Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(6):1165-1175. doi:10.7150/ijms.94566 This issue Cite
Review
PI3K/AKT Signaling Pathway Mediated Autophagy in Oral Carcinoma - A Comprehensive Review
1. Department of Biological Sciences, College of Science, King Faisal University, Al-Ahsa, 31982, Saudi Arabia.
2. Centre of Molecular Medicine and Diagnostics (COMManD), Department of Biochemistry, Saveetha Dental College & Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai, 600 077, Tamil Nadu, India.
3. Department of Oral Pathology & Oral Microbiology, Meenakshi Ammal Dental College and Hospital, MAHER, Alapakkam Main Road, Maduravoyal, Chennai-600095, India.
4. Department of Oral and Maxillofacial Surgery, Meenakshi Ammal Dental College and Hospital, MAHER, Alapakkam Main Road, Maduravoyal, Chennai-600095, India.
5. Department of Botany and Microbiology, Faculty of Science, Cairo University, Cairo, 12613, Egypt.
6. Department of Zoology, Faculty of Science, Assiut University, Assiut, 71515, Egypt.
7. Botany and Microbiology Department, Faculty of Science, Assiut University, Assiut 71516, Egypt.
* Equal First Authorship.
Received 2024-1-22; Accepted 2024-4-13; Published 2024-4-29
Abstract
Oral cancer is the most heterogeneous cancer at clinical and histological levels. PI3K/AKT/mTOR pathway was identified as one of the most commonly modulated signals in oral cancer, which regulates major cellular and metabolic activity of the cell. Thus, various proteins of PI3K/AKT/mTOR pathway were used as therapeutic targets for oral cancer, to design more specific drugs with less off-target toxicity. This review sheds light on the regulation of PI3K/AKT/mTOR, and its role in controlling autophagy and associated apoptosis during the progression and metastasis of oral squamous type of malignancy (OSCC). In addition, we reviewed in detail the upstream activators and the downstream effectors of PI3K/AKT/mTOR signaling as potential therapeutic targets for oral cancer treatment.
Keywords: oral cancer, PI3K/AKT, autophagy, metastasis
1. Introduction
Head and neck cancer stands as the seventh most common type of cancer world-wide and oral cancer (OC) ranks seventeenth, globally. Every year about 0.66 million new cases and about 0.32 million deaths were recorded [1]. In terms of overall population, oral cancer ranks third in India, considering both genders combined. In India, approximately 130,000 new cases of oral cancer are recorded annually, with around 72,000 recorded deaths. India contributes about one-third of the total oral cancer patients, globally [2]. Malignancy involving the tongue, floor of the mouth and gingivo-buccal sulcus are the most commonly occurring sub sites of oral cancer. Squamous type of malignancy (OSCC) is the most common type of oral cancer [3]. Apart from OSCC the other type of malignancy encountered in oral cavity includes melanoma, lymphoma, sarcoma and salivary gland tumours [4]. There is a changing trend in incidence pattern of OSCC. Earlier individuals above 60 years were most commonly affected by the disease, but recently, younger individuals in the middle age group were also affected. Combined genetic and epigenetic factors thought to play a major role in this changing pattern of the incidence [5]. Various risk factors including tobacco, alcohol, diet, microbes, inflammation, poor oral hygiene, socioeconomic status and age showed to play an important role as a causative or triggering agent in OSCC. Genetic disorders such as Cowden syndrome and dyskeratosis congenita ought to increase the risk of OSCC [6]. Reactive oxygen species (ROS) such as singlet oxygen, hydroxyl radical, superoxide, peroxide ion, nitric oxide and hydrogen peroxide formed as a by-product in various mechanism and promotes carcinogenesis. In addition, ROS induces oxidative damage causing random mutations, that induces various epigenetic modulations [7]. Despite tremendous discoveries of various biomarkers for early detection of OSCC, the five-year survivability decreased. The increased death rate relates to diagnosis of the disease at the later stages of the disease [8]. PI3K/AKT pathway plays an important role in gene expression, protein synthesis, cell proliferation and survival. Numerous genetic and epigenetic factors were reported to regulate the PI3K/AKT pathway and influence cell proliferation and apoptosis [9]. The most common management protocol for OSCC includes surgical resection with or without radiotherapy and chemotherapy. The main chemotherapeutic drugs used in OSCC are cisplatin, 5-fluorouracil, Paclitaxel, Docetaxel, Hydroxyurea. Chemotherapeutic drug resistance occurs commonly due to disordered PI3K/AKT pathway as it mainly controls proliferation and apoptosis [10]. This review demonstrated PI3K/AKT as a potential target to induce autophagy in oral squamous cell carcinoma.
2. PI3K/AKT pathway
PI3K/AKT acts as a major regulating pathway to maintain the survival of cells in tumour environment with cellular stress [11]. This pathway is commonly activated by growth factors such as epidermal growth factor, fibroblastic growth factor and the receptor is of enzymatic type tyrosine kinase (RTK). RTKs are transmembrane proteins, with two different subunits, once the signalling molecules attach to RTK (receptor), it is phosphorylated and support the cross phosphorylation causing dimerization. Dimerization of these receptors activates the PI3Ks. PI3Ks belong to lipid kinase family that has the potency to phosphorylate 3'-OH group in inositol phospholipids [12]. The PI3Ks are classified into three different classes based on the protein domains and their regulatory sub-units. Class I group of PI3Ks has catalytic subunits p110 α, β, γ, δ and regulatory subunits p85, p55, p10, p84, p87, p50. Class II group with catalytic subunits PI3KC2 α, β, γ. Class III has Vps34 catalytic subunit and Vps15 regulatory subunit. PI3Ks are very much substrate specific and has distinct mechanism of action and tissue distribution [13], which converts the membrane embedded molecule PIP2 (Phosphatidylinositol-bisphosphate) to PIP3 (Phosphatidylinositol-triphosphate). PTEN (Phosphatase and Tensin homolog) inhibits the process of conversion of PIP2 to PIP3. PIP3 in-turn activates AKT, which helps the cells to proliferate, and divide by modulating GSK3β. Secondly, it also inhibits the apoptotic pathway by modulating MDM2, NF-кB, BAD and FKHR. AKT in turn recruits mTOR that modulates the transcription factors such as S6K1, 4EBP, which further induces protein synthesis, accelerates cell cycle and inhibits the apoptotic initiators [14]. AKT accelerates the function of mTOR by inhibiting Tsc1/2 and thereby upregulating Rheb with the help of GTP. mTOR activates the enzyme S6K and downregulates 4EBP1 to promote synthesis of proteins to accelerate DNA repair, upregulate Glut 1, LAT 1, HIF-1α and cyclin D1 and inhibit apoptosis and autophagy [15].
During carcinogenesis, PI3K-AKT pathway activity accelerates to support the nutritional as well as metabolic requirement. It also hyperactivates the cell division, angiogenesis, growth and proliferation and silences the apoptotic and autophagy upregulating proteins. This resulted in consistent tumour growth and decelerated cell death, and thereby, maintaining all the requisites of Hallmarks of cancer [16].
The catalytic subunit of class I PI3K has four isoforms named p110 α, β, δ, ϒ. Although all are functionally distinct and tissue specific, it was observed that mutations of p110 are related to many cancers especially p110α, resulting in abnormal kinase activity. Mutations in PIK3CA, the oncogene coding for p110α seems to be the most commonly mutated gene in various cancer types like liver, colorectal and breast. It is common in prostate cancer, that somatic mutation causing loss of function of the tumour suppressor gene coding PTEN (Phosphatase and tensin homolog) [12,13].
3. PI3K/AKT in various cancer
Lung cancer is one of the most common cancers that occurs among men with smoking habits. Mutations of RTKs and EGFR are commonly observed in NSCLC type of lung cancer. In about 67% of the individuals with EGFR mutation, the AKT/mTOR pathway was hyperactivated. Patients with squamous cell carcinoma of the lung were observed with increased mutation in PIK3CA, that codes for catalytic subunit, and another component PIK3R1 which codes for regulatory sub unit of PI3K pathway. Genes including AKT and PTEN were also reported to be mutated in lung cancer patients that modulate the PI3K/mTOR pathway [17].
Breast cancer is the most commonly diagnosed malignancy. Although the diagnosis and treatment protocols show tremendous development towards improving the survival rate, many patients die from drug resistance. The PI3K/AKT/mTOR pathway is strongly associated with drug resistance. Increased mutation of PIK3CA is evident in luminal A subtype, HER2- and ER-positive tumours. Although PTEN mutations were significantly associated with various types of breast cancer, mutations of PIK3CA, AKT and PTEN usually coexist [18]. Oesophageal cancers are commonly seen in Asia and rank sixth most common worldwide. Oesophageal squamous cell carcinoma (ESCC) is the most common type of malignancy reported in the oesophagus. PIK3CA seems to be the most common type of mutation in ESCC, with frequencies ranging from 4 to 25%. Single nucleotide polymorphism (SNPs) of AKT1 was reported to play a major role in ESCC incidence, especially among females and non-alcoholics. Other genes which were found to be commonly mutated are PTEN, FRAP1 and mTOR [19]. Cervical cancer and human papilloma virus (HPV) are related to tobacco and oral cancer. Oncoproteins E7, E6 of the HPV were reported to modulate PI3K/AKT/mTOR. Patients with PIK3CA mutation of cervical squamous cell carcinoma showed increased survival rate in comparison to those without PIK3CA mutation. Knockdown of DEPTOR and RAGE has been shown to inhibit PI3K/AKT pathway in cervical cancer by decelerating proliferation and inducing apoptosis [20].
Prostate cancer is one of the leading causes of cancer death among men. More than 50% of the castration-resistant prostate tumours had PTEN mutation or deletion. Progression of prostate cancer and multi-drug-resistant tumour was significantly associated with PI3kinase pathway hyperactivation, either due to PTEN modulation or due to hyperactivation of PIK3CA, AKT, mTOR or any other proteins of the pathway [21].
It is evident in gastric cancer that as the severity of the disease increases the frequency of PIK3CA mutation also increases. It was shown by experiment that knockdown of PIK3R3 effectively reduced the progression of tumour. pAKT expression was detected in 78% of gastric cancers. Apart from the above-mentioned proteins and genes, increased activity of GSK3β was significantly correlated to better prognosis [22].
4. PI3K/AKT in Head and Neck cancers
Head and Neck cancers (HNC) are one of the most common types of cancers encountered. HNC includes laryngeal cancer, pharyngeal cancer, oesophageal cancer, oral cancer, tongue cancer and other unidentified cancers of head and neck region. Oral squamous cell carcinoma (OSCC) is the most common type of oral cancer. Tobacco consumption habit stands the most common cause of OSCC. Alcohol acts as an adjuvant to tobacco habit. Apart from the tobacco by-products and many synthetic carcinogens such as 4-NQO, various single nucleotide polymorphisms (SNP) were also shown to activate PI3K/AKT pathway [23].
Targeted therapy to inhibit epidermal growth factor (EGFR), one of the ligands of RTKs, was thought to be a promising therapeutic option. However, due to increase in resistance to EGFR therapy, its usage is limited clinically. In OSCC often the PI3K/AKT/mTOR pathway is directly or indirectly activated, targeted therapies to modulate this pathway could cause a ground breaking change in the therapeutic aspect of OC [24]. Many proteins are involved in the PI3K/AKT pathway, PTEN plays a very important role in controlling the conversion of PIP2 to PIP3 thereby accelerating growth and division. AKT has four isoforms and each one of them plays a very distinct role in cell survival [25]. Roy et al., studied the specific role of isoforms in OC and they concluded that AKT1 and AKT2 are the frequently isoforms hyperexpressed in OC. Silencing of these isoforms resulted in cell cycle arrest at G2-M phase thereby reducing cell survivability by inhibiting survivin, cyclooxygenase-2 (COX-2), cyclin D1 and Bcl-2, the anti-apoptotic protein in OC [26].
Patel et al. 2005, profiled the activity of EGFR in OSCC cell line in comparison to normal cell using a modified western blot technique to identify the potential therapeutic targets in PI3K/AKT pathway [27]. Matsuo et al. 2018, investigated the role of various key proteins of PI3K/AKT pathway such as AKT, mTOR, GSK3β in cervical lymph node metastasis, using immunohistochemistry. Expression pattern of all the three proteins was higher in patients with metastasis in comparison to those individuals without metastasis and normal, and GSK3β was significantly associated as a predictor marker of poor prognosis [28]. Georgy et al. 2015, studied the potential role of GRHL3 in suppression of HNC. They reported that the GRHL3 silencing triggered the loss of PTEN causing abnormal proliferation resulting in aggressive form of squamous cell carcinoma. Their study concluded that the targeting of GRHL3/GSK3B/c-MYC would be rationally strategic [29]. Zhu et al. 2014, studied the role of LB1 in enhancing the treatment efficacy of chemotherapeutic drug cisplatin both in vivo and in vitro. They concluded that the efficacy of chemotherapy with cisplatin and radiotherapy can be effectively increased by LB1 by modulating MDM2, p53 and AKT [30]. Hu et al. 2013, studied the role of 3p-RNA in inducing apoptosis by activation of RIG-1. In this study, the activation of retinoic acid inducible gene like receptor using viral dsRNA showed to upregulate apoptotic pathway at high doses in comparison to low one [31].
5. PI3K/AKT and Autophagy
Autophagy is the process that occurs by the formation of autophagosome. It helps in degradation of proteins and organelles of the cell and the products are recycled to yield raw materials for further metabolism and cell survival [32]. Excessive autophagy can result in apoptosis. Autophagy is the process that regulate the turnover of proteins and organelles inside the cell. In case of extreme hypoxia, endoplasmic reticulum stress, oxidative or cellular stress, autophagic process induces necrosis or apoptosis resulting in autophagic death of the cell [33]. Autophagy and apoptosis communication aids in identification of dead cell antigen by immune cells as well physiological clearance of dying cells. Autophagy is activated during accumulation of unfavourable or misfolded proteins. Autophagy regulatory genes (Atg), which in-turn is regulated by ULK1/2 complex and class III PI3 kinase complex (Beclin1, vps34 and Ambra1) govern the process of autophagy [34]. Autophagy maintains cellular balance by constantly working on selective or bulk degradation of cellular and metabolic waste. Lack of autophagy may cause genotoxicity, while increased autophagy may result in constant remodelling causing increased energy supply and also facilitates escape from immune surveillance and targeted drug therapy [35].
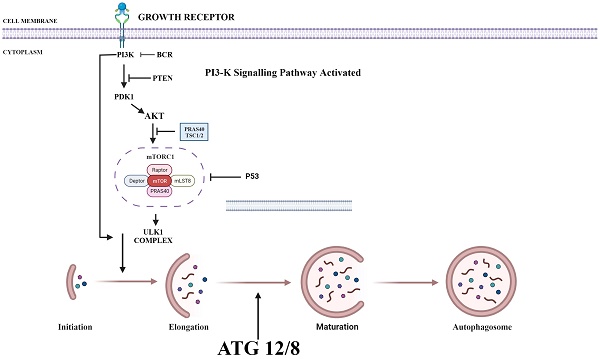
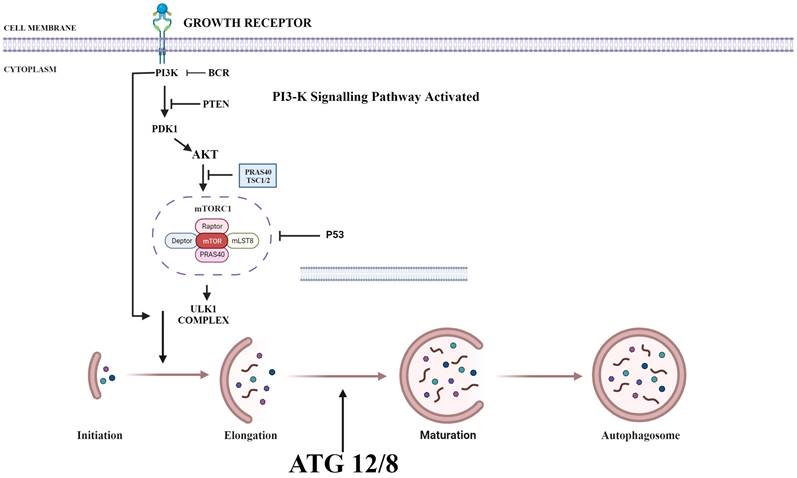
PI3K/AKT/mTOR plays a key role in regulating autophagy. mTOR plays an important inhibitory role in regulating autophagy. Autophagy process is initiated by the formation of phagophore commonly at the juncture of endoplasmic reticulum and mitochondria. Components such as Golgi, other cytoplasmic organelles and plasma membrane also contribute in formation of autophagosomes [36]. mTOR phosphorylates autophagy-related protein 13 to prevent the formation of autophagosome. It also promotes the adhesion of ribosome to endoplasmic reticulum which prevents the formation of autophagosome membrane. Thus, upon suppression of PI3K, both AKT and mTOR are blocked and activate autophagy process (Fig. 1) [37].
In the tumorigenic environment the somatic mutations of AKT enhances its activity causing inhibition of proapoptotic signals such as Bad and Bax. Downregulation of Bcl-2-associated death promoter-Bad in turn hyperactivates Bcl-xL-B cell lymphoma extra-large (anti-apoptotic) protein, which further shuts down the apoptosis [38]. Similarly, AKT inactivates both Caspases and FOXO-1 (forkhead box protein O1) which regulates the proapoptotic genes like FasL. Along with FOXO, the activity of glycogen synthase kinase 3β will be deregulated and leads to the activation of cyclin D1 as well cyclin-dependent kinase 4 and 6, thereby facilitating the cell entering replication phase. AKT also increases cytoplasmic localization of p27 which plays an important role in metastasis [39]. AKT also modulates mTOR along with the above said proteins. AKT inhibits TSC-2 tuberous sclerosis complex-2 by phosphorylation thereby preventing the activation of RHEB (Ras homolog enriched in brain), which eventually leads to activation of mTORC1 mammalian target of rapamycin complex 1 and eukaryotic translation initiation factor 4 complex (eIF4). Activation of mTORC1 and eIF4 accelerates cell cycle, tumour progression, angiogenesis and altered apoptosis [40].
The activation of mTORC1 results in complete activation of AKT by phosphorylation on C-terminal region at serine residues- Ser472, Ser473, Ser 474 of AKT3, AKT1 and AKT2, respectively. mTOR associated proteins including DEP- Dishevelled, Egl-10 and Pleckstrin, mLST- mammalian lethal with SEC13 protein 8 and DEPTOR-domain-containing mTOR-interacting protein signal the mTOR complexes. On the other hand, MAPKAP1-mitogen-activated protein kinase-associated protein 1, RICTOR-Rapamycin-Insensitive Companion on mTOR, PRAS40- proline rich AKT substrate of 40kDa and RAPTOR- regulatory-associated protein of mTOR interact with mTORC1 and mTORC2 to modulate the access to active sites of mTORC1 and mTORC2 [41]. mTORC1's key role in various cell processes is regulated by the activation of S6, which also modulates translation of 4E-BP1, the binding protein that regulates cell proliferation, protein synthesis, metabolism and apoptosis. The MAPKAP1 along with PI3K protein activates and regulates the function of mTORC2, which further enhances the above-mentioned cell processes [42]. Another important function of AKT/mTOR axis is that, it deregulates GSK-3β which helps in glycogen synthesis, modulates fatty acid synthesis by governing ATP citrate lyase and also regulates the proteins involved in glucose transport (PIP5K, AS160) and glycolysis (Hexokinase, 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 2 (PFKFB2), Fructose-2) to further enable the suitable environment for tumorigenesis [43].
6. Role of ROS in Autophagy
Reactive Oxygen Species (ROS) are a by-product of various cellular metabolic activity. The metabolic activity is higher and continuous in cancerous cells to constantly keep the pace of cellular replication, hence leading to increased production of ROS [44]. On the other hand, increased accumulation of ROS in normal or cancerous cell can cause detrimental effects. To combat those effects, the ROS removed from the cell by autophagic machineries. Autophagy in such circumstances not only remove the damaged components of the cell, but also provide the raw materials for mitophagy. Increased nutrients in a cell activates mitochondria resulting in increased ROS production that activates Beclin1 and ATG genes to induce macro-autophagy [45]. Beclin1 is bounded to BH3 domain of antiapoptotic molecule Bcl-2 in its inactive state. Increased accumulation of ROS dissociates Beclin1 from Bcl-2 and thus, initiating the process of autophagy. Increased regulation of Beclin1 also leads to apoptosis by binding to the proapoptotic factors in the mitochondrial membrane [46]. Apart from the endogenous ROS, those that are derived exogenously such as flavonoids, ionizing radiation can also promote autophagy/apoptosis by activating PTEN and inhibiting PI3K/AKT pathway. ROS also regulate signal that modulate MAPK/JNK, Ras, Raf, ERK, MEK, which ultimately activates autophagy. Therefore, the utility of these interactions in radiotherapy and chemotherapy resistant oral squamous cell carcinoma are reviewed in this article [47].
7. PI3K/Akt Autophagy in Oral Squamous Cell Carcinoma
The role of autophagy in tumour progression and metastasis has been frequently investigated in OSCC. Chen et al. 2023, studied the efficacy of nanoparticles incorporated hydroxychloroquine by inhibiting autophagy in oral cancer individuals. The study demonstrated that the effect of nanoparticles reducing the scavenging reactive oxygen species helps in success of targeted therapy [48]. Vo et al. 2021, studied the role of surfactin in inducing cell cycle arrest, apoptosis and autophagy in human OSCC cell line. They reported that surfactin could regulate autophagy and associated with apoptosis and also cause cell cycle arrest [49]. Qiu et al. 2023, investigated the role of (FBXW7) F-box and WD repeat domain containing 7 in OSCC cell line. They observed, that FBXW7 expression was lessened, which in-turn increased the autophagic activity by accelerating Atg7, Beclin1, BCL2, BAX and BAK. In xenograft tumour model, it was also observed that FBXW7 inhibits cell proliferation and promotes autophagy [50]. Borges et al. 2023, studied the role of 16 naphthoquinones, which inhibits PKM2 to induce autophagy via PI3K/Akt pathway and its associated apoptosis. It was concluded, that the compound 6a was the most potent drug used in the treatment of cancer and was well tolerated by the animals, extremely selective and had promising pharmacokinetic properties [51]. Wen et al. 2023, reported that Rho-associated protein kinase (ROCK) inhibitor Y-27632 modulated Akt/mTOR pathway to induce autophagy and further tumour progression. They concluded that Y-27632 could act as a potent therapeutic drug in OSCC [52].
Various Inhibitors that are used as therapeutic agent to target various proteins in PI3/AKT pathway in order to activate autophagy. PI3K: Phosphoinositide-3-kinase; BCR: B-cell antigen receptors; PTEN: Phosphatase and TENsin homolog; PDK1: 3-Phosphoinositide-dependent kinase 1; Akt: Akt serine/threonine kinase family; ULK1: Unc-51 Like Autophagy Activating Kinase 1; ATG: Autophagy Related Gene. (Indicates inhibition…….; Indicates Stimulation →). Created with Biorender in September 2023.
Zhang et al. 2023, reported that ITGA6 could act as an excellent prognostic biomarker in OSCC and plays a very important role in tumour progression by modulating mTOR associated autophagy and apoptosis [53]. Ma et al. 2023, developed ATPscore based on differentially expressed genes in relation to autophagy and identified its regulation pattern. Based on these, the authors identified the suitable genes for autophagy targeted therapy and immunotherapy to increase the survival rate in OSCC individuals [54]. Yang et al. 2023, investigated the autophagy and apoptotic activity of Antrodia salmonea (AS) in head and neck squamous cell carcinoma cell line. It was observed that AS was a potent inducer of reactive oxygen species mediated autophagy and associated apoptosis [55]. Abirami et al. 2018, evaluated the expression pattern of p-mTOR in various grades of OSCC in comparison to normal tissue to identify the dyspegulation in PI3K/Akt/mTOR pathway and identified p-mTOR as an effective prognostic marker [56]. Kapoor et al. 2014, studied the anti-cancer activity of Erufosine by inducing autophagy via modulation of mTOR. Apart from autophagy, the compound showed effective therapeutic effect on the entire PI3K/Akt/mTOR pathway causing cell cycle arrest by downregulation of cyclin D1 and also induced apoptosis [57]. Inui et al. 2013, studied the expression pattern of p62 and its association with the survival rate by modulating PI3K/Akt pathway [58]. Tang et al. 2013, studied the endogenous expression of LC3-II in 90 tumour specimens of oral cavity. In this context, high LC3-II expression correlated with poor disease survival [59]. Similarly, Sakakura et al. 2015, studied the LC3-II expression and correlated the role of immunology in prognosis of OSCC [60].
Wu et al. 2014, studied the role of ionizing radiation in inducing autophagy in OSCC cell lines. They also observed that autophagy induced apoptosis via PI3K/Akt was stimulated by ionising radiation [61]. Weng et al. 2014, also studied the role of Beclin1 in tongue squamous cell carcinoma. They concluded that knockdown of Beclin1 resulted in increased proliferation and invasion of tumour cells due to downregulation of autophagy associated proteins [62]. Ahn et al. 2011, studied the autophagic activity of Apicidin in OSCC cell lines. Apicidin was found to increase the expression of LC3-II, ATG5 and also to increase the accumulation of acidic vesicular organelles thereby induced autophagy [63] (Table 1).
8. Regulation of PI3/Akt through epigenetic modification
The PI3K/AKT signaling pathway not only plays a crucial role in regulating cell survival, proliferation, and apoptosis but also has significant involvement in autophagy, a cellular process that degrades and recycles cellular components. Autophagy is a critical cellular mechanism for maintaining cellular homeostasis, responding to nutrient starvation, and removing damaged organelles or pathogens.
The epigenetic regulation of the PI3K/AKT pathway impacts autophagy, influencing cellular health and disease states by DNA methylation which can regulate the expression of genes involved in the PI3K/AKT pathway and autophagy. The methylation status of the promoter regions of certain autophagy-related genes (ATGs) can influence their expression.
The role of different compounds, genes and proteins in regulating autophagy via PI3K/Akt/mTOR signalling.
| S.NO | COMPOUND/ PROTEIN/ GENE STUDIED | METHOD | FUNCTION | REF |
|---|---|---|---|---|
| 1 | Co-Fc coated nanoparticles | CAL-27 Cell line | Safe and effective delivery system | 48 |
| 2 | Surfactin | SCC4 & SCC25 Human OSCC Cell line | Effectively induce apoptosis via autophagy | 49 |
| 3 | FBXW7 | CAL27 Xenograft tumour model | Promotes autophagy | 50 |
| 4 | lawsone, arylaldehydes, and benzylamine | OSCC4, OSCC9, OSCC25 Cell line | Compound 6a was found to be the most potent autophagic agent | 51 |
| 5 | Y-27632 | Tca8113 and CAL-27 Cell line | Potent ROCK inhibitor | 52 |
| 6 | ARG | OSCC tissue | CCL2, CDKN2A, CTSB, CTSD, CXCR4, ITGA6, MAP1LC3A, MAPK3, PARP1, and RAB11A. ITGA6 identified as efficient biomarker | 53 |
| 7 | SRPX | FaDu & CAL-27 Cell line | Application of autophagy targeted therapy with immunotherapy in OSCC could be of more therapeutic value | 54 |
| 8 | Antrodia salmonea | FaDu Cell line | Antrodia salmonea is a potent anti-tumour agent. | 55 |
| 9 | p-mTOR | OSCC tissue | Potent prognostic marker | 56 |
| 10 | Erufosine | CAL-27, FaDu, SCC 9, SCC 25 Cell line | Potent inducer of autophagy and apoptosis. | 57 |
| 11 | p62/SQSTM1 | OSCC Tissue | Act as an early indicator of carcinogenesis and multi-drug resistance. | 58 |
| 12 | LC3 | OSCC Tissue | LC3 expression indicates poor survival rate. | 59 |
| 13 | LC3, p62/SQSTM1, Beclin-1 | OSCC Tissue | Expression of proteins LC3, p62/SQSTM1,Beclin-1 indicates poor prognosis. | 60 |
| 14 | LC3-II | OSCC OC3, SAS Cell line | Irradiation could increase the autophagic effect in tumour cells. | 61 |
| 15 | Beclin 1 | TSCC, SCC9, SCC15 Cell line | Potential target for tongue squamous cell carcinoma | 62 |
| 16 | Apicidin | YD-8, YD-10B Cell line | Potent inducer of autophagy and apoptosis | 63 |
Moreover, the methylation of genes encoding components of the PI3K/AKT pathway can alter its activity, indirectly affecting autophagy. For instance, hypermethylation and subsequent silencing of PTEN (a negative regulator of the PI3K/AKT pathway) can enhance AKT signalling, which typically inhibits autophagy by activating mTOR, a key negative regulator of autophagy. Histone modifications can also influence the expression of genes related to the PI3K/AKT pathway and autophagy. Histone acetylation and methylation can either promote or repress the transcription of specific genes, depending on the specific modification and the site on the histone tails. Acetylation of histones near ATG genes can promote their expression and thus enhance autophagy. Conversely, specific histone deacetylases (HDACs) can suppress autophagy by deacetylating histones near ATG genes, leading to chromatin condensation and gene silencing. Non-coding RNAs, including microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), can regulate the PI3K/AKT pathway and autophagy at the post-transcriptional level. Certain miRNAs can target mRNAs encoding components of the PI3K/AKT pathway or directly target mRNAs of autophagy-related genes, modulating their stability and translation. For instance, miRNAs that inhibit the expression of AKT can indirectly promote autophagy by reducing the activation of mTOR. Similarly, lncRNAs can interact with miRNAs, mRNAs, or proteins to modulate the expression of genes involved in the PI3K/AKT pathway and autophagy, acting as scaffolds, decoys, or guides. The interplay between the PI3K/AKT pathway and autophagy is complex and can be influenced by various epigenetic mechanisms. Dysregulation of these epigenetic processes can lead to altered autophagy, contributing to the pathogenesis of numerous diseases, including cancer, neurodegeneration, and metabolic disorders. Understanding the epigenetic regulation of the PI3K/AKT pathway and autophagy provides insights into potential therapeutic targets for modulating autophagy in disease treatment and prevention.
9. PI3K/Akt- a potential target
Oral cancer seems to have the most heterogenous characteristics, both clinically, histologically and have highly variable mutations at genetic level. Despite such heterogenicity, the PI3K/Akt/mTOR pathway was found to be the most commonly modulated [64]. This might be due to involvement of number of proteins that regulate major cellular and metabolic activity of the cell via this pathway. Activation of PI3K/Akt pathway seems to activate autophagy due to its effect on mTOR. PI3K/Akt/mTOR pathway has a very complex function and offers several therapeutic targets which mainly include mTOR and PI3K inhibitors. PI3K/Akt can be altered through various factors such as genomic alterations of PIK3CA or ligands such as EGFR, IGR, FGFR, VEGFR, TLR, BCR or PTEN - known as the upstream activators. The downstream effectors such as GSK3, mTOR, FOXO, MDM2, Akt or Beclin1 can alter the pathway. PI3K/Akt also modulates other pathways such as Ras, Raf, NF-кB, thence mutations in PI3K/Akt may further modulate other pathways positively enhancing the tumour environment [43]. PI3K/Akt also regulates metabolic process through glycolytic pathway, inflammation, immunity, and angiogenesis. PI3K/Akt (PIP3, β-catenin, E-cadherin) plays an important role in metastasis by regulating the crosstalk between epithelium and mesenchyme, causing Epithelial Mesenchymal Transition [65].
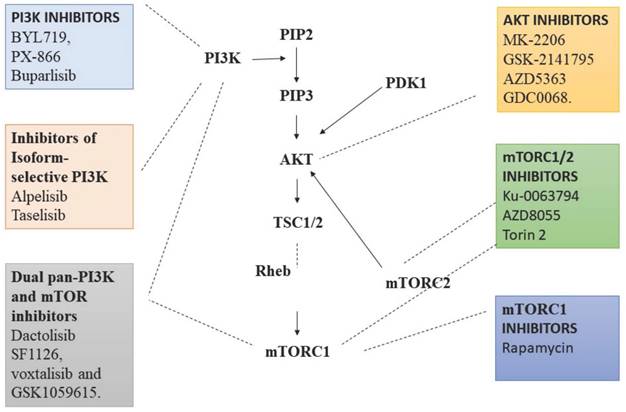
Various therapies target PI3K/Akt pathway, including the PI3K inhibitors such as Pan-PI3K inhibitors that targets p110 subunits of class IA PI3Ks. For examples, BYL719, PX-866 and buparlisib, which have better anti-tumour activity with less toxicity [66]. Inhibitors of isoform-selective PI3K, these therapeutic agents exhibit more specificity to tumour types with less off-target toxicity in comparison to pan-PI3K inhibitors. These includes alpelisib and taselisib [67]. Dual pan-PI3K and mTOR inhibitors exert improved effect, as they inhibit both mTOR and PI3K. The combined therapeutic effect helps in the inhibition of entire PI3K/Akt/mTOR pathway. For examples, dactolisib, SF1126, voxtalisib and GSK1059615. Besides their efficacy they are less specific and exert more toxic effects, but they inhibit p110 subunit completely and prevents the feedback mechanism by mTORC1 [68]. Similarly, Akt inhibitors are currently under trial, including compounds like MK-2206, GSK-2141795, AZD5363 and GDC0068. These act on all three subunits of Akt. mTORC1 and mTORC2 inhibitors are very promising and hotspot therapeutic targets under research [69]. Rapamycin are 1st generation mTOR inhibitors which bind to FKBP-12 and mTOR to form a complex, and inhibits the phosphorylation of mTORC1 S6K1 resulting in down streaming of mTORC1 activity. Although these drugs are very promising, varied doses of Rapamycin are required for individuals to visualise the expected outcome [70]. ATP-competitive mTOR inhibitors are a group of compounds that act by blocking the active sites of mTOR kinase thereby completely preventing the phosphorylation of Akt. AZD8055 is a recent ATP-competitive mTOR inhibitor, which has both tumour suppressing activity and induces autophagy. It seems to be more potent in rapamycin resistant tumours also [71]. In addition, many combination strategies were employed to deliver better outcome. PI3K/Akt/mTOR inhibitors along with GFR inhibitors, MAPK inhibitors and in combination with chemotherapy as well radiotherapy is being tried in drug resistance individuals (Fig. 2) [72].
Targets on PI3K/Akt that inhibit the function of various proteins in the pathway to decelerate cell proliferation, angiogenesis, epithelial-mesenchymal transition and promote autophagy-apoptosis axis. Created with Biorender in September 2023.
10. Promises and Challenges
Chemotherapy in OSCC causes increased systemic toxicity, which necessitates a more personalised and targeted treatment. Cetuximab, an anti-EGFR targeted drug is the only approved agent for treating squamous cell carcinoma of head and neck region [73]. It shows improved survival rate in comparison to standard chemotherapy regimen. Despite its promising results, EGFR resistance seems to be a concerning obstacle. A large number of studies has been conducted to improve the survival rate of OSCC individuals by modulating PI3K/Akt/mTOR associated autophagy [74]. Only few drugs such as cisplatin that induce autophagy are licensed for use in oral cancer despite the invention of many compounds due to many contraindications. Many natural compounds, such as areca nut extract [75], curcumin [76], Carfilzomib and ONX 0912 [77], G15 [78], Tetrandrine [79], Sulfasalazine [80] and other synthetic components [81-84] have been shown to modulate autophagy and associated apoptosis. These effects lead to decreases in tumour growth and increases in survival rate. However, no effective drug has been identified due to the drawback that increased activation of PI3K/Akt causes increased resistance to chemotherapies. On the aspect of immunotherapy, inhibitors of immune checkpoints PD-1, PD-L1 and CTLA-4 are the most successful therapeutic targets. These immune checkpoints are majorly governed by PI3K/Akt. Yet, many cancers such as lung, ovary, breast, prostate have acquired multi-drug resistance (MDR). MDR seems to be the major reason for tumour progression and treatment failure. Hyperactivation of PI3K/Akt could be the major reason for MDR causing poor survival rate. Thereby, targeted therapies must be used with caution without exploitation [85].
11. Conclusion
The field of theranostics is a hotspot field of research. Despite the huge effort made in this field, the prognosis of OSCC individuals is very poor and their survival rate seems to be very low in comparison to other malignancies. Tailored treatment modules with less toxicity and better outcomes are need. To meet these requirements, more targeted therapies should be identified with caution, as this could be a double-edged sword. Appropriate targets belonging to the PI3K/Akt/mTOR pathway are highly favourable to achieve better pharmacokinetic results.
Acknowledgements
Deanship of Scientific Research at King Faisal University, Saudi Arabia.
Funding
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (GRANT6,188).
Author contributions
Conceptualization, P.R. and R.S.; methodology, P.R, R.S. and BMA; software, S.B.; validation, S.B., E.M.A and S.A.A.; formal analysis, A.M.M.; investigation, P.R and R.S.; resources, P.S.D.; data curation, B.M.A.; writing—original draft preparation, R.S.; writing—review and editing, P.R and B.M.A.; visualization, S.B. and P.S.D; supervision, P.R.; project administration, P.R and R.S.; funding acquisition, P.R All authors have read and agreed to the published version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Kawakita D, Oze I, Iwasaki S, Matsuda T, Matsuo K, Ito H. Trends in the Incidence of Head and Neck Cancer by Subsite between 1993 and 2015 in Japan. Cancer Medicine. 2022Mar;11(6):1553-60
2. Borse V, Konwar AN, Buragohain P. Oral cancer diagnosis and perspectives in India. Sensors International. 2020Jan1;1:100046
3. Montero PH, Patel SG. Cancer of the oral cavity. Surgical Oncology Clinics. 2015Jul1;24(3):491-508
4. Fuoad SA, Mohammad DN, Hamied MA, Garib BT. Oro-facial malignancy in north of Iraq: a retrospective study of biopsied cases. BMC Oral Health. 2021Dec;21:10
5. Ferreira e Costa R, Leão ML, Sant'Ana MS, Mesquita RA, Gomez RS, Santos-Silva AR, Khurram SA, Tailor A, Schouwstra CM, Robinson L, van Heerden WF. Oral Squamous Cell Carcinoma Frequency in Young Patients from Referral Centers Around the World. Head and Neck Pathology. 2022Sep;16(3):755-62
6. Aghiorghiesei O, Zanoaga O, Nutu A, Braicu C, Campian RS, Lucaciu O, Berindan Neagoe I. The world of Oral cancer and its risk factors viewed from the aspect of MicroRNA expression patterns. Genes. 2022Mar26;13(4):594
7. Collin F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. International journal of molecular sciences. 2019May15;20(10):2407
8. González-Moles MÁ, Aguilar-Ruiz M, Ramos-García P. Challenges in the Early Diagnosis of Oral Cancer, Evidence Gaps and Strategies for Improvement: A Scoping Review of Systematic Reviews. Cancers. 2022Oct10;14(19):4967
9. Rascio F, Spadaccino F, Rocchetti MT, Castellano G, Stallone G, Netti GS, Ranieri E. The pathogenic role of PI3K/AKT pathway in cancer onset and drug resistance: An updated review. Cancers. 2021Aug5;13(16):3949
10. Ketabat F, Pundir M, Mohabatpour F, Lobanova L, Koutsopoulos S, Hadjiiski L, Chen X, Papagerakis P, Papagerakis S. Controlled drug delivery systems for oral cancer treatment—current status and future perspectives. Pharmaceutics. 2019Jun30;11(7):302
11. Hemmings BA, Restuccia DF. Pi3k-pkb/akt pathway. Cold Spring Harbor perspectives in biology. 2012Sep1;4(9):a011189
12. Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010Jun25;141(7):1117-34
13. Backer JM. The regulation of class IA PI 3-kinases by inter-subunit interactions. Phosphoinositide 3-kinase in Health and Disease: Volume 1. 2011:87-114
14. Faes S, Dormond O. PI3K and AKT: unfaithful partners in cancer. International journal of molecular sciences. 2015Sep;16(9):21138-52
15. Chrienova Z, Nepovimova E, Kuca K. The role of mTOR in age-related diseases. Journal of Enzyme Inhibition and Medicinal Chemistry. 2021Jan1;36(1):1678-92
16. Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nature Reviews Cancer. 2020Feb;20(2):74-88
17. Sanaei MJ, Razi S, Pourbagheri-Sigaroodi A, Bashash D. The PI3K/Akt/mTOR pathway in lung cancer; oncogenic alterations, therapeutic opportunities, challenges, and a glance at the application of nanoparticles. Translational Oncology. 2022Apr1;18:101364
18. Bhushan A, Gonsalves A, Menon JU. Current state of breast cancer diagnosis, treatment, and theranostics. Pharmaceutics. 2021May14;13(5):723
19. Luo Q, Du R, Liu W, Huang G, Dong Z, Li X. PI3K/Akt/mTOR signaling pathway: role in esophageal squamous cell carcinoma, regulatory mechanisms and opportunities for targeted therapy. Frontiers in oncology. 2022Mar22;12:852383
20. Zhang L, Wu J, Ling MT, Zhao L, Zhao KN. The role of the PI3K/Akt/mTOR signalling pathway in human cancers induced by infection with human papillomaviruses. Molecular cancer. 2015Dec;14(1):1-3
21. Cham J, Venkateswaran AR, Bhangoo M. Targeting the PI3K-AKT-mTOR pathway in castration resistant prostate cancer: a review article. Clinical Genitourinary Cancer. 2021Dec1;19(6):563-e1
22. Matsuoka T, Yashiro M. The role of PI3K/Akt/mTOR signaling in gastric carcinoma. Cancers. 2014Jul7;6(3):1441-63
23. Johnson DE, Burtness B, Leemans CR, Lui VW, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nature reviews Disease primers. 2020Nov26;6(1):92
24. Yamaoka T, Ohba M, Ohmori T. Molecular-targeted therapies for epidermal growth factor receptor and its resistance mechanisms. International journal of molecular sciences. 2017Nov15;18(11):2420
25. Carnero A, Paramio JM. The PTEN/PI3K/AKT pathway in vivo, cancer mouse models. Frontiers in oncology. 2014Sep23;4:252
26. Roy NK, Monisha J, Padmavathi G, Lalhruaitluanga H, Kumar NS, Singh AK, Bordoloi D, Baruah MN, Ahmed GN, Longkumar I, Arfuso F. Isoform-specific role of akt in oral squamous cell carcinoma. Biomolecules. 2019Jun27;9(7):253
27. Patel V, Ramesh A, Traicoff JL, Baibakov G, Emmert-Buck MR, Gutkind JS, Knezevic V. Profiling EGFR activity in head and neck squamous cell carcinoma by using a novel layered membrane Western blot technology. Oral oncology. 2005May1;41(5):503-8
28. Matsuo FS, Andrade MF, Loyola AM, da Silva SJ, Silva MJ, Cardoso SV, de Faria PR. Pathologic significance of AKT, mTOR, and GSK3β proteins in oral squamous cell carcinoma-affected patients. Virchows Archiv. 2018Jun;472:983-97
29. Georgy SR, Cangkrama M, Srivastava S, Partridge D, Auden A, Dworkin S, McLean CA, Jane SM, Darido C. Identification of a novel proto-oncogenic network in head and neck squamous cell carcinoma. JNCI: Journal of the National Cancer Institute. 2015 Sep 1;107(9)
30. Zhu D.W, Yuan Y.X, Qiao J.K, Yu C, Yang X, Wang L.Z, Zhang Z.Y, Zhong L.P. Enhanced anticancer activity of a protein phosphatase 2A inhibitor on chemotherapy and radiation in head and neck squamous cell carcinoma. Cancer Lett. 2015;356:773-780 doi: 10.1016/j.canlet.2014.10.024
31. Hu J, He Y, Yan M, Zhu C, Ye W, Zhu H, Chen W, Zhang C, Zhang Z. Dose dependent activation of retinoic acid-inducible gene-I promotes both proliferation and apoptosis signals in human head and neck squamous cell carcinoma. PLoS One. 2013Mar4;8(3):e58273
32. Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxidants & redox signaling. 2014Jan20;20(3):460-73
33. Marino G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nature reviews Molecular cell biology. 2014Feb;15(2):81-94
34. Cui B, Lin H, Yu J, Yu J, Hu Z. Autophagy and the immune response. Autophagy: Biology and Diseases: Basic Science. 2019:595-634
35. Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. The Journal of pathology. 2010May;221(1):3-12
36. Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. The Journal of clinical investigation. 2015Jan2;125(1):25-32
37. Alers S, Löffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Molecular and cellular biology. 2012Jan1;32(1):2-11
38. Henshall DC, Araki T, Schindler CK, Lan JQ, Tiekoter KL, Taki W, Simon RP. Activation of Bcl-2-associated death protein and counter-response of Akt within cell populations during seizure-induced neuronal death. Journal of Neuroscience. 2002Oct1;22(19):8458-65
39. Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2011Nov1;1813(11):1978-86
40. Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochemical Society Transactions. 2009Feb1;37(1):217-22
41. Moore SF, Hunter RW, Hers I. mTORC2 protein-mediated protein kinase B (Akt) serine 473 phosphorylation is not required for Akt1 activity in human platelets. Journal of Biological Chemistry. 2011Jul15;286(28):24553-60
42. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017Mar9;168(6):960-76
43. Harsha C, Banik K, Ang HL, Girisa S, Vikkurthi R, Parama D, Rana V, Shabnam B, Khatoon E, Kumar AP, Kunnumakkara AB. Targeting AKT/mTOR in oral cancer: mechanisms and advances in clinical trials. International journal of molecular sciences. 2020May6;21(9):3285
44. Lin CS, Wang YC, Huang JL, Hung CC, Chen JY. Autophagy and reactive oxygen species modulate cytotoxicity induced by suppression of ATM kinase activity in head and neck cancer cells. Oral Oncol. 2012;48:1152-8
45. Dong L, He J, Luo L, Wang K. Targeting the Interplay of Autophagy and ROS for Cancer Therapy: An Updated Overview on Phytochemicals. Pharmaceuticals. 2023Jan;16(1):92
46. Decuypere JP, Parys JB, Bultynck G. Regulation of the autophagic bcl-2/beclin 1 interaction. Cells. 2012Jul6;1(3):284-312
47. Gupta SC, Hevia D, Patchva S, Park B, Koh W, Aggarwal BB. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxidants & redox signaling. 2012Jun1;16(11):1295-322
48. Chen J, Zhu Z, Pan Q, Bai Y, Yu M, Zhou Y. Targeted Therapy of Oral Squamous Cell Carcinoma with Cancer Cell Membrane Coated Co-Fc Nanoparticles Via Autophagy Inhibition. Advanced Functional Materials. 2023Mar;16:2300235
49. Vo TT, Wee Y, Cheng HC, Wu CZ, Chen YL, Tuan VP, Liu JF, Lin WN, Lee IT. Surfactin induces autophagy, apoptosis, and cell cycle arrest in human oral squamous cell carcinoma. Oral Diseases. 2023Mar;29(2):528-41
50. Qiu B, Sun Y, Nie W, Yang Q, Guo X. FBXW7 promotes autophagy and inhibits proliferation of oral squamous cell carcinoma. Immunity, Inflammation and Disease. 2023May;11(5):e845
51. Borges AA, de Souza MP, da Fonseca AC, Wermelinger GF, Ribeiro RC, Amaral AA, de Carvalho CJ, Abreu LS, de Queiroz LN, de Almeida EC, Rabelo VW. Chemoselective Synthesis of Mannich Adducts from 1, 4-Naphthoquinones and Profile as Autophagic Inducers in Oral Squamous Cell Carcinoma. Molecules. 2023Jan;28(1):309
52. Wang ZM, Yang DS, Liu J, Liu HB, Ye M, Zhang YF. ROCK inhibitor Y-27632 inhibits the growth, migration, and invasion of Tca8113 and CAL-27 cells in tongue squamous cell carcinoma. Tumor Biology. 2016Mar;37:3757-64
53. Zhang C, Cai Q, Ke J. Poor Prognosis of Oral Squamous Cell Carcinoma Correlates With ITGA6. international dental journal. 2023Apr1;73(2):178-85
54. Ma B, Li H, Zheng M, Cao R, Yu R. A novel autophagy-related subtypes to distinguish immune phenotypes and predict immunotherapy response in head and neck squamous cell carcinoma. Biomolecules and Biomedicine. 2023 May 31
55. Yang HL, Lin YA, Pandey S, Liao JW, Way TD, Yeh YL, Chen SJ, Hseu YC. In vitro and in vivo anti-tumor activity of Antrodia salmonea against twist-overexpressing HNSCC cells: Induction of ROS-mediated autophagic and apoptotic cell death. Food and Chemical Toxicology. 2023Feb1;172:113564
56. Abirami M. Immunohistochemical evaluation of p-mTOR (Phosphorylated Mammalian Target of Rapamycin) in oral squamous cell carcinoma (Doctoral dissertation, Adhiparasakthi Dental College and Hospital, Melmaruvathur)
57. Kapoor V, Zaharieva MM, Berger MR. Erufosine Induces Autophagy and Apoptosis in Oral Squamous Cell Carcinoma: Role of the Akt-mTOR Signaling Pathway. InAutophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging. 2014 Jan 1 (pp. 229-245). Academic Press
58. Inui T, Chano T, Takikita-Suzuki M, Nishikawa M, Yamamoto G, Okabe H. Association of p62/SQSTM1 excess and oral carcinogenesis. PLoS ONE. 2013;8:e74398
59. Tang JY, Hsi E, Huang YC, Hsu NC, Chu PY, Chai CY. High LC3 expression correlates with poor survival in patients with oral squamous cell carcinoma. Hum Pathol. 2013;44:2558-62
60. Sakakura K, Takahashi H, Kaira K, Toyoda M, Oyama T, Chikamatsu K. Immunological significance of the accumulation of autophagy components in oral squamous cell carcinoma. Cancer Sci. 2015;106:1-8
61. Wu SY, Liu YW, Wang YK, Lin TH, Li YZ, Chen SH. et al. Ionizing radiation induces autophagy in human oral squamous cell carcinoma. J BUON: Official J Balkan Union Oncol. 2014;19:137-44
62. Weng J, Wang C, Wang Y, Tang H, Liang J, Liu X. et al. Beclin 1 inhibits proliferation, migration and invasion in tongue squamous cell carcinoma cell lines. Oral Oncol. 2014;50:983-90
63. Ahn MY, Ahn SG, Yoon JH. Apicidin, a histone deaceylase inhibitor, induces both apoptosis and autophagy in human oral squamous carcinoma cells. Oral oncology. 2011Nov1;47(11):1032-8
64. Tian T, Li X, Zhang J. mTOR signaling in cancer and mTOR inhibitors in solid tumor targeting therapy. International journal of molecular sciences. 2019Feb11;20(3):755
65. Fedorova O, Parfenyev S, Daks A, Shuvalov O, Barlev NA. The Role of PTEN in Epithelial-Mesenchymal Transition. Cancers. 2022Aug3;14(15):3786
66. Mishra R, Patel H, Alanazi S, Kilroy MK, Garrett JT. PI3K inhibitors in cancer: clinical implications and adverse effects. International Journal of Molecular Sciences. 2021Mar27;22(7):3464
67. Castel P, Toska E, Engelman JA, Scaltriti M. The present and future of PI3K inhibitors for cancer therapy. Nature cancer. 2021Jun;2(6):587-97
68. Wu X, Xu Y, Liang Q, Yang X, Huang J, Wang J, Zhang H, Shi J. Recent advances in dual PI3K/mTOR inhibitors for tumour treatment. Frontiers in Pharmacology. 2022 13
69. Brown JS, Banerji U. Maximising the potential of AKT inhibitors as anti-cancer treatments. Pharmacology & therapeutics. 2017Apr1;172:101-15
70. Polivka Jr J, Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacology & therapeutics. 2014May1;142(2):164-75
71. Schenone S, Brullo C, Musumeci F, Radi M, Botta M. ATP-competitive inhibitors of mTOR: an update. Current medicinal chemistry. 2011Jul1;18(20):2995-3014
72. Peng Y, Wang Y, Zhou C, Mei W, Zeng C. PI3K/Akt/mTOR pathway and its role in cancer therapeutics: are we making headway? Frontiers in oncology. 2022Mar24;12:819128
73. Cheng Y, Li S, Gao L, Zhi K, Ren W. The molecular basis and therapeutic aspects of cisplatin resistance in oral squamous cell carcinoma. Frontiers in oncology. 2021:4340.
74. Liu YP, Zheng CC, Huang YN, He ML, Xu WW, Li B. Molecular mechanisms of chemo-and radiotherapy resistance and the potential implications for cancer treatment. MedComm. 2021Sep;2(3):315-40
75. Yen CY, Chiang WF, Liu SY, Cheng PC, Lee SY, Hong WZ. et al. Long-term stimulation of areca nut components results in increased chemoresistance through elevated autophagic activity. J Oral Pathol Med: Official Publ Int Assoc Oral Pathol Am Acad Oral Pathol. 2014;43:91-6
76. Kim JY, Cho TJ, Woo BH, Choi KU, Lee CH, Ryu MH. et al. Curcumin-induced autophagy contributes to the decreased survival of oral cancer cells. Arch Oral Biol. 2012;57:1018-25
77. Zang Y, Thomas SM, Chan ET, Kirk CJ, Freilino ML, DeLancey HM. et al. Carfilzomib and ONX 0912 inhibit cell survival and tumor growth of head and neck cancer and their activities are enhanced by suppression of Mcl-1 or autophagy. Clin Cancer Res: Official J Am Assoc Cancer Res. 2012;18:5639-49
78. Bai LY, Weng JR, Hu JL, Wang D, Sargeant AM, Chiu CF. G15, a GPR30 antagonist, induces apoptosis and autophagy in human oral squamous carcinoma cells. Chem Biol Interact. 2013;206:375-84
79. Huang AC, Lien JC, Lin MW, Yang JS, Wu PP, Chang SJ. et al. Tetrandrine induces cell death in SAS human oral cancer cells through caspase activationdependent apoptosis and LC3-I and LC3-II activation-dependent autophagy. Int J Oncol. 2013;43:485-94
80. Han HY, Kim H, Jeong SH, Lim DS, Ryu MH. Sulfasalazine induces autophagic cell death in oral cancer cells via Akt and ERK pathways. Asian Pacific J Cancer Prevention: APJCP. 2014;15:6939-44
81. Ahn MY, Yoon HE, Kwon SM, Lee J, Min SK, Kim YC. et al. Synthesized Pheophorbide a-mediated photodynamic therapy induced apoptosis and autophagy in human oral squamous carcinoma cells. J Oral Pathol Med: Official Publ Int Assoc Oral Pathol Am Acad Oral Pathol. 2013;42:17-25
82. Hsieh MT, Chen HP, Lu CC, Chiang JH, Wu TS, Kuo DH. et al. The novel pterostilbene derivative ANK-199 induces autophagic cell death through regulating PI3 kinase class III/beclin 1/Atgrelated proteins in cisplatinresistant CAR human oral cancer cells. Int J Oncol. 2014;45:782-94
83. Takeuchi R, Hoshijima H, Nagasaka H, Chowdhury SA, Kikuchi H, Kanda Y. et al. Induction of non-apoptotic cell death by morphinone in human promyelocytic leukemia HL-60 cells. Anticancer Res. 2006;26:3343-8
84. Sekine T, Takahashi J, Nishishiro M, Arai A, Wakabayashi H, Kurihara T. et al. Tumor-specificity and type of cell death induced by trihaloacetylazulenes in human tumor cell lines. Anticancer Res. 2007;27:133-43
85. Housman G, Byler S, Heerboth S. et al. Drug resistance in cancer: an overview. Cancers (Basel). 2014;6(3):1769-1792
Author contact
![]() Corresponding authors: Dr. Peramaiyan Rajendran, Department of Biological Sciences, No.9. College of Science, King Faisal University, University Road, Al-Ahsa, 31982, Saudi Arabia. Email: prajendranedu.sa; drramya.oralpathologyedu.in.
Corresponding authors: Dr. Peramaiyan Rajendran, Department of Biological Sciences, No.9. College of Science, King Faisal University, University Road, Al-Ahsa, 31982, Saudi Arabia. Email: prajendranedu.sa; drramya.oralpathologyedu.in.

 Global reach, higher impact
Global reach, higher impact