3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2023; 20(9):1240-1246. doi:10.7150/ijms.86601 This issue Cite
Research Paper
Correlation between coronary heart disease severity and subsequent chronic rhinosinusitis severity: A retrospective cohort study
1. Division of Cardiology, Department of Internal Medicine, Changhua Christian Hospital, Yunlin Branch, Yunlin, Taiwan.
2. Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan.
3. Department of Post-Baccalaureate Medicine, College of Medicine, National Chung Hsing University, Taichung, Taiwan.
4. Department of Otolaryngology, Chung Shan Medical University Hospital, Taichung, Taiwan.
5. School of Medicine, Chung Shan Medical University, Taichung, Taiwan.
6. Department of Otolaryngology, St. Martin De Porres Hospital, Chiayi, Taiwan.
7. Department of Ophthalmology, Nobel Eye Institute, Taipei, Taiwan.
8. Department of Ophthalmology, Jen-Ai Hospital Dali Branch, Taichung, Taiwan.
9. Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan.
10. Department of Psychiatry, Chung Shan Medical University Hospital, Taichung, Taiwan.
Received 2023-5-29; Accepted 2023-7-25; Published 2023-8-6
Abstract
Coronary heart disease (CHD) is associated with the development of several diseases. This retrospective population-based cohort study investigated the association between CHD severity and subsequent chronic rhinosinusitis (CRS) of varying severity. We used data from Taiwan's National Health Insurance Research Database. CHD was categorized as severe if treated using a coronary artery bypass graft (CABG) and as mild if treated with percutaneous coronary intervention (PCI). The primary outcome of this study was the development of CRS or severe CRS treated using functional endoscopic sinus surgery. Cox proportional hazards regression was used to calculate adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) for CRS and severe CRS in different patient groups. We included 31,784 patients who received PCI surgery (the CHD-PCI group) and 15,892 patients who received CABG surgery (the CHD-CABG group). A total of 813 and 482 episodes of CRS occurred in the CHD-PCI and CHD-CABG groups, respectively, and 45 and 16 severe CRS events occurred in the CHD-PCI and CHD-CABG groups, respectively. Our multivariable analysis demonstrated that the incidence of CRS in the CHD-CABG group was significantly higher than that in the CHD-PCI group (aHR: 1.196, 95% CI: 1.064-1.280, P = 0.0402), but the two groups had similar incidence rates of severe CRS (aHR: 0.795, 95% CI: 0.456-1.388, P = 0.5534). Subgroup analyses revealed that the association between CHD severity and CRS development was more significant among men (P = 0.0016). In conclusion, we determined that severe CHD treated with CABG was associated with a higher incidence of subsequent CRS, and this association was more prominent among men.
Keywords: coronary heart disease, chronic rhinosinusitis, severity, male, epidemiology
Introduction
Coronary heart disease (CHD) is characterized by coronary artery stenosis and subsequent coronary blood flow reduction and myocardial ischemia [1]. The incidence of CHD is lower among women; nevertheless, the prognosis for women with CHD is poorer than that for men with CHD [2, 3]. CHD can be managed through medical treatment, including the administration of antiplatelet and antihypertensive drugs and anticoagulants [1]. Treating advanced CHD involving main coronary artery obstruction necessitates the use of percutaneous coronary intervention (PCI) with a coronary artery bypass graft (CABG) to restore the impaired coronary circulation [4-6].
In addition to affecting the myocardium, CHD contributes to the occurrence of comorbidities outside the heart [7, 8]. For example, CHD was reported to be significantly associated with hypertension [9] and was determined to be correlated with metabolic syndrome [10]. Moreover, patients with periodontitis were indicated to be at a significant risk of CHD episodes [11]. High CHD severity levels were also reported to be associated with a reduction in subfoveal choroidal thickness and an impairment of the choroidal vasculature [12].
Chronic rhinosinusitis (CRS) is a chronic inflammatory disorder of the nasal and paranasal mucosa that often involves the formation of nasal polyps [13]. CRS had been demonstrated to be associated with several inflammatory diseases, including asthma and dry eye disease [13, 14]. Moreover, Wang et al., reported that CRS patients were at higher risk for acute myocardial infarction occurrence [15]. Wu and co-workers highlighted that CHD is considered a comorbid medical disorder for sinusitis patients [16]. However, the association between CHD and CRS has yet to be investigated. Because patients with CHD and those with CRS present with inflammatory responses, an association may exist between both conditions.
To fill the aforementioned research gap, the present study investigated the possible association between CHD severity and subsequent CRS by using data from Taiwan's National Health Insurance Research Database (NHIRD). The study determined CHD severity and CRS severity levels on the basis of associated surgical procedures.
Materials and Methods
Data Source
This study adhered to the guidelines of the Declaration of Helsinki. The study was approved by the National Health Insurance Administration of Taiwan and the Institutional Review Board of Chung Shan Medical University Hospital (CS1-23044). The requirement for informed consent was waived by these administrative bodies. Taiwan's NHIRD contains claims data from Taiwan's National Health Insurance system. This database contains the medical records of 23 million Taiwanese patients. For this study, patient data for the period from January 1, 2014, to December 31, 2020, were included for analysis. The available patient data included International Classification of Diseases, Ninth Revision (ICD-9) and International Classification of Diseases, Tenth Revision (ICD-10) diagnostic codes, age, sex, place of residence, education level, laboratory exam codes, medical department visit records, imaging exam codes, surgical codes, procedure codes, and Anatomical Therapeutic Chemical (ATC) codes for medical prescriptions.
Patient Selection
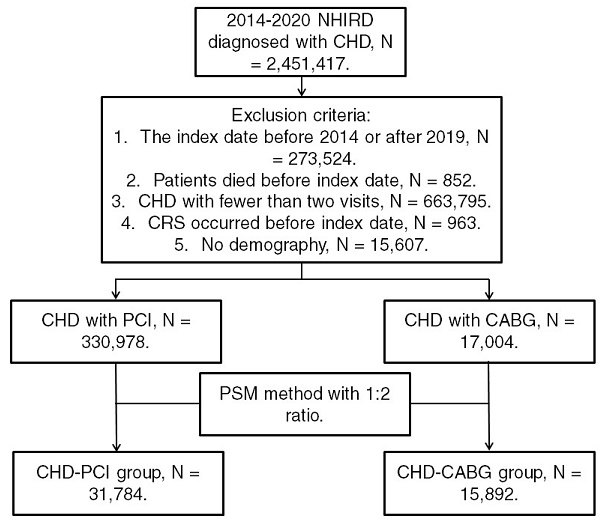
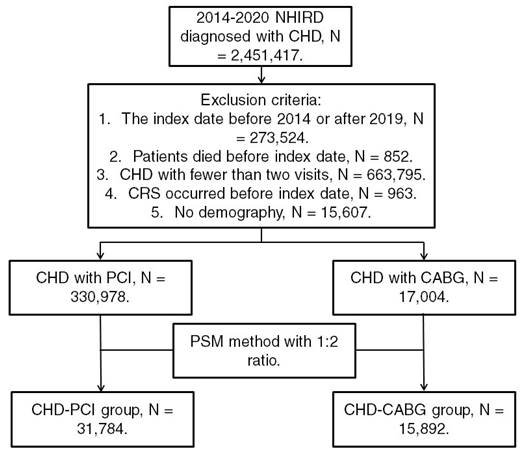
Patients from the NHIRD were considered to have CHD and included in the study if they met the following criteria: (1) receiving a CHD diagnosis based on ICD-9 or ICD-10 codes during 2014-2019; (2) undergoing complete blood cell count, cholesterol, triglyceride, high-density lipoprotein, low-density lipoprotein, white blood cell differentiation, cardiac angiography, and electrocardiography tests before CHD diagnosis; and (3) undergoing follow-up assessments in the internal medicine, family medicine, or cardiovascular department for >2 months. The index date was set as 6 months after CHD diagnosis. Patients were excluded if they met the following criteria: (1) having an index date before 2015 or after 2019, (2) having died before the index date, (3) undergoing fewer than two follow-up assessments for CHD in a medical department, (4) having had CRS before the index date, and (5) having no available demographic data. We categorized CHD severity on the basis of the type of surgery used for CHD treatment; specifically, CHD was categorized as severe if treated using CABG surgery and as mild if treated using PCI. Moreover, we matched each patient who received CABG surgery for CHD with two patients who received PCI surgery for CHD by using propensity score matching (PSM) adjusted for demographics, systemic diseases, and medical prescriptions. Accordingly, after the PSM process, we obtained a total of 31,784 patients who received PCI surgery (the CHD-PCI group) and 15,892 patients who received CABG surgery (the CHD-CABG group). Figure 1 illustrates the patient selection flowchart.
Primary Outcome
The primary outcome was the presence of CRS that met the following criteria: (1) CRS diagnosed using relevant ICD-9 or ICD-10 diagnostic codes, (2) CRS diagnosed after computed tomography and endoscopic examination in accordance with examination codes, and (3) CRS diagnosed by an otorhinolaryngologist. Severe CRS was defined as CRS that met the aforementioned criteria and required functional endoscopic sinus surgery. Only CRS episodes that occurred after the index date were considered in the determination of the primary outcome.
Flowchart of patient selection. NHIRD: National Health Insurance Research Database; CHD: coronary heart disease; N: number; CRS: chronic rhinosinusitis; PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft; PSM: propensity score matching
Demographic and Systemic Confounding Factors
To reduce the effect of confounding factors on the CRS development, we adjusted for the following demographic characteristics, systemic disorders, and prescriptions in our multivariable analyses: age, sex, occupation, hypertension, hyperlipidemia, diabetes mellitus, peripheral vascular disease, cerebrovascular disease, rheumatoid arthritis, systemic lupus erythematosus, Sjögren syndrome, systemic corticosteroids, clopidogrel, aspirin, alpha blockers, beta blockers, calcium channel blockers, angiotensin receptor blockers, angiotensin converting enzyme inhibitors, and statins. The presence of these confounding factors was assessed using ICD-9/ICD-10 codes, insurance codes, and ATC codes in the patients' records. To ensure that these confounding factors had the potential to sufficiently influence CRS development, only comorbidities and prescriptions that persisted for >2 years before the index date were considered confounding comorbidities or prescriptions. The patients in our cohort study were observed until the appearance of CRS, withdrawal from the Taiwan National Health Insurance program, or December 31, 2020 (the end of the follow-up period in this study).
Statistical Analysis
All statistical analyses were conducted using SAS (version 9.4; SAS Institute, Cary, NC, USA). Descriptive analyses were used to compare demographic characteristics, comorbidities, and medical prescriptions between the CHD-PCI and CHD-CABG groups, and the absolute standardized difference (ASD) was used to compare the distribution of confounding factors between the CHD-PCI and CHD-CABG groups; an ASD value of >0.1 was considered to indicate a significant difference. Furthermore, Cox proportional hazards regression was used to calculate and compare adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) for CRS and severe CRS between the CHD-PCI and CHD-CABG groups. The effects of age, sex, occupation, systemic comorbidities, and medical prescriptions were adjusted for in the Cox proportional hazards regression. We conducted subgroup analyses by dividing the patients with CHD into subgroups according to age and sex; we then used Cox proportional hazards regression to determine and compare the incidence of CRS and severe CRS in the CHD subgroups. In addition, interaction tests were used to analyze differences in the incidence of CRS and severe CRS between the subgroups. A P value of <0.05 indicated significance.
Results
Table 1 presents the characteristics of the CHD-PCI and CHD-CABG groups. The two groups had similar sex (ASD: 0.0000) and age (ASD: 0.0017) distributions, which can be attributed to the use of PSM. Moreover, the two groups did not differ significantly in terms of occupation, systemic comorbidities, or medical prescriptions (all ASD < 0.1; Table 1).
Characteristics of patients receiving percutaneous coronary intervention and coronary artery bypass graft surgery
| Character | CHD-PCI group (N = 31,784) | CHD-CABG group (N = 15,892) | ASD |
|---|---|---|---|
| Sex | 0.0000 | ||
| Male | 22287 (70.12%) | 11143 (70.12%) | |
| Female | 9497 (29.88%) | 4749 (29.88%) | |
| Age | 0.0017 | ||
| <40 | 801 (2.52%) | 267 (1.68%) | |
| 40-49 | 2689 (8.46%) | 1049 (6.60%) | |
| 50-59 | 6061 (19.07%) | 3145 (19.79%) | |
| 60-69 | 9538 (30.01%) | 5839 (36.74%) | |
| ≥70 | 12695 (39.94%) | 5592 (35.19%) | |
| Occupation | 0.0025 | ||
| Government employee | 1491 (4.69%) | 623 (3.92%) | |
| Worker | 16375 (51.52%) | 8181 (51.48%) | |
| Farmer and fisherman | 7857 (22.47%) | 2520 (15.86%) | |
| Low-income | 346 (1.09%) | 323 (2.03%) | |
| Others | 5715 (20.23%) | 4245 (26.71%) | |
| Co-morbidities | |||
| Hypertension | 24582 (77.34%) | 12812 (80.62%) | 0.0038 |
| Diabetes mellitus | 14567 (45.83%) | 9462 (59.54%) | 0.0254 |
| Hyperlipidemia | 18791 (59.12%) | 10056 (63.28%) | 0.0107 |
| Cerebrovascular disease | 2972 (9.35%) | 2012 (12.66%) | 0.0331 |
| Peripheral vascular disease | 1405 (4.42%) | 828 (5.21%) | 0.0076 |
| Rheumatoid arthritis | 496 (1.56%) | 138 (0.87%) | 0.0198 |
| Systemic lupus erythematosus | 130 (0.41%) | 99 (0.62%) | 0.0005 |
| Sicca/Sjogren syndrome | 407 (1.28%) | 133 (0.84%) | 0.0010 |
| Medications | |||
| Systemic corticosteroids | 7835 (24.65%) | 10343 (32.54%) | 0.0145 |
| Aspirin | 20396 (64.17%) | 22621 (71.17%) | 0.0397 |
| Clopidogrel | 6954 (21.88%) | 11204 (35.25%) | 0.0627 |
| Alpha-blockers | 2762 (8.69%) | 3585 (11.28%) | 0.0288 |
| Beta-blockers | 17570 (55.28%) | 19979 (62.86%) | 0.0186 |
| CCB | 18241 (57.39%) | 16315 (51.33%) | 0.0130 |
| ACEi | 12482 (39.27%) | 15485 (48.72%) | 0.0228 |
| ARB | 2552 (8.03%) | 3433 (10.80%) | 0.0084 |
| Statin | 16518 (51.97%) | 21454 (67.50%) | 0.0386 |
N: number; CHD: coronary heart disease; PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft; ASD: absolute standardized difference; CCB: calcium channel blocker; ACEi: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker
Throughout the follow-up period (2014-2020), 813 and 482 episodes of CRS occurred in the CHD-PCI and CHD-CABG groups, respectively. Our multivariable analysis demonstrated that the incidence of CRS in the CHD-CABG group was significantly higher than that in the CHD-PCI group (aHR: 1.196, 95% CI: 1.064-1.280, P = 0.0402). Furthermore, a total of 45 and 16 severe CRS events occurred in the CHD-PCI and CHD-CABG groups, respectively. The risks of severe CRS were similar between the CHD-PCI and CHD-CABG groups (aHR: 0.795, 95% CI: 0.456-1.388, P = 0.5534; Table 2).
Our age-based subgroup analysis revealed that among patients aged <60 years, the risk of CRS was significantly higher in the CHD-CABG population than in the CHD-PCI population (aHR: 1.180, 95% CI: 1.119-1.244). However, the age-based subgroups had similar risks of CRS (P = 0.8182). Furthermore, our sex-based subgroup analysis indicated that among male patients, the CHD-CABG population exhibited a higher incidence of CRS than did the CHD-PCI population (aHR: 1.206, 95% CI: 1.007-1.243), and the correlation between CHD severity and CRS development was significantly higher among male patients (P = 0.0016). The occurrence of severe CRS did not differ between the subgroups (both P < 0.05; Table 3).
Risk of chronic rhinosinusitis in patients with coronary heart disease treated with percutaneous coronary intervention or coronary artery bypass grafts
| Event | CHD-PCI group | CHD-CABG group | P value |
|---|---|---|---|
| CRS | |||
| Person-months | 1098512 | 422078 | |
| Event | 813 | 482 | |
| Crude HR (95% CI) | Reference | 1.512 (1.246-1.835)* | |
| aHR (95% CI) | Reference | 1.196 (1.064-1.280)* | 0.0402* |
| Severe CRS | |||
| Person-months | 1002136 | 415645 | |
| Event | 45 | 16 | |
| Crude HR (95% CI) | Reference | 0.801 (0.463-1.385) | |
| aHR (95% CI) | Reference | 0.795 (0.456-1.388) | 0.5534 |
CHD: coronary heart disease; PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft; CRS: chronic rhinosinusitis; HR: hazard ratio; CI: confidence interval; aHR: adjusted hazard ratio. The results obtained from the Cox regression model.* significant difference between groups
Subgroup analyses of chronic rhinosinusitis incidence by age and sex
| Subgroup | aHR | 95% CI | Interaction P value |
|---|---|---|---|
| CRS | |||
| Age | 0.8182 | ||
| <60 | 1.180 | 1.119-1.244 | |
| ≥60 | 1.125 | 0.899-1.409 | |
| Sex | 0.0016* | ||
| Male | 1.206 | 1.007-1.243 | |
| Female | 0.755 | 0.483-1.382 | |
| Severe CRS | |||
| Age | 0.2575 | ||
| <60 | 1.097 | 0.933-1.291 | |
| ≥60 | 0.571 | 0.140-2.332 | |
| Sex | 0.3346 | ||
| Male | 0.803 | 0.408-1.454 | |
| Female | 0.555 | 0.383-2.182 |
CRS: chronic rhinosinusitis; aHR: adjusted hazard ratio; CI: confidence interval.
The results obtained from the Cox regression model.* significant difference between groups.
Discussion
This study determined that the CHD-CABG group had a higher risk of CRS than did the CHD-PCI group. Moreover, the CHD-PCI and CHD-CABG groups had similar incidence rates of severe CHD treated using functional endoscopic sinus surgery. The study also determined that the association between severe CHD and subsequent CRS was more prominent in male patients.
Several pathways contribute to the development and progression of CHD [17]. Inflammation is a major mechanism of CHD development, and CHD was reported to be associated with relatively high inflammatory cytokine levels [18]. A previous study revealed that patients with CHD had elevated lipoprotein-associated phospholipase A2 and C-reactive protein levels [19]. Additionally, the neutrophil-to-lymphocyte ratio, the platelet-to-lymphocyte ratio, and C-reactive protein levels can predict CHD severity [20]. Atherosclerotic plaques also constitute a major factor contributing to CHD development; such plaques are caused by macrophages and high expression of vascular cell adhesion molecules and matrix E-selectin [17]. In addition to inflammation, elevated serum lipid levels can contribute to the risk of CHD; this risk can be attenuated by using statins to lower serum low-density lipoprotein concentrations [21]. CRS is also associated with inflammation; specifically, CRS is associated with relatively high expression levels of genes producing interleukin and intercellular adhesion molecules [22]. Moreover, CRS involves local aggregation of immune cells such as eosinophils, natural killer cells, and neutrophils in nasal polyps [23]. Apart from molecular mechanisms contributing to CHD development, conditions such as obesity and asthma are commonly associated with CHD or CRS [24-27]. The advancement of CHD may be associated with the severity of subsequent CRS, and this is attributable to their similar pathophysiology and associated comorbidities [17, 24-28]. Our findings support this possible association.
Our study revealed that severe CHD was associated with a higher incidence of subsequent CRS. Previous studies have reported cases of nasal diseases in patients with CHD [29, 30]. However, no large-scale study with an adequate sample size has investigated the association between CHD and nasal diseases. To the best of our knowledge, our study is the first to demonstrate a positive association between CHD severity and CRS development. To exclude the influence of preexisting CRS on our findings, we excluded patients with CRS that occurred before CHD diagnosis or within 6 months after CHD diagnosis; moreover, we adjusted for multiple risk factors for CRS, including age, sex, and systemic inflammatory disorders, in our Cox proportional hazards regression [2, 24, 31]. Despite these adjustments, we observed that the association between CHD severity and CRS occurrence still remained significant. Consequently, CHD severity may be an independent risk factor for subsequent CRS. A previous study indicated that the severity of CHD was associated with the presence of inflammatory respiratory diseases such as chronic obstructive pulmonary disease [32]. Accordingly, we may reasonably assume that CHD severity can affect the occurrence of other inflammatory diseases in the respiratory tract, such as CRS. We observed that the incidence of severe CRS requiring functional endoscopic sinus surgery did not differ significantly between the CHD-PCI and CHD-CABG groups. This phenomenon can be attributed to two possible reasons. First, the increase in inflammation occurring in severe CHD may be inadequate to trigger a significant progression of CRS because other factors are key to the development of severe CRS. Second, the numbers of patients with severe CRS were low in both groups, which may have led to statistical bias.
In our subgroup analyses, the age-based subgroups of patients with CHD had similar risks of CRS. However, our sex-based subgroup analysis revealed that the correlation between CHD severity and CRS occurrence was significantly higher among male patients than among female patients. A previous study reported age to be a risk factor for CHD [24]; however, another study indicated that age was not a risk factor for CRS, showing that the severity of CRS was greater among young individuals [33]. The correlation between severe CHD and subsequent CRS development in the present study was significant but did not differ by age. However, previous studies have reported that the male sex was associated with a relatively high prevalence of CHD and CRS, rendering the male sex a prominent risk factor for both CHD and CRS [2, 34]. The influence of severe CHD may be greater in patients at a high risk of CRS, including men [2, 34], and this assumption is supported by our findings of a stronger correlation between severe CHD and CRS among male patients. We observed nonsignificant associations between severe CRS and CHD severity in all age- and sex-based subgroups, and these observations were noted to be consistent with our overall results.
The prevalence of CHD is >6% globally and is as high as 30% in Northern European men [24, 35]. CHD is the second leading cause of all-cause mortality in the United Kingdom [36] and the leading cause of death in the United States, despite a reduction in the mortality rate associated with CHD [37]. Additionally, CRS is globally prevalent and is the most common chronic disease in the United States [13]. Although death directly caused by CRS is rare, CRS adversely affects daily life, and many patients cannot fully recover from CRS, leading to a substantial economic burden [25]. Both CHD and CRS affect many individuals and can lead to substantial health problems [13, 38]; hence, identifying any correlation between them is crucial.
This study has some limitations. First, we used data from the NHIRD, which does not contain real medical records. Therefore, we could not obtain or analyze data on several crucial factors such as imaging results for CHD, serum lipid levels for CHD, degrees of arterial stenosis in CHD, treatment details for CHD, the prognosis of CHD, nasal imaging results for CRS, surgical outcomes for CRS, and therapeutic outcomes for CRS. Second, we applied a retrospective study design, and the health of the patients may have changed; nevertheless, PSM was used to minimize the influence of this. Third, we determined disease severity by considering the treatment procedure used rather than conducting a clinical evaluation, which may have engendered a substantial bias because PCI can be used to treat advanced coronary artery stenosis with acceptable outcomes [39, 40]. Finally, we excluded a considerable number of patients with CHD treated with PCI during the matching process. Nonetheless, our sample size is not smaller than those in previous population-based studies [14, 24]; hence, the influence of this limitation might be minimal.
In conclusion, we observed that severe CHD treated with CABG was associated with a higher incidence of subsequent CRS when compared with mild CHD treated with PCI. Accordingly, CRS-related examinations may be recommended for individuals with severe CHD undergoing surgical management. Further large-scale prospective research is warranted to determine whether the severity of CHD influences therapeutic outcomes for CRS of varying severity.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Lorenzen US, Buggeskov KB, Nielsen EE, Sethi NJ, Carranza CL, Gluud C. et al. Coronary artery bypass surgery plus medical therapy versus medical therapy alone for ischaemic heart disease: a protocol for a systematic review with meta-analysis and trial sequential analysis. Syst Rev. 2019;8:246
2. Khamis RY, Ammari T, Mikhail GW. Gender differences in coronary heart disease. Heart. 2016;102:1142-1149
3. Lee YH, Fang J, Schieb L, Park S, Casper M, Gillespie C. Prevalence and Trends of Coronary Heart Disease in the United States, 2011 to 2018. JAMA Cardiol. 2022;7:459-462
4. South T. Coronary artery bypass surgery. Crit Care Nurs Clin North Am. 2011;23:573-585
5. Montrief T, Koyfman A, Long B. Coronary artery bypass graft surgery complications: A review for emergency clinicians. Am J Emerg Med. 2018;36:2289-2297
6. Banning AP, Baumbach A, Blackman D, Curzen N, Devadathan S, Fraser D. et al. Percutaneous coronary intervention in the UK: recommendations for good practice 2015. Heart. 2015;101(Suppl 3):1-13
7. Cymerman RM, Skolnick AH, Cole WJ, Nabati C, Curcio CA, Smith RT. Coronary Artery Disease and Reticular Macular Disease, a Subphenotype of Early Age-Related Macular Degeneration. Curr Eye Res. 2016;41:1482-1488
8. Wirtz PH, von Känel R. Psychological Stress, Inflammation, and Coronary Heart Disease. Curr Cardiol Rep. 2017;19:111
9. Escobar E. Hypertension and coronary heart disease. J Hum Hypertens. 2002;16(Suppl 1):S61-63
10. Rana JS, Nieuwdorp M, Jukema JW, Kastelein JJ. Cardiovascular metabolic syndrome - an interplay of, obesity, inflammation, diabetes and coronary heart disease. Diabetes Obes Metab. 2007;9:218-232
11. Priyamvara A, Dey AK, Bandyopadhyay D, Katikineni V, Zaghlol R, Basyal B. et al. Periodontal Inflammation and the Risk of Cardiovascular Disease. Curr Atheroscler Rep. 2020;22:28
12. Seo WW, Yoo HS, Kim YD, Park SP, Kim YK. Choroidal vascularity index of patients with coronary artery disease. Sci Rep. 2022;12:3036
13. Wang X, Cutting GR. Chronic rhinosinusitis. Adv Otorhinolaryngol. 2011;70:114-121
14. Lee CY, Yang KL, Sun CC, Huang JY, Chen HC, Chen HC. et al. The Development of Dry Eye Disease After Surgery-Indicated Chronic Rhinosinusitis: A Population-Based Cohort Study. Int J Environ Res Public Health. 2020 17
15. Wang PC, Lin HC, Kang JH. Chronic rhinosinusitis confers an increased risk of acute myocardial infarction. Am J Rhinol Allergy. 2013;27:e178-182
16. Wu CW, Chao PZ, Hao WR, Liou TH, Lin HW. Risk of stroke among patients with rhinosinusitis: a population-based study in Taiwan. Am J Rhinol Allergy. 2012;26:278-282
17. Li H, Sun K, Zhao R, Hu J, Hao Z, Wang F. et al. Inflammatory biomarkers of coronary heart disease. Front Biosci (Schol Ed). 2018;10:185-196
18. Harrington RA. Targeting Inflammation in Coronary Artery Disease. N Engl J Med. 2017;377:1197-1198
19. Sarwar N, Thompson AJ, Di Angelantonio E. Markers of inflammation and risk of coronary heart disease. Dis Markers. 2009;26:217-225
20. Liu Y, Ye T, Chen L, Jin T, Sheng Y, Wu G. et al. Systemic immune-inflammation index predicts the severity of coronary stenosis in patients with coronary heart disease. Coron Artery Dis. 2021;32:715-720
21. Shaya GE, Leucker TM, Jones SR, Martin SS, Toth PP. Coronary heart disease risk: Low-density lipoprotein and beyond. Trends Cardiovasc Med. 2022;32:181-194
22. Khurana N, Pulsipher A, Jedrzkiewicz J, Ashby S, Pollard CE, Ghandehari H. et al. Inflammation-driven vascular dysregulation in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2021;11:976-983
23. Klingler AI, Stevens WW, Tan BK, Peters AT, Poposki JA, Grammer LC. et al. Mechanisms and biomarkers of inflammatory endotypes in chronic rhinosinusitis without nasal polyps. J Allergy Clin Immunol. 2021;147:1306-1317
24. Voutilainen A, Brester C, Kolehmainen M, Tuomainen TP. Epidemiological analysis of coronary heart disease and its main risk factors: are their associations multiplicative, additive, or interactive? Ann Med. 2022;54:1500-1510
25. Zhang L, Zhang R, Pang K, Liao J, Liao C, Tian L. Prevalence and risk factors of chronic rhinosinusitis among Chinese: A systematic review and meta-analysis. Front Public Health. 2022;10:986026
26. Nam JS, Roh YH, Fahad WA, Noh HE, Ha JG, Yoon JH. et al. Association between obesity and chronic rhinosinusitis with nasal polyps: a national population-based study. BMJ Open. 2021;11:e047230
27. Wang L, Gao S, Yu M, Sheng Z, Tan W. Association of asthma with coronary heart disease: A meta analysis of 11 trials. PLoS One. 2017;12:e0179335
28. Sedaghat AR, Kuan EC, Scadding GK. Epidemiology of Chronic Rhinosinusitis: Prevalence and Risk Factors. J Allergy Clin Immunol Pract. 2022;10:1395-1403
29. Chien CY, Tai SY, Wang LF, Lee CT. Chronic obstructive pulmonary disease predicts chronic rhinosinusitis without nasal polyps: A population-based study. Am J Rhinol Allergy. 2015;29:e75-80
30. Li YE, Ren J. Association between obstructive sleep apnea and cardiovascular diseases. Acta Biochim Biophys Sin (Shanghai). 2022;54:882-892
31. Wu XF, Huang JY, Chiou JY, Chen HH, Wei JC, Dong LL. Increased risk of coronary heart disease among patients with primary Sjögren's syndrome: a nationwide population-based cohort study. Sci Rep. 2018;8:2209
32. Svendsen CD, Kuiper KKJ, Ostridge K, Larsen TH, Nielsen R, Hodneland V. et al. Factors associated with coronary heart disease in COPD patients and controls. PLoS One. 2022;17:e0265682
33. Holmes T, Makary C, Unsal AA, Biddinger P, Reyes-Gelves C, Kountakis SE. How Does Age Impact Presentation and Outcomes in Chronic Rhinosinusitis? Ann Otol Rhinol Laryngol. 2020;129:872-877
34. Kim YS, Kim NH, Seong SY, Kim KR, Lee GB, Kim KS. Prevalence and risk factors of chronic rhinosinusitis in Korea. Am J Rhinol Allergy. 2011;25:117-121
35. Zhu KF, Wang YM, Zhu JZ, Zhou QY, Wang NF. National prevalence of coronary heart disease and its relationship with human development index: A systematic review. Eur J Prev Cardiol. 2016;23:530-543
36. Bhatnagar P, Wickramasinghe K, Wilkins E, Townsend N. Trends in the epidemiology of cardiovascular disease in the UK. Heart. 2016;102:1945-1952
37. Duggan JP, Peters AS, Trachiotis GD, Antevil JL. Epidemiology of Coronary Artery Disease. Surg Clin North Am. 2022;102:499-516
38. Dalen JE, Alpert JS, Goldberg RJ, Weinstein RS. The epidemic of the 20(th) century: coronary heart disease. Am J Med. 2014;127:807-812
39. Akbari T, Al-Lamee R. Percutaneous Coronary Intervention in Multi-Vessel Disease. Cardiovasc Revasc Med. 2022;44:80-91
40. Hoole SP, Bambrough P. Recent advances in percutaneous coronary intervention. Heart. 2020;106:1380-1386
Author contact
![]() Corresponding author: Ming-Hong Hsieh, School of Medicine, Chung Shan Medical University, Taichung 402, Taiwan; E-mail: mhhpsycom.
Corresponding author: Ming-Hong Hsieh, School of Medicine, Chung Shan Medical University, Taichung 402, Taiwan; E-mail: mhhpsycom.

 Global reach, higher impact
Global reach, higher impact