zhu P, Ma Q, Toan S, Wang J. et al. Inhibitory effect of melatonin on necroptosis via repressing the Ripk3-PGAM5-CypD-mPTP pathway attenuates cardiac microvascular ischemia-reperfusion injury. Journal of pineal research. 2018;65:e12503
33. Deng Y, Wang H, Guo X, Jiang S, Cai J. Long-term blood pressure outcomes of laparoscopic adrenalectomy in trHTN patients. J Transl Int Med. 2023;11:275-81
34. Dou L, Lu E, Tian D, Li F, Deng L, Zhang Y. Adrenomedullin induces cisplatin chemoresistance in ovarian cancer through reprogramming of glucose metabolism. J Transl Int Med. 2023;11:169-77
35. Zhou B, Wang Z, Dou Q, Li W, Li Y, Yan Z. et al. Long-term outcomes of esophageal and gastric cancer patients with cardiovascular and metabolic diseases: A two-center propensity score-matched cohort study. J Transl Int Med. 2023;11:234-45
36. Zhou H, Shi C, Hu S, Zhu H, Ren J, Chen Y. BI1 is associated with microvascular protection in cardiac ischemia reperfusion injury via repressing Syk-Nox2-Drp1-mitochondrial fission pathways. Angiogenesis. 2018;21:599-615
37. Chang X, Zhang Q, Huang Y, Liu J, Wang Y, Guan X. et al. Quercetin inhibits necroptosis in cardiomyocytes after ischemia-reperfusion via DNA-PKcs-SIRT5-orchestrated mitochondrial quality control. Phytother Res. 2024;38:2496-517
38. Liu Y, Liu Y, Ye S, Feng H, Ma L. A new ferroptosis-related signature model including messenger RNAs and long non-coding RNAs predicts the prognosis of gastric cancer patients. J Transl Int Med. 2023;11:145-55
39. Wang X, Chen JD. Therapeutic potential and mechanisms of sacral nerve stimulation for gastrointestinal diseases. J Transl Int Med. 2023;11:115-27
40. Luan Y, Huang E, Huang J, Yang Z, Zhou Z, Liu Y. et al. Serum myoglobin modulates kidney injury via inducing ferroptosis after exertional heatstroke. J Transl Int Med. 2023;11:178-88
41. Jiang L, Chen T, Xiong L, Xu JH, Gong AY, Dai B. et al. Knockdown of m6A methyltransferase METTL3 in gastric cancer cells results in suppression of cell proliferation. Oncol Lett. 2020;20:2191-8
42. Zhang L, Jiang B, Zhu N, Tao M, Jun Y, Chen X. et al. Mitotic checkpoint kinase Mps1/TTK predicts prognosis of colon cancer patients and regulates tumor proliferation and differentiation via PKCα/ERK1/2 and PI3K/Akt pathway. Med Oncol. 2019;37:5
43. Yu W, Qin X, Zhang Y, Qiu P, Wang L, Zha W. et al. Curcumin suppresses doxorubicin-induced cardiomyocyte pyroptosis via a PI3K/Akt/mTOR-dependent manner. Cardiovasc Diagn Ther. 2020;10:752-69
44. Shao Y, Zhao T, Zhang W, He J, Lu F, Cai Y. et al. Presence of the apolipoprotein E-ε4 allele is associated with an increased risk of sepsis progression. Sci Rep. 2020;10:15735
45. Chen L, Tian Q, Shi Z, Qiu Y, Lu Q, Liu C. Melatonin Alleviates Cardiac Function in Sepsis-Caused Myocarditis via Maintenance of Mitochondrial Function. Front Nutr. 2021;8:754235
46. Zhao Z, Jiao Y, Yang S, Zhou A, Zhao G, Guo S. et al. Endoscopic diagnosis and treatment of superficial non-ampullary duodenal epithelial tumors: A review. J Transl Int Med. 2023;11:206-15
47. Gao WL, Li XH, Dun XP, Jing XK, Yang K, Li YK. Grape Seed Proanthocyanidin Extract Ameliorates Streptozotocin-induced Cognitive and Synaptic Plasticity Deficits by Inhibiting Oxidative Stress and Preserving AKT and ERK Activities. Curr Med Sci. 2020;40:434-43
48. E IR, Bakarozi M, Dimas I, Galanis K, Lygoura V, N KG. et al. Total and individual PBC-40 scores are reliable for the assessment of health-related quality of life in Greek patients with primary biliary cholangitis. J Transl Int Med. 2023;11:246-54
49. Chen L, Zhan CZ, Wang T, You H, Yao R. Curcumin Inhibits the Proliferation, Migration, Invasion, and Apoptosis of Diffuse Large B-Cell Lymphoma Cell Line by Regulating MiR-21/VHL Axis. Yonsei Med J. 2020;61:20-9
50. Pu X, Zhang Q, Liu J, Wang Y, Guan X, Wu Q. et al. Ginsenoside Rb1 ameliorates heart failure through DUSP-1-TMBIM-6-mediated mitochondrial quality control and gut flora interactions. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2024;132:155880
51. Chang X, Zhou S, Liu J, Wang Y, Guan X, Wu Q. et al. Zishen Tongyang Huoxue decoction (TYHX) alleviates sinoatrial node cell ischemia/reperfusion injury by directing mitochondrial quality control via the VDAC1-β-tubulin signaling axis. Journal of ethnopharmacology. 2024;320:117371
52. Chang X, Li Y, Liu J, Wang Y, Guan X, Wu Q. et al. ß-tubulin contributes to Tongyang Huoxue decoction-induced protection against hypoxia/reoxygenation-induced injury of sinoatrial node cells through SIRT1-mediated regulation of mitochondrial quality surveillance. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2023;108:154502
53. Chang X, Toan S, Li R, Zhou H. Therapeutic strategies in ischemic cardiomyopathy: Focus on mitochondrial quality surveillance. EBioMedicine. 2022;84:104260
54. Chang X, Li Y, Cai C, Wu F, He J, Zhang Y. et al. Mitochondrial quality control mechanisms as molecular targets in diabetic heart. Metabolism: clinical and experimental. 2022;137:155313
55. Chang X, Lochner A, Wang HH, Wang S, Zhu H, Ren J. et al. Coronary microvascular injury in myocardial infarction: perception and knowledge for mitochondrial quality control. Theranostics. 2021;11:6766-85
56. Wang Y, Jasper H, Toan S, Muid D, Chang X, Zhou H. Mitophagy coordinates the mitochondrial unfolded protein response to attenuate inflammation-mediated myocardial injury. Redox biology. 2021;45:102049
57. Chang X, Liu J, Wang Y, Guan X, Liu R. Mitochondrial disorder and treatment of ischemic cardiomyopathy: Potential and advantages of Chinese herbal medicine. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2023;159:114171
58. Pang B, Dong G, Pang T, Sun X, Liu X, Nie Y. et al. Emerging insights into the pathogenesis and therapeutic strategies for vascular endothelial injury-associated diseases: focus on mitochondrial dysfunction. Angiogenesis. 2024
59. Ke R, Kumar S, Singh SK, Rana A, Rana B. Molecular insights into the role of mixed lineage kinase 3 in cancer hallmarks. Biochimica et biophysica acta Reviews on cancer. 2024;1879:189157
60. Zhao J, Cheng W, Dai Y, Li Y, Feng Y, Tan Y. et al. Excessive accumulation of epicardial adipose tissue promotes microvascular obstruction formation after myocardial ischemia/reperfusion through modulating macrophages polarization. Cardiovasc Diabetol. 2024;23:236
61. Chen Y, Li S, Guan B, Yan X, Huang C, Du Y. et al. MAP4K4 exacerbates cardiac microvascular injury in diabetes by facilitating S-nitrosylation modification of Drp1. Cardiovasc Diabetol. 2024;23:164
62. Zhao D, Han C, Mammadova-Bach E, Watanabe-Kusunoki K, Bandeira Honda TS, Li Y. et al. Inhibition of complement factor C5a or C5aR for cholesterol crystal embolism-related vascular thrombosis with microvascular injury and its consequences. Kidney Int. 2024 S0085-2538(24)00543-X
63. Kravitz MS, Kattouf N, Stewart IJ, Ginde AA, Schmidt EP, Shapiro NI. Plasma for prevention and treatment of glycocalyx degradation in trauma and sepsis. Crit Care. 2024;28:254
64. Tiller C, Holzknecht M, Lechner I, Oberhollenzer F, von der Emde S, Kremser T. et al. Association of Circulating PCSK9 With Ischemia-Reperfusion Injury in Acute ST-Elevation Myocardial Infarction. Circ Cardiovasc Imaging. 2024;17:e016482
65. Suehiro JI, Kimura T, Fukutomi T, Naito H, Kanki Y, Wada Y. et al. Endothelial specific LAT1 ablation normalizes tumor vasculature. JCI insight. 2024: e171371.
66. Aksan B, Kenkel AK, Yan J, Sánchez Romero J, Missirlis D, Mauceri D. VEGFD signaling balances stability and activity-dependent structural plasticity of dendrites. Cellular and molecular life sciences: CMLS. 2024;81:354
67. Zhang W, Liu J, Li P, Wang X, Tang B. Reversible Fluorescent Probes for Dynamic Imaging of Liver Ischemia-Reperfusion Injury. Acc Chem Res. 2024
68. Liu L, Yao Y, Liu Y, Hong B, Li Z, Chen X. et al. Targeted H(2)S-Mediated Gas Therapy with pH-Sensitive Release Property for Myocardial Ischemia-Reperfusion Injury by Platelet Membrane. Biomater Res. 2024;28:0061
69. Nicolai L, Pekayvaz K, Massberg S. Platelets: Orchestrators of immunity in host defense and beyond. Immunity. 2024;57:957-72
70. Zhao BH, Ruze A, Zhao L, Li QL, Tang J, Xiefukaiti N. et al. The role and mechanisms of microvascular damage in the ischemic myocardium. Cellular and molecular life sciences: CMLS. 2023;80:341
71. McCallinhart PE, Chade AR, Bender SB, Trask AJ. Expanding landscape of coronary microvascular disease in co-morbid conditions: Metabolic disease and beyond. Journal of molecular and cellular cardiology. 2024;192:26-35
72. Wang T, Wang X, Fu T, Ma Y, Wang Q, Zhang S. et al. Roles of mitochondrial dynamics and mitophagy in diabetic myocardial microvascular injury. Cell Stress Chaperones. 2023;28:675-88
73. Zhi F, Zhang Q, Liu L, Chang X, Xu H. Novel insights into the role of mitochondria in diabetic cardiomyopathy: molecular mechanisms and potential treatments. Cell Stress Chaperones. 2023;28:641-55
74. Xiang Q, Geng ZX, Yi X, Wei X, Zhu XH, Jiang DS. PANoptosis: a novel target for cardiovascular diseases. Trends Pharmacol Sci. 2024;45:739-56
75. Yang QQ, Liu SJ, Huang W, Peng C, Han B. Exploring Protein Bioconjugation: A Redox-Based Strategy for Tryptophan Targeting. Research (Wash D C). 2024;7:0410
76. Zhou T, Fang YL, Tian TT, Wang GX. Pathological mechanism of immune disorders in diabetic kidney disease and intervention strategies. World J Diabetes. 2024;15:1111-21
77. Ding L, Lu S, Zhou Y, Lyu D, Ouyang C, Ma Z. et al. The 3' Untranslated Region Protects the Heart from Angiotensin II-Induced Cardiac Dysfunction via AGGF1 Expression. Mol Ther. 2020;28:1119-32
78. Wang A, Wan X, Zhu F, Liu H, Song X, Huang Y. et al. Habitual Daily Intake of Fried Foods Raises Transgenerational Inheritance Risk of Heart Failure Through NOTCH1-Triggered Apoptosis. Research (Wash D C). 2024;7:0401
79. Dong J, Ruan B, Zhang L, Wei A, Li C, Tang N. et al. DNA Methylation-Mediated GPX4 Transcriptional Repression and Osteoblast Ferroptosis Promote Titanium Particle-Induced Osteolysis. Research (Wash D C). 2024;7:0457
80. Peng Y, Wang Y, Zhou C, Mei W, Zeng C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front Oncol. 2022;12:819128
81. Wang D, Jiang J, Wang M, Li K, Liang H, Wang N. et al. Mitophagy Promotes Hair Regeneration by Activating Glutathione Metabolism. Research (Wash D C). 2024;7:0433
82. Singh VP, Jaiswal S, Wang Y, Feng S, Tripathi DK, Singh S. et al. Evolution of reactive oxygen species cellular targets for plant development. Trends in plant science. 2024;29:865-77
83. Yan Y, Dai T, Guo M, Zhao X, Chen C, Zhou Y. et al. A review of non-classical MAPK family member, MAPK4: A pivotal player in cancer development and therapeutic intervention. Int J Biol Macromol. 2024;271:132686
84. Malamos P, Papanikolaou C, Gavriatopoulou M, Dimopoulos MA, Terpos E, Souliotis VL. The Interplay between the DNA Damage Response (DDR) Network and the Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway in Multiple Myeloma. International journal of molecular sciences. 2024;25:6991
85. Li L, Zhang G, Yang Z, Kang X. Stress-Activated Protein Kinases in Intervertebral Disc Degeneration: Unraveling the Impact of JNK and p38 MAPK. Biomolecules. 2024;14:393
86. Wu M, Wang S, Ma P, Li B, Hu H, Wang Z. et al. Dual roles of the MPK3 and MPK6 mitogen-activated protein kinases in regulating Arabidopsis stomatal development. Plant Cell. 2024: koae225.
87. Zhou H, Lin B, Yang J, Wei X, Fu W, Ding Z. et al. Analysis of the mechanism of Buyang Huanwu Decoction against cerebral ischemia-reperfusion by multi-omics. Journal of ethnopharmacology. 2023;305:116112
Author contact
![]() Corresponding author: Hao Zhou, email: zhouhaoorg; Hang Zhu, email: zhuhang301com; Junyan Wang, email: junyan_wangcom.
Corresponding author: Hao Zhou, email: zhouhaoorg; Hang Zhu, email: zhuhang301com; Junyan Wang, email: junyan_wangcom.

 Global reach, higher impact
Global reach, higher impact