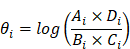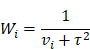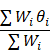Impact Factor
ISSN: 1449-1907
Int J Med Sci 2023; 20(8):1009-1023. doi:10.7150/ijms.83963 This issue Cite
Research Paper
Circulating microRNAs as diagnostic biomarkers for ischemic stroke: evidence from comprehensive analysis and real-world validation
1. Department of Neurology, The First Affiliated Hospital of Shenzhen University, Shenzhen Second People's Hospital, Shenzhen 518000, China.
2. Research and Development Unit, Shenzhen GenDo Medical Technology Co., Ltd., Dapeng, Shenzhen 518000, China.
3. SDIVF R&D Centre, 209,12W, HKSTP, Shatin, Hong Kong, China.
4. Department of Epidemiology and Biostatistics, School of Public Health, Guangdong Medical University, Dongguan 523808, China.
5. Faculty of Education, Health and Wellbeing, University of Wolverhampton, Wolverhampton WV1 1QU, UK.
6. CUHK-SDU Joint Laboratory on Reproductive Genetics, School of Biomedical Sciences, The Chinese University of Hong Kong, Hong Kong SAR, China.
*These authors contributed equally to this work.
Received 2023-3-1; Accepted 2023-5-16; Published 2023-6-4
Abstract
Ischemic stroke (IS) is the majority of strokes which remain the second leading cause of deaths in the last two decades. Circulating microRNAs (miRNAs) have been suggested as potential diagnostic and therapeutic tools for IS by previous studies analyzing their differential expression. However, inconclusive and controversial conclusions of these results have to be addressed. In this study, comprehensive analysis and real-world validation were performed to assess the associations between circulating miRNAs and IS. 29 studies with 112 miRNAs were extracted after manual selection and filtering, 12 differentially expressed miRNAs were obtained from our results of meta-analysis. These miRNAs were evaluated in 20 IS patients, compared to 20 healthy subjects. 4 miRNAs (hsa-let-7e-5p, hsa-miR-124-3p, hsa-miR-17-5p, hsa-miR-185-5p) exhibited the significant expression level in IS patient plasma samples. Pathway and biological process enrichment analysis for the target genes of the 4 validated miRNAs identified cellular senescence and neuroinflammation as key post-IS response pathways. The results of our analyses closely correlated with the pathogenesis and implicated pathways observed in IS subjects suggested by the literature, which may provide aid in the development of circulating diagnostic or therapeutic targets for IS patients.
Keywords: circulating microRNAs, ischemic stroke, biomarkers, comprehensive analysis, real-world validation
Introduction
Stroke has been ranked as the second leading cause of death globally in the last two decades [1]. The majority of strokes are ischemic stroke (IS) which are caused by the blockage of blood supply to the brain due to embolism or other vascular diseases such as atherosclerosis, leading to the lack of oxygen and nutrients supply and brain damage. Current diagnosis for IS is mainly based on the use of computed tomography scan or magnetic resonance imaging after the patient is hospitalized [2]. In the past decade, studies have emerged suggesting other potential diagnostic approaches for different diseases including the dysregulation of microRNAs (miRNAs) and circular RNAs [3]. miRNAs have become one of the most promising types of biomarkers among these approaches and were extensively studied for their potential use in cancers, nervous system disorders and cardiovascular diseases [4]. miRNAs are a class of non-coding, small RNAs composed of around 20-25 nucleotides. They function to regulate the degradation of mRNAs and transcription and translation of their target genes [5, 6]. Multiple sources for extracting miRNAs have been suggested, such as plasma, serum, peripheral blood mononuclear cells (PBMCs), cerebrospinal fluid (CSF), saliva, and urine. Circulating miRNAs have been the focus among them due to its ease to detect, high accuracy, specificity and stability [7].
The understanding of the role of miRNAs in stroke patients has been improved in the recent years. Studies have shown that the expression levels of miRNAs were associated with the prognosis of stroke [8, 9]. Differentially expressed miRNAs in the circulation or CSF of stroke patients were suggested, raising the potential of using these miRNAs as early diagnostic biomarkers or treatments [10, 11]. Studies have also shown the value of particular circulating miRNAs as the predictive tools for stroke risk by the combination of multiple clinical risk factors such as age, sex, smoking status, blood pressure and body mass index. Some of these miRNAs include miR-6124, miR-5196-5p, miR-4292, of which the expression levels in serum were associated with the risk of stroke [12, 13]. Previous studies have shown the differential expression levels of many other miRNAs (miR-9, miR-29b, miR124 and miR-125b) and suggested their potential clinical value [14]. Despite this, consistency and reliability of the results should be addressed in terms of the differences in number of sample sizes, subjects and miRNA profiling methods. Therefore, we assess the existing studies for circulating miRNAs in stroke patients by performing meta-analysis and experimental validation.
Early prediction and diagnosis for IS is of vital importance to reduce the risk of permanent brain damage due to the delay in prevention and treatments. This study identified 12 miRNAs by meta-analysis as potential circulating biomarkers for IS. 4 miRNAs (hsa-let-7e-5p, hsa-miR-124-3p, hsa-miR-17-5p, hsa-miR-185-5p) exhibited the significant expression level in IS subjects compared to the healthy subjects. The target genes of these miRNAs were closely related to the cellular senescence and neuroinflammation pathways implicated in ischemia. This study will provide a direction for future studies in developing diagnostic and therapeutic targets for IS patients.
Materials and methods
Data Collection
Studies were collected on PubMed from 1st January 1994 until 31st December 2022. Studies were searched on PubMed using the following search terms: ("stroke") AND ("Blood" OR "Serum" OR "Plasma" OR "Circulat*" OR "Peripheral" OR "PBMC") AND ("miRNA" OR "microRNA" OR "miR"). The collected studies' title, abstract and full texts were then screened and selected according to the eligibility criteria. Information of each relevant study was recorded in a standardised form including the PubMed ID, first author, publication year, region, specimen(s), miRNA profiling method(s), number of cases and controls, dysregulated miRNAs, their dysregulation states, and p-values.
Eligibility Criteria
Studies were included in the present meta-analysis if they (1) were primary studies; (2) were case-controlled studies; (3) performed profiling of human miRNAs in the circulation of IS patients; (4) reported the type(s) of specimens used; (5) reported the sample sizes of case and control groups; (6) reported the miRNA profiling methods; (7) reported the statistical significance of each miRNA and their dysregulation states; (8) did not perform irrelevant comparisons (e.g., treatment-naïve vs treatment group); (9) were written in English. Studies that were excluded if they (1) were review articles; (2) did not contain case and control (stroke and healthy) samples; (3) performed profiling of miRNAs in animal models or cell lines other than human blood, plasma or serum; (4) did not report the type(s) of specimens used; (5) did not report the sample sizes of case and control groups; (6) did not report the miRNA profiling methods; (7) did not report the statistical significance of each miRNA and their dysregulation states; (8) performed comparisons other than stroke vs healthy controls; (9) written in languages other than English.
Meta-analysis to identify differentially expressed miRNAs
Meta-analysis was performed for each miRNA that was differentially expressed in IS patients in more than one study. The names of the miRNAs were standardized by the R package miRNAmeConverter (Version 1.10.0) [15] according to the miRBase database (Version 22.0) [16]. R package metafor (Version 3.0.2) was used to calculate the effect sizes for each qualified miRNA in each study (θi) independently as log Odds Ratios (logORs) using a random-effects model [17]. The logOR for the ith study was calculated by:
where in a 2x2 table, Ai, Bi, Ci and Di represent the number of up-regulation events in the disease group, down-regulation events in the disease group, up-regulation events in the control group, and down-regulation events in the control group, respectively. For this meta-analysis, since there were no differentially expressed miRNAs in the control group, Ci represents the number of controls and Di would be zero. 0.5 was added to all zero values in the equation. Other outcomes include p-values, tau square (τ2), I2 and the sample variance (vi). τ2 and I2 were used to estimate the heterogeneity of each miRNA. The weight of the ith study (Wi) was calculated by:
The overall effect size for a miRNA in the associated studies was then calculated by:
The result was considered significant if the p-value was lower than 0.05. The miRNA was considered up-regulated or down-regulated if the overall effect size was greater or smaller than 0, respectively.
Tissue-specific expression analysis of 12 miRNAs
miRNAs identified from meta-analysis were subjected to tissue enrichment analysis using data obtained from Human miRNA tissue atlas [18]. Quantile-normalized data was used to visualize the expression levels of each miRNA in a total of 31 tissues. Expression levels of multiple samples obtained from the same tissue were grouped into their average values. Relative expression levels of each miRNA across the tissues were calculated as z-scores for visualization.
RNA extraction from plasma
Human blood plasma was prepared from peripheral blood as described previously [19]. Briefly, at 24 hour following stroke onset, blood samples were obtained using EDTA tubes using standard procedures. The samples were placed on ice immediately and centrifuged at 1000 g for 15 minutes at 4°C. Total RNA was extracted from 200 μl plasma using the Universal Extraction Kit following the manufacturer's instructions (GeneDotech, #GD-101). Total RNA was eluted by adding 15 μl of nuclease-free water and stored at -80oC. 20 IS patients and 20 healthy subjects were enrolled for this study. The ischemic patients recruited were defined by an acute focal neurological deficit in combination with a diffusion weighted imaging-positive lesion on magnetic resonance imaging or a new lesion on a delayed CT scan. The collection time point was at 24 hour after stroke onset. The collection and use of specimens in this experiment were all signed and confirmed by patients and healthy subjects. The study design was approved by the appropriate ethics review board of The Second People's Hospital of Shenzhen (No. 20200601022-FS01). The consent form was approved by the Medical Ethics Committee of The Second People's Hospital of Shenzhen.
cDNA synthesis and real time PCR
5 μl eluted RNA was reverse transcribed in 20 μl reactions according to manufacturer's instructions (GeneDotech, #GD-102). Briefly, 5 μl of RNA in a final volume of 20 μl including transcription mastermix was incubated at 42°C for 1 hour followed by enzyme inactivation at 95°C for 5 minutes. The cDNA was diluted and assayed in 10 μl PCR reactions according to the instruction for the PCR master mix (Probe) (GeneDotech, #GD-105). Quantitation of miRNAs was carried out using Probe based Real-Time PCR. The amplification was performed in StepOne plus Detection System (Applied Biosystems) in 96 well plates, each sample is performed in triplicate. The amplification curves were analyzed using the ABI SDS software, both for determination of Ct. The gene expression levels of selected miRNAs are presented as ΔCt relative to the mean Ct values of the external references including cel-miR-39, 54 and 238. Fold change was calculated relative to that of healthy individuals' group. The raw average Ct values of measured miRNAs and external references were displayed in supplementary Table S1.
Biological Significance
Target genes for each miRNA that was identified from the meta-analyses were retrieved by the R package multiMiR (Version 1.4.0) [20]. Only the validated miRNA-target gene interactions obtained from mirTarBase were included. Pathway enrichment analysis for these target genes was performed by R packages ReactomePA (Version 1.26.0) and clusterProfiler (Version 3.10.1) based on Reactome and Kyoto Encyclopedia of Genes and Genomes (KEGG), respectively [21, 22]. Gene Ontology (GO) analysis was performed to analyze the enrichment of biological processes. A pathway or biological process was considered significantly enriched if its associated False Discovery Rate (FDR)-adjusted p-value was less than 0.05. Ingenuity Pathway Analysis (IPA) was used to further study the miRNA-mRNA interactions and their associated pathways using its microRNA Target Filter module [23]. The interactions and their associated pathways were further filtered. Only those with strong confidence (experimentally observed), implicated in cardiovascular disease, neuroinflammatory response or neurological disease, and human interactions were selected. Subsequent miRNA-mRNA interactions network was plotted using PathDesigner included in IPA software.
Receiver operating characteristics analysis
Receiver operating characteristics (ROC) curve analysis was performed using plasma samples collected from 20 IS patients and 20 healthy individuals from the hospital. Logistic model was built on the normalized expression levels for each miRNA and disease group. R package “pROC” was used to calculate AUC values for each model and visualise the ROC curves.
Statistical analysis
GraphPad Prism 8.2 and SPSS 19.0 statistical packages were used for statistical analysis, and the Student's t test (two-tailed) was used in qRT-PCR analysis between two groups of data sets. P-value <0.05 was considered statistically significant.
Results
Included literatures
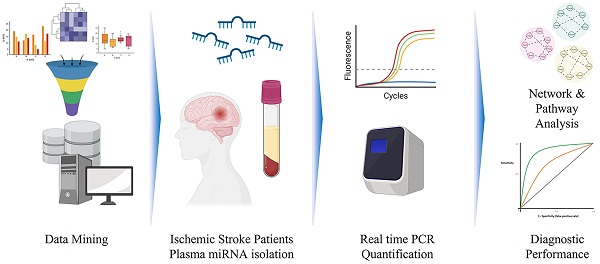
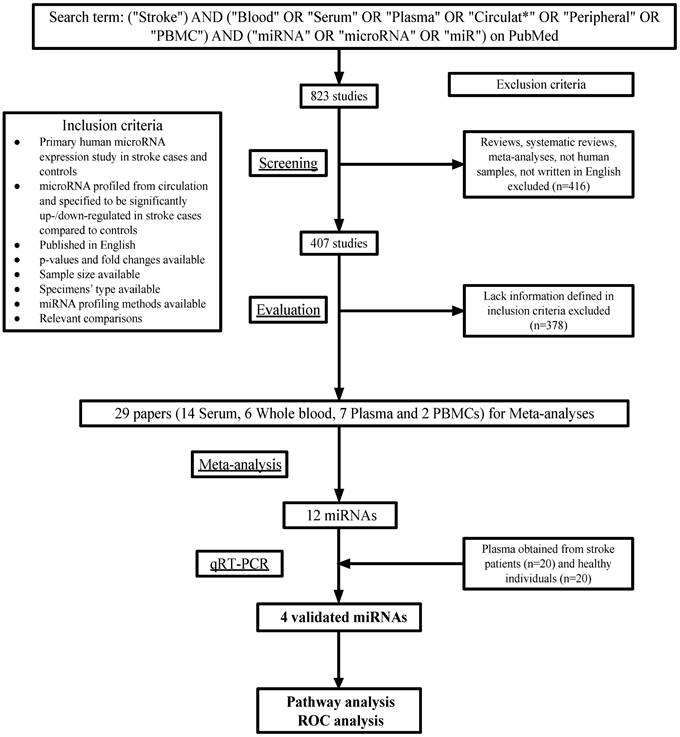
A total of 823 articles were found on PMC using the search terms. After excluding the studies that did not match the eligibility criteria, 29 studies were left (Figure 1). Among these studies, 14 measured the levels of miRNAs in patients' serum, 7 in plasma, 6 in whole blood and 2 in peripheral blood mononuclear cells (Table 1).
Workflow of meta-analysis and real-world validation stage.
Characteristics of the included studies and the involved miRNAs.
| PMID | Author | Year | Country | miRNAs | Dysregulation state | Sample size | Specimen | Technique | |
|---|---|---|---|---|---|---|---|---|---|
| Stroke | Healthy | ||||||||
| 19888324 | Tan, K. S. | 2009 | Singapore | hsa-miR-126-3phsa-miR-144-3phsa-miR-16-5phsa-miR-21-5phsa-miR-223-3phsa-miR-320a-3p | DownUpUpUpUpUp | 19 | 5 | Whole blood | microarray, qRT-PCR |
| 21622133 | Zeng, L. | 2011 | China | hsa-miR-210-3p | Down | 112 | 60 | Whole blood | qRT-PCR |
| 24237608 | Long, G. | 2013 | China | hsa-miR-126-3phsa-miR-30a-5p | DownDown | 38 | 50 | Plasma | qRT-PCR |
| 24911610 | Jickling, G. C. | 2014 | USA | hsa-let-7i-5phsa-miR-122-5phsa-miR-148a-3phsa-miR-19a-3phsa-miR-320dhsa-miR-363-3phsa-miR-4429hsa-miR-487b-3p | DownDownDownDownDownUpDownUp | 24 | 24 | PBMCs | microarray, qRT-PCR |
| 25257664 | Liu, Y. | 2015 | China | hsa-miR-124-3phsa-miR-9-5p | DownDown | 31 | 11 | Serum | qRT-PCR |
| 25287657 | Wang, W. | 2014 | China | hsa-miR-106b-5phsa-miR-320dhsa-miR-320ehsa-miR-4306 | UpDownDownUp | 136 | 116 | Plasma | microarray, qRT-PCR |
| 25410304 | Li, P. | 2015 | China | hsa-miR-1246hsa-miR-1299hsa-miR-1913hsa-miR-224-3phsa-miR-3149hsa-miR-32-3phsa-miR-377-5phsa-miR-423-5phsa-miR-451ahsa-miR-4739hsa-miR-518bhsa-miR-532-5p | UpUpDownDownUpUpDownUpUpUpDownDown | 117 | 82 | Serum | microarray, qRT-PCR |
| 26044809 | Li, S. | 2015 | China | hsa-miR-146a-5phsa-miR-185-5p | Down Down | 60 | 30 | Whole blood | microarray, qRT-PCR |
| 26096228 | Jia, L. | 2015 | China | hsa-miR-145-5phsa-miR-221-3phsa-miR-23a-3p | UpDownDown | 146 | 96 | Serum | qRT-PCR |
| 26459744 | Zeng, Y. | 2015 | China | hsa-miR-124-3phsa-miR-218-5phsa-miR-22-3phsa-miR-23a-3phsa-miR-30a-5phsa-miR-33a-5phsa-miR-330-3phsa-miR-9-5p | DownDownUpUpDownDownDownDown | 10 | 10 | Serum | qRT-PCR |
| 26885038 | Wu, J. | 2015 | China | hsa-miR-15a-5phsa-miR-16-5phsa-miR-17-5p | UpUpUp | 106 | 120 | Serum | qRT-PCR |
| 27545688 | Liang, T. | 2016 | China | hsa-miR-34a-5p | Up | 102 | 97 | Plasma | qRT-PCR |
| 27776139 | Huang, S. | 2016 | China | hsa-let-7e-5p | Up | 302 | 302 | Whole blood | qRT-PCR |
| 28111007 | Wang, Y. | 2017 | China | hsa-miR-221-3phsa-miR-382-5p | DownDown | 68 | 39 | Serum | qRT-PCR |
| 28168424 | Bam, M. | 2018 | USA | hsa-miR-130a-3phsa-miR-320ahsa-miR-376c-3phsa-miR-432-5phsa-miR-4656hsa-miR-487hsa-miR-503-5phsa-miR-874-3p | UpUpUpUpUpUpUpDown | 19 | 20 | PBMCs | microarray, qRT-PCR |
| 28875333 | Jin, F. | 2017 | China | hsa-miR-126-3phsa-miR-130a-3phsa-miR-185-5phsa-miR-218-5phsa-miR-222-3phsa-miR-378a-5p | DownDownUpUpUpDown | 106 | 110 | Plasma | qRT-PCR |
| 29402769 | Chen, Z. | 2018 | China | hsa-miR-146b-5p | Up | 128 | 102 | Serum | qRT-PCR |
| 29701837 | Vijayan, M. | 2018 | USA | hsa-miR-122-5phsa-miR-211-5phsa-miR-22-3phsa-miR-23a-3phsa-miR-30d-5p | UpUpDownDownDown | 34 | 11 | Serum | Illumina deep sequencing, qRT-PCR |
| 30030634 | Jin, F. | 2018 | China | hsa-miR-126-3phsa-miR-130a-3phsa-miR-185-5phsa-miR-219-5phsa-miR-222-3p | DownDownUpUpUp | 148 | 148 | Plasma | qRT-PCR |
| 30112629 | Yoo, H. | 2019 | Korea | hsa-let-7e-5phsa-miR-1229-3phsa-miR-1238-5phsa-miR-1270hsa-miR-1294hsa-miR-1301-3phsa-miR-140-5phsa-miR-142-3phsa-miR-144-3phsa-miR-186-5phsa-miR-18b-5phsa-miR-19a-3phsa-miR-301a-3phsa-miR-32-5phsa-miR-335-5phsa-miR-340-5phsa-miR-362-3phsa-miR-505-5phsa-miR-517b-3phsa-miR-544ahsa-miR-579-3phsa-miR-628-5phsa-miR-660-5phsa-miR-664a-5phsa-miR-877-5p | UpUpUpUpUpUpDownDownDownDownDownDownDownDownDownDownDownUpDownUpDownUpDownUpUp | 10 | 11 | Whole blood | microarray, TaqMan miRNA assay |
| 30617992 | van Kralingen, J. C. | 2019 | UK | hsa-miR-17-5phsa-miR-20b-5phsa-miR-27b-3phsa-miR-93-5p | UpUpUpUp | 139 | 34 | Serum | microarray, qRT-PCR |
| 30678250 | Giordano, M. | 2019 | Italy | hsa-miR-195-5phsa-miR-451a | UpUp | 18 | 20 | Serum | qRT-PCR |
| 30899379 | Geng, W. | 2019 | China | hsa-miR-126-3p | Down | 13 | 17 | Plasma | qRT-PCR |
| 31496785 | Kotb, H. G. | 2019 | Egypt | hsa-miR-146a-5p | Down | 44 | 22 | Serum | qRT-PCR |
| 31935511 | Li, L. | 2020 | China | hsa-miR-1275 | Down | 279 | 279 | Whole blood | microarray, qRT-PCR |
| 32406219 | Li, S. | 2020 | China | hsa-miR-128-3p | Up | 80 | 60 | Serum | qRT-PCR |
| 35018114 | Guo, C. | 2022 | China | hsa-miR-185-5phsa-miR-424-5p | UpUp | 142 | 50 | Serum | qRT-PCR |
| 35328807 | Aldous, E. K. | 2022 | Qatar | hsa-miR-451ahsa-miR-574-5phsa-miR-4446-3phsa-miR-142-3phsa-miR-6721-5phsa-miR-676-3phsa-miR-379-5phsa-miR-485-3phsa-miR-411-5phsa-miR-149-5p | UpDownDownDownDownDownDownDownDownDown | 47 | 96 | Serum | RNA-sequencing |
| 35562921 | Eyileten, C. | 2022 | Poland | hsa-miR-19a-3phsa-let-7f-5p | UpDown | 28 | 35 | Plasma | qRT-PCR |
Differential expressed miRNAs from meta-analysis
One hundred and twelve unique miRNAs were included initially by these studies. After standardization, a list of 96 unique miRNAs was generated for this meta-analysis (Table 1). Twenty-three of these miRNAs were suggested by more than 1 study, of which 15 were qualified for performing meta-analysis as their differential expression states were derived from the same blood elements (Table 2). Twelve miRNAs were identified as significantly dysregulated by the meta-analysis with p < 0.05. Eight of them were upregulated (hsa-let-7e-5p, hsa-miR-17-5p, hsa-miR-185-5p, hsa-miR-218-5p, hsa-miR-222-3p, hsa-miR-451a, hsa-miR-487b-3p, hsa-miR-9-5p) while 4 were downregulated (hsa-miR-124-3p, hsa-miR-126-3p, hsa-miR-130a-3p, hsa-miR-221-3p).
Tissue-specific expression levels of 12 miRNAs
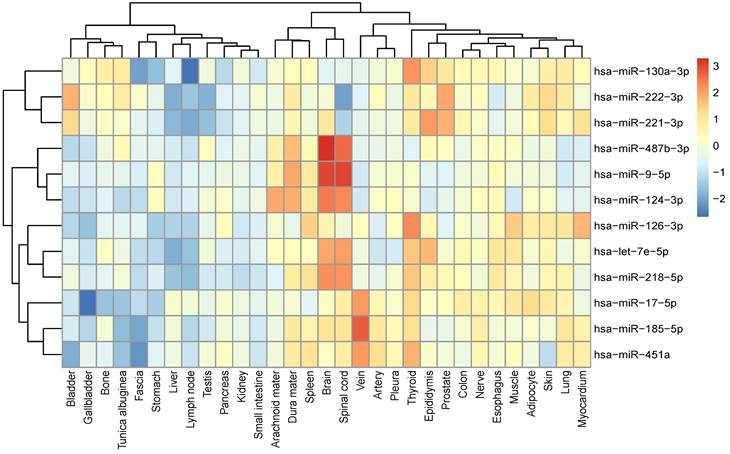
To investigate the relationship between tissue specificity and IS, the expression levels of 12 identified miRNAs in multiple tissues were studied. hsa-miR-487b-3p, hsa-miR-9-5p and hsa-miR-124-3p are specifically expressed in the Central Nervous System (CNS) tissues including arachnoid mater, brain, dura mater and spinal cord, while hsa-let-7e-5p and hsa-miR-218-5p are also highly expressed in the CNS tissues. hsa-miR-17-5p, hsa-miR-185-5p and hsa-miR-451a, on the other hand, are found to be highly expressed in veins (Figure 2).
Tissue-specific expression of 12 miRNAs. Relative expression of each validated miRNA in each tissue was plotted using z-scores.
Meta-analysis results for qualified miRNAs.
| miRNAs | Study | Specimen | τ^2 | I^2 | Weight | P-value | LogOR | 95% CI |
|---|---|---|---|---|---|---|---|---|
| hsa-let-7e-5p | Huang, S., 2016Yoo, H., 2019 | Whole blood | 17.89 | 81.37% | 50.20%49.80% | 4.13E-03 | 9.51 | [3.01, 16.01] |
| hsa-miR-124-3p | Liu, Y., 2015Zeng, Y., 2015 | Serum | 0 | 0.00% | 50.43%49.57% | 3.46E-06 | -6.69 | [-9.51, -3.86] |
| hsa-miR-126-3p | Long, G., 2013Jin, F., 2017Jin, F., 2018Geng, W., 2019 | Plasma | 0.09 | 2.14% | 25.13%25.17%25.20%24.50% | 1.75E-22 | -9.92 | [-11.91, -7.93] |
| hsa-miR-130a-3p | Jin, F., 2017Jin, F., 2018 | Plasma | 0 | 0.00% | 49.97%50.03% | 5.51E-15 | -11.07 | [-13.85, -8.30] |
| hsa-miR-144-3p | Tan, K. S., 2009Yoo, H., 2019 | Whole blood | 57.53 | 94.20% | 49.44%50.56% | 9.31E-01 | 0.47 | [-10.36, 11.30] |
| hsa-miR-17-5p | Wu, J., 2015van Kralinge, J. C., 2019 | Serum | 0 | 0.00% | 50.11%49.89% | 2.90E-13 | 10.36 | [7.58, 13.14] |
| hsa-miR-185-5p | Jin, F., 2017Jin, F., 2018 | Plasma | 0 | 0.00% | 49.97%50.03% | 5.51E-15 | 11.07 | [8.30, 13.85] |
| hsa-miR-218-5p | Jin, F., 2017Jin, F., 2018 | Plasma | 0 | 0.00% | 49.97%50.03% | 5.51E-15 | 11.07 | [8.30, 13.85] |
| hsa-miR-22-3p | Zeng, Y., 2015Vijayan, M., 2018 | Serum | 86.41 | 95.41% | 49.98%50.02% | 9.24E-01 | -0.64 | [-13.83, 12.55] |
| hsa-miR-221-3p | Jia, L., 2015Wang, Y., 2017 | Serum | 0 | 0.00% | 50.14%49.86% | 1.01E-12 | -10.12 | [-12.90, -7.34] |
| hsa-miR-222-3p | Jin, F., 2017Jin, F., 2018 | Plasma | 0 | 0.00% | 49.97%50.03% | 5.51E-15 | 11.07 | [8.30, 13.85] |
| hsa-miR-23a-3p | Jia, L., 2015Zeng, Y., 2015Vijayan, M., 2018 | Serum | 76.5 | 94.90% | 33.37%33.30%33.33% | 4.31E-01 | -4.08 | [-14.24, 6.08] |
| hsa-miR-451a | Li, P., 2015Giordano, M., 2019 | Serum | 1.19 | 22.67% | 50.39%49.61% | 3.24E-08 | 8.96 | [5.78, 12.13] |
| hsa-miR-487b-3p | Jickling, G. C., 2014Bam, M., 2018 | PBMCs | 0 | 0.00% | 50.11%49.89% | 1.15E-07 | 7.58 | [4.78, 10.38] |
| hsa-miR-9-5p | Liu, Y., 2015Zeng, Y., 2015 | Serum | 0 | 0.00% | 50.43%49.57% | 3.46E-06 | 6.69 | [3.86, 9.51] |
Relative expression levels of 12 miRNAs obtained from plasma from IS patients (Stroke) and healthy individuals (Control). The relative expression levels of the miRNAs are presented as ΔCt relative to the external references including cel-miR-39, 54 and 238. Fold change was calculated relative to that of healthy individuals' group. *: p < 0.05.
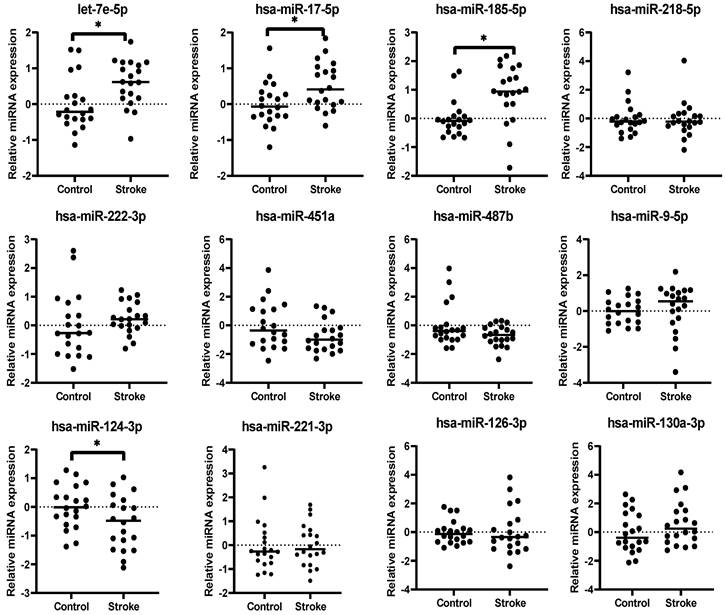
Mean relative expression levels of 12 miRNAs in plasma of IS patients (n=20) and healthy individuals (n=20).
| miRNA | Stroke | Healthy | delta delta CT | Fold-change | p-value |
|---|---|---|---|---|---|
| hsa-let-7e-5p | 0.582 ± 0.143 | -5e-04 ± 0.164 | 0.58 | 1.50 | 0.0108 |
| hsa-miR-17-5p | 0.509 ± 0.147 | 0.0025 ± 0.135 | 0.51 | 1.42 | 0.0153 |
| hsa-miR-185 | 0.87 ± 0.221 | -0.004 ± 0.139 | 0.87 | 1.83 | 0.0019 |
| hsa-miR-218 | -0.0685 ± 0.273 | 0.002 ± 0.244 | -0.07 | 0.95 | 0.8484 |
| hsa-miR-222 | 0.244 ± 0.123 | -5e-04 ± 0.247 | 0.24 | 1.18 | 0.3809 |
| has-miR-451a | -0.756 ± 0.243 | -0.0025 ± 0.359 | -0.76 | 0.59 | 0.0897 |
| hsa-miR-487b | -0.693 ± 0.157 | 0.001 ± 0.332 | -0.69 | 0.62 | 0.0664 |
| hsa-miR-9-5p | 0.138 ± 0.301 | -5e-04 ± 0.163 | 0.14 | 1.10 | 0.6881 |
| hsa-miR-124-3p | -0.552 ± 0.207 | 0.0035 ± 0.168 | -0.55 | 0.68 | 0.0441 |
| hsa-miR-221-3p | 0.0055 ± 0.197 | 0.0035 ± 0.248 | 0.01 | 1.00 | 0.995 |
| hsa-miR-126-3p | 0.064 ± 0.356 | 0.0025 ± 0.185 | 0.06 | 1.05 | 0.8789 |
| hsa-miR-130a-3p | 0.592 ± 0.34 | -0.001 ± 0.316 | 0.59 | 1.51 | 0.2093 |
Validation of miRNAs in plasma of IS patients using qRT-PCR
The 12 miRNAs identified from meta-analysis were subjected to qRT-PCR validation using plasma samples. Relative expression levels of each miRNA were measured in both IS (n=20) and healthy (n=20) samples (Figure 3). The expression levels of 4 miRNAs were significantly dysregulated in IS patients when compared to the healthy controls, with 3 upregulated (hsa-let-7e-5p, Fold-change[FC] = 1.50, p = 0.011; hsa-miR-17-5p, FC = 1.42, p = 0.015; hsa-miR-185-5p, FC = 1.83, p = 0.0019) and 1 downregulated (hsa-miR-124-3p, FC = 0.68, p = 0.044) (Table 3). The dysregulation states of these miRNAs were in the same directions as obtained from the results of meta-analysis, further suggesting that these 4 miRNAs could be potential biomarkers for IS patients.
Biological significance of validated miRNAs
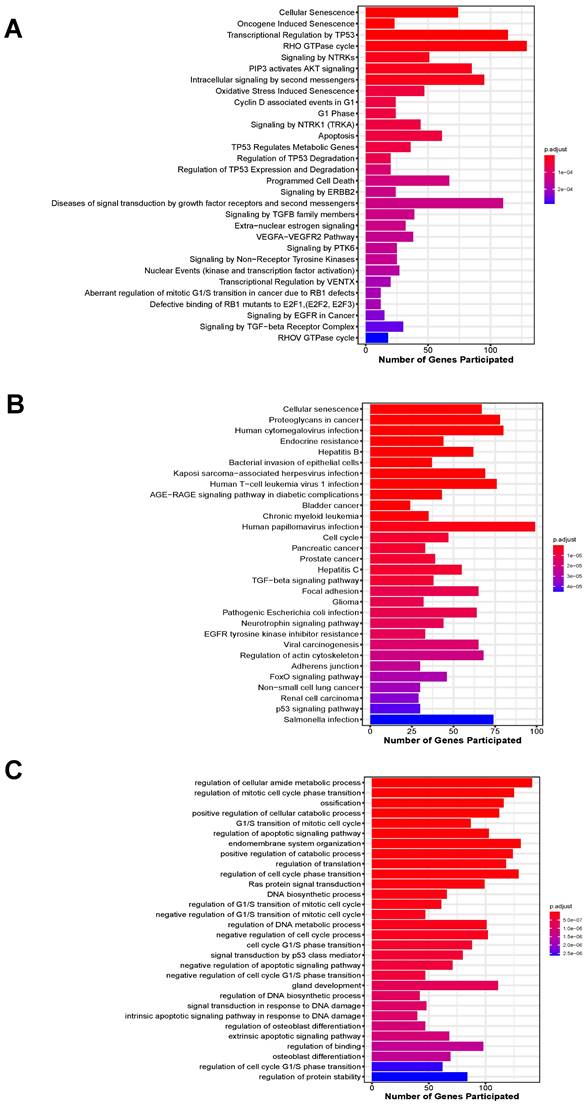
In total, 3201 unique target genes were identified from the 4 validated miRNAs (Suppl. Table S2). Pathway enrichment analysis identified 310 and 103 significantly enriched Reactome and KEGG pathways, respectively (Suppl. Table S3-S4). For enriched Reactome pathways, many of them are involved in the response to the depletion of oxygen and glucose caused by IS, including the responses to stress, pathways involved in cellular senescence, regulation of cell-cycle progression, VEGF signaling and AKT signaling (Figure 4A). Other pathways related to neuroinflammatory responses were also implicated, such as Forkhead box protein (FoxO)-mediated transcription and Toll-like receptor (TLR) cascades. These pathways are involved in apoptosis, which was also enriched, as well as mediating cell proliferation and angiogenesis to compensate for the lack of blood supply to vital organs. For KEGG-enriched pathways, similar observations were also obtained, with the addition of other cancer-associated and immune system-related pathways (Figure 4B).
Moreover, a wide range of biological processes were enriched by the target genes for the 4 miRNAs (Suppl. Table S5). Target genes of all the 4 miRNAs were implicated in the top 10 significantly enriched biological processes, including regulation of cellular metabolic and catabolic processes, ossification, cell cycle progression, apoptosis, endomembrane system organization and translation (Figure 4C).
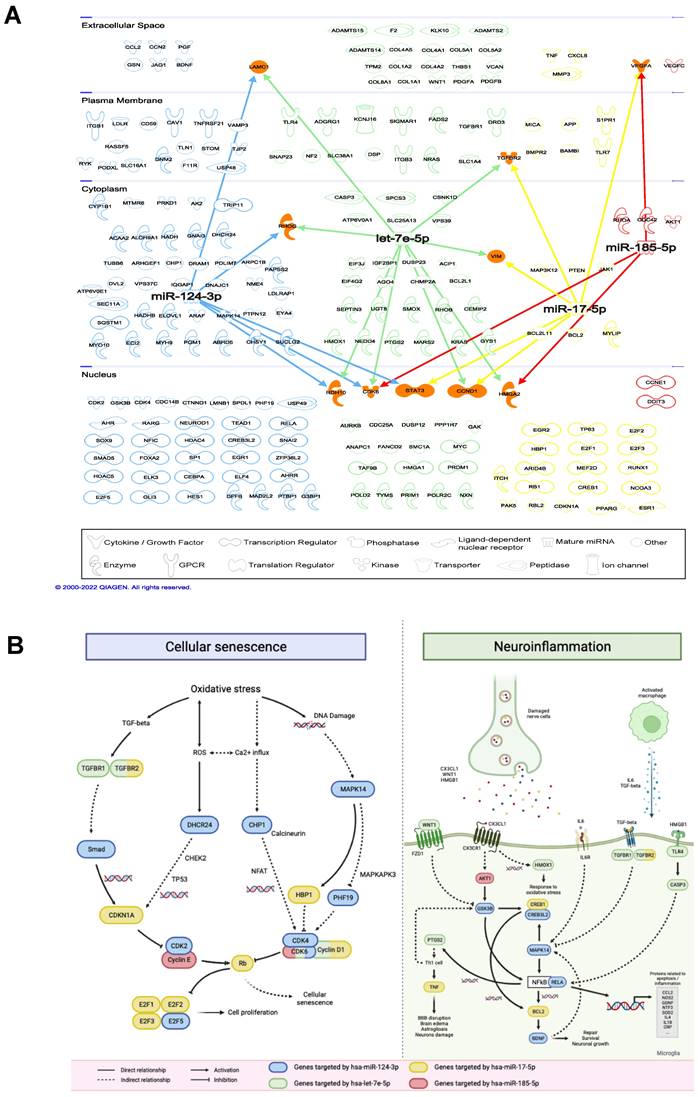
IPA analysis showed that 221 mRNAs known to be implicated in inflammatory response, cardiovascular or neurological diseases were targeted by the 4 miRNAs (Figure 5A, Suppl. Table S6). It was found that different mRNAs were commonly targeted by multiple miRNAs, including cardiac growth- and cell cycle-relevant genes [24, 25]. For all 221 mRNAs, IS-related pathways were identified, including cellular senescence and neuroinflammation (Figure 5B). Target genes of the 4 miRNAs are involved in multiple steps in the two illustrated pathways. Genes that play important roles from activating and transducing the signaling cascades to causing cellular senescence directly are targeted by these miRNAs. In particular, TGFBR2 is targeted by hsa-let-7e-5p and hsa-miR-17-5p that causes the transcription of CDKN1A, CDK6 is targeted by hsa-let-7e-5p, hsa-miR-124-3p and hsa-miR-185-5p which inhibits the retinoblastoma protein (pRB). In neuroinflammation, multiple signaling cascades are activated by the binding of ligands released by the damaged nerve cells and macrophages to the receptors expressed on microglia. Particularly, hsa-miR-124-3p targets multiple important genes in neuroinflammation, including GSK3B, CREB3L2, MAPK14, RELA and BDNF. These genes interact with each other directly or indirectly to mediate the production of pro- or anti-inflammatory proteins that cause further neuronal damage or neuronal growth and repair.
Diagnostic value of 4 validated miRNAs
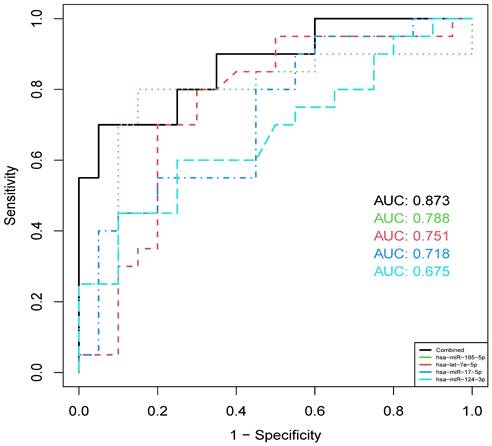
To investigate the diagnostic value of identified miRNAs, cross-validation ROC curve analysis was performed on each of the 4 validated miRNAs in the plasma of all 40 samples collected from the hospital. The analysis indicated that hsa-miR-185-5p showed highest diagnostic value (AUC = 0.788), followed by hsa-let-7e-5p (AUC = 0.751), hsa-miR-17-5p (AUC = 0.718) and hsa-miR-124-3p (AUC = 0.675). When combining the 4 miRNAs, we found that the AUC was improved to 0.873 (Figure 6), suggesting the potential use of a panel of these circulating miRNAs in the diagnosis of IS.
Biological processes enriched by target genes of all the 4 miRNAs. FDR; False discovery rate.
| GO term | Biological Process | FDR |
|---|---|---|
| GO:0034248 | Regulation of cellular amide metabolic process | 7.75E-10 |
| GO:1901990 | Regulation of mitotic cell cycle phase transition | 1.16E-08 |
| GO:0001503 | Ossification | 1.50E-08 |
| GO:0031331 | Positive regulation of cellular catabolic process | 1.50E-08 |
| GO:0000082 | G1/S transition of mitotic cell cycle | 2.47E-08 |
| GO:2001233 | Regulation of apoptotic signaling pathway | 2.65E-08 |
| GO:0010256 | Endomembrane system organization | 3.49E-08 |
| GO:0009896 | Positive regulation of catabolic process | 3.86E-08 |
| GO:0006417 | Regulation of translation | 5.73E-08 |
| GO:1901987 | Regulation of cell cycle phase transition | 5.73E-08 |
Discussion
The development of circulating biomarkers for IS is urgently needed to aid in predicting the risk of disease onset and early diagnosis for better clinical outcomes. miRNAs have been identified from previous studies demonstrating the potential use of these biomarkers to diagnose patients with cancers by expression profiling [26-28]. In addition to diagnosis, miRNAs may also exhibit anti-tumour properties, as suggested by a study of miR-146b inhibiting cell growth of malignant glioma [29]. Studies have demonstrated the therapeutic potential of miRNAs by identifying the miRNA-mRNA interactions and gene regulatory networks. For example, high-throughput sequencing of RNAs isolated by crosslinking immunoprecipitation was used to identify the miRNA-target mRNA interaction sites [30]. CRISPR screening was used to identify the essential miRNA binding sites [31], while multiple in silico approaches could also be used to predict miRNA-target interactions [32].
Top 30 significantly enriched pathways by target genes of the 4 miRNAs ranked by their adjusted p-value. (A) Enriched Reactome pathways; (B) Enriched KEGG pathways; (C) Enriched biological processes.
Interactions between miRNAs and target mRNAs. (A) Network visualization of 4 validated miRNAs and their target mRNAs. (B) Cellular senescence (left) and neuroinflammation (right) pathways involving miRNAs and their target mRNAs.
ROC curves of 4 validated miRNAs and their combined effect in distinguishing IS and healthy individuals.
Furthermore, miRNA replacement therapy and inhibitory antimiRs have also been suggested to directly target the dysregulated miRNAs as therapeutics [33]. Moreover, the miRNA expression levels between disease subtypes could be distinguished, raising the potential of using miRNAs to discover personalized therapeutic targets [34]. In this study, meta-analysis was performed to identify the associations between circulating miRNAs expression levels and IS. Twelve miRNAs were identified from publicly available differential expression studies as potential biomarkers, of which 4 were validated using plasma samples obtained from IS patients. Previous studies have suggested that the time of samples collection could lead to variations in the expression of circulating miRNAs [35, 36]. In line with our validation results, the studies that provided the qualified miRNAs in our meta-analysis which collected the samples at 6, 48 and 72 hours did not yield significant differences in expression levels (i.e., hsa-miR-487b and hsa-miR-221-3p). This further suggests that when using circulating miRNAs as early diagnostic biomarkers, the time after stroke onset plays a key role in determining the accuracy and reliability of the diagnostic panel.
Following the events of ischemia, a series of cellular responses is activated. First, stress signals trigger neuroinflammatory responses, leading to the generation of reactive oxygen species and reactive nitrogen species which eventually causes neuronal cell death. Matrix metalloproteinase is also activated by stress signals, damaging the endothelial cells and causing dysfunction of the blood-brain barrier. Second, ion imbalance in brain cells due to reduced blood flow causes the release of glutamate and calcium ion influx. Consequently, degradative enzymes are activated, inducing dysfunction of mitochondria, cell and DNA damage, resulting in neuronal cell apoptosis [37]. The target genes for the miRNAs identified by this meta-analysis are implicated in multiple pathways and biological processes that correspond to these cellular responses to IS.
Neuroinflammation is known to be associated with IS due to the release of cytokines and chemokines by the damaged neuronal cells and immune cells [38, 39]. Microglia dysregulation has been associated with neuroinflammation induced by the binding of CX3CL1, IL-6, TGF-β and other cytokines [40-42]. Activated glial cells then initiate intracellular signaling cascades to trigger the release of both pro- and anti-inflammatory signals [43]. In this study, we identified several miRNAs in the circulation of IS patients that contribute to these mechanisms. First, binding of CX3CL1 to CX3CR1 activates the PI3K/GSK3β/NF-kβ signaling pathway which has been associated with neuroinflammation in multiple neurological disorders to regulate the release of pro- and anti-inflammatory cytokines [44]. We identified hsa-miR-185-5p and hsa-miR-124-3p that target AKT1 and GSK3B which both play a crucial role in this pathway. hsa-miR-124-3p also targets RELA, which is a subunit of the NFkB dimer that regulates the transcription of BDNF, where increased BDNF production promotes neuronal growth and survival [45]. This pathway has also been suggested to promote the resolution of neuroinflammation and increase tissue repair [46]. Our finding agrees with previous studies that down-regulation of hsa-miR-124-3p was observed in an Alzheimer's Disease (AD) model, contributing to neuroprotection via the regulation of PI3K/Akt/GSK3β pathway in neurological diseases to reduce the release of pro-inflammatory cytokines [47]. hsa-miR-185-5p was also reported to participate in the neuro-protective axes in response to brain injury and AD [48, 49]. Second, hsa-let-7e-5p targets WNT1 and multiple receptors that initiate the signaling cascades in neuroinflammation including TGFBR1/2 and TLR4. hsa-let-7e-5p also targets HMOX1 that is responsible for reducing oxidative stress and tissue damage [50, 51]. hsa-miR-17-5p targets TNF-α which is a pro-inflammatory cytokine known to be involved in neuroinflammation [52]. Similar to hsa-miR-124-3p and hsa-miR-185-5p, hsa-miR-17-5p was upregulated in both IS and AD patients [53]. Other studies suggested the relationship between miR-17-5p, SMAD7, and TNF-α, where overexpression of miR-17-5p negatively regulates the expression of SMAD7 that increases the release of TNF-α and other cytokines [53, 54]. Therefore, up-regulation of miR-17-5p in the circulation may be a potential biomarker to reflect the neuroinflammatory response post-IS.
Cellular senescence plays an important role in the response to IS. As a response to the stressful stimuli such as oxidative stress and DNA damage, cellular senescence leads to impaired cell replication. The association between cellular senescence and IS was recently suggested by a study observing the cellular senescence-associated secretory phenotype in mice and human with IS [55]. The relationship between the shortening of cell-cycle, especially G1-phase, and IS patients was also suggested as a result of increased stroke-induced neurogenesis. Our results are in agreement with previous studies. hsa-miR-17-5p which targets CDKN1A and pRB was identified in this study. It has been well known that both CDKN1A and pRB play a crucial role in inhibiting cell cycle progression and inducing senescence [56, 57]. Evidence has shown that another hsa-miR-17-5p's target, HBP1, contributes to premature cellular senescence by either activating p16 or repressing DNMT1, that indirectly inhibits the transcription of cyclin-D1 [58]. Cyclin-D1, encoded by CCND1, is targeted by both hsa-let-7e-5p and hsa-miR-17-5p. This protein is overexpressed in senescent cells due to its action of preventing the cells from entering S phase [59]. Agreeing with previous studies, the let-7 family members are highly implicated in cancers by acting as tumor suppressors and mediating cell cycle dynamics by regulating different checkpoints [60, 61].
Apart from the pathways regulating cell cycle progression, the VEGF-associated pathways implicated by the target genes of miRNAs regulating angiogenesis correspond to the literatures suggesting as one of the mainly affected pathways in post-IS patients [62]. Neurotrophin signaling pathway is responsible for the repair and regeneration of neuronal cells after ischemic or traumatic brain injury [63]. Moreover, FoxO signaling pathway was also implicated. Its roles in IS have also been suggested, including the induction of apoptosis, inflammation and affecting the blood-brain barrier [64].
Therefore, our results correlate with existing literature demonstrating the associations between specific pathways and IS. Our study identified 4 reliable associations between the miRNAs and IS, highlighting the potential of targeting them or their target genes for developing diagnostic or therapeutic tools.
Abbreviations
IS: ischemic stroke; miRNAs: microRNAs; PBMC: peripheral blood mononuclear cells; CSF: cerebrospinal fluid; OR: odds ratios; KEGG: Kyoto Encyclopedia of Genes and Genomes; GO: Gene Ontology; FDR: False Discovery Rate; IPA: Ingenuity Pathway Analysis; ROC: Receiver operating characteristics.
Supplementary Material
Supplementary tables.
Acknowledgements
This work was supported by Shenzhen-Hong Kong Innovation Circle Joint R&D Project (SGLH20180628161804465); Innovation and Technology Fund of the Hong Kong Government grants (ITF-GHX-010-18SZ) to CUHK; Shenzhen Second People's Hospital Clinical Research Project (20203357035); Special Funds for Science Technology Innovation and Industrial Development of Shenzhen Dapeng New District (KJYF202001-21).
Author contributions
YW and XS: conceptualization, data curation, and writing-original draft. GHL, BR, QX and JZ: investigation, resources, and validation. QZ, and LY: investigation and resources. GL, WC and LR: supervision and writing-review and editing. All authors contributed to the article and approved the submitted version.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Collaborators GBDS. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795-820
2. Chalela JA, Kidwell CS, Nentwich LM, Luby M, Butman JA, Demchuk AM. et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293-8
3. Siracusa C, Sabatino J, Leo I, Eyileten C, Postula M, De Rosa S. Circular RNAs in Ischemic Stroke: Biological Role and Experimental Models. Biomolecules. 2023 13
4. Condrat CE, Thompson DC, Barbu MG, Bugnar OL, Boboc A, Cretoiu D. et al. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells. 2020 9
5. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215-33
6. Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12:99-110
7. Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL. et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513-8
8. Powers CJ, Dickerson R, Zhang SW, Rink C, Roy S, Sen CK. Human cerebrospinal fluid microRNA: temporal changes following subarachnoid hemorrhage. Physiol Genomics. 2016;48:361-6
9. Zheng HW, Wang YL, Lin JX, Li N, Zhao XQ, Liu GF. et al. Circulating MicroRNAs as potential risk biomarkers for hematoma enlargement after intracerebral hemorrhage. CNS Neurosci Ther. 2012;18:1003-11
10. Iwuchukwu I, Nguyen D, Sulaiman W. MicroRNA Profile in Cerebrospinal Fluid and Plasma of Patients with Spontaneous Intracerebral Hemorrhage. CNS Neurosci Ther. 2016;22:1015-8
11. Wang W, Sun G, Zhang L, Shi L, Zeng Y. Circulating microRNAs as novel potential biomarkers for early diagnosis of acute stroke in humans. J Stroke Cerebrovasc Dis. 2014;23:2607-13
12. Mens MMJ, Heshmatollah A, Fani L, Ikram MA, Ikram MK, Ghanbari M. Circulatory MicroRNAs as Potential Biomarkers for Stroke Risk: The Rotterdam Study. Stroke. 2021;52:945-53
13. Sonoda T, Matsuzaki J, Yamamoto Y, Sakurai T, Aoki Y, Takizawa S. et al. Serum MicroRNA-Based Risk Prediction for Stroke. Stroke. 2019;50:1510-8
14. Burlacu CC, Ciobanu D, Badulescu AV, Chelaru VF, Mitre AO, Capitanescu B. et al. Circulating MicroRNAs and Extracellular Vesicle-Derived MicroRNAs as Predictors of Functional Recovery in Ischemic Stroke Patients: A Systematic Review and Meta-Analysis. Int J Mol Sci. 2022 24
15. Haunsberger SJ, Connolly NM, Prehn JH. miRNAmeConverter: an R/bioconductor package for translating mature miRNA names to different miRBase versions. Bioinformatics. 2017;33:592-3
16. Kozomara A, Birgaoanu M, Griffiths-Jones S. miRBase: from microRNA sequences to function. Nucleic Acids Res. 2019;47:D155-D62
17. Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. Journal of Statistical Software. 2010;36:1-48
18. Ludwig N, Leidinger P, Becker K, Backes C, Fehlmann T, Pallasch C. et al. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016;44:3865-77
19. Su XW, Chan AH, Lu G, Lin M, Sze J, Zhou JY. et al. Circulating microRNA 132-3p and 324-3p Profiles in Patients after Acute Aneurysmal Subarachnoid Hemorrhage. PLoS One. 2015;10:e0144724
20. Ru Y, Kechris KJ, Tabakoff B, Hoffman P, Radcliffe RA, Bowler R. et al. The multiMiR R package and database: integration of microRNA-target interactions along with their disease and drug associations. Nucleic Acids Res. 2014;42:e133
21. Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27-30
22. Griss J, Viteri G, Sidiropoulos K, Nguyen V, Fabregat A, Hermjakob H. ReactomeGSA - Efficient Multi-Omics Comparative Pathway Analysis. Mol Cell Proteomics. 2020;19:2115-25
23. Kramer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30:523-30
24. Kim YK, Yu J, Han TS, Park SY, Namkoong B, Kim DH. et al. Functional links between clustered microRNAs: suppression of cell-cycle inhibitors by microRNA clusters in gastric cancer. Nucleic Acids Res. 2009;37:1672-81
25. Liu X, Xiao J, Zhu H, Wei X, Platt C, Damilano F. et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015;21:584-95
26. Cui M, Wang H, Yao X, Zhang D, Xie Y, Cui R. et al. Circulating MicroRNAs in Cancer: Potential and Challenge. Front Genet. 2019;10:626
27. Ng EK, Chong WW, Jin H, Lam EK, Shin VY, Yu J. et al. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58:1375-81
28. Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D. et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834-8
29. Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O. et al. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335:201-4
30. Chi SW, Zang JB, Mele A, Darnell RB. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature. 2009;460:479-86
31. Yang B, McJunkin K. CRISPR screening strategies for microRNA target identification. FEBS J. 2020;287:2914-22
32. Arif KT, Okolicsanyi RK, Haupt LM, Griffiths LR. A combinatorial in silico approach for microRNA-target identification: Order out of chaos. Biochimie. 2021;187:121-30
33. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203-22
34. Kaduthanam S, Gade S, Meister M, Brase JC, Johannes M, Dienemann H. et al. Serum miR-142-3p is associated with early relapse in operable lung adenocarcinoma patients. Lung Cancer. 2013;80:223-7
35. Long G, Wang F, Li H, Yin Z, Sandip C, Lou Y. et al. Circulating miR-30a, miR-126 and let-7b as biomarker for ischemic stroke in humans. BMC Neurol. 2013;13:178
36. Giordano M, Ciarambino T, D'Amico M, Trotta MC, Di Sette AM, Marfella R. et al. Circulating MiRNA-195-5p and -451a in Transient and Acute Ischemic Stroke Patients in an Emergency Department. J Clin Med. 2019 8
37. Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF. Pathogenic mechanisms following ischemic stroke. Neurol Sci. 2017;38:1167-86
38. Jian Z, Liu R, Zhu X, Smerin D, Zhong Y, Gu L. et al. The Involvement and Therapy Target of Immune Cells After Ischemic Stroke. Front Immunol. 2019;10:2167
39. Kim JY, Park J, Chang JY, Kim SH, Lee JE. Inflammation after Ischemic Stroke: The Role of Leukocytes and Glial Cells. Exp Neurobiol. 2016;25:241-51
40. Lauro C, Chece G, Monaco L, Antonangeli F, Peruzzi G, Rinaldo S. et al. Fractalkine Modulates Microglia Metabolism in Brain Ischemia. Front Cell Neurosci. 2019;13:414
41. Suzumura A, Sawada M, Yamamoto H, Marunouchi T. Transforming growth factor-beta suppresses activation and proliferation of microglia in vitro. J Immunol. 1993;151:2150-8
42. Maysinger D, Zhang I. Nutritional and Nanotechnological Modulators of Microglia. Front Immunol. 2016;7:270
43. Balke EC, Healy WM, Ullah T. An assessment of efficient water heating options for an all-electric single family residence in a mixed-humid climate. Energy Build. 2016;133:371-80
44. Maixner DW, Weng HR. The Role of Glycogen Synthase Kinase 3 Beta in Neuroinflammation and Pain. J Pharm Pharmacol (Los Angel). 2013;1:001
45. Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci. 2015;11:1164-78
46. Cianciulli A, Porro C, Calvello R, Trotta T, Lofrumento DD, Panaro MA. Microglia Mediated Neuroinflammation: Focus on PI3K Modulation. Biomolecules. 2020 10
47. Kang Q, Xiang Y, Li D, Liang J, Zhang X, Zhou F. et al. MiR-124-3p attenuates hyperphosphorylation of Tau protein-induced apoptosis via caveolin-1-PI3K/Akt/GSK3beta pathway in N2a/APP695swe cells. Oncotarget. 2017;8:24314-26
48. Ding L, Yang X, Xia X, Li Y, Wang Y, Li C. et al. Exosomes Mediate APP Dysregulation via APP-miR-185-5p Axis. Front Cell Dev Biol. 2022;10:793388
49. Xiong LL, Xue LL, Du RL, Zhou HL, Tan YX, Ma Z. et al. Vi4-miR-185-5p-Igfbp3 Network Protects the Brain From Neonatal Hypoxic Ischemic Injury via Promoting Neuron Survival and Suppressing the Cell Apoptosis. Front Cell Dev Biol. 2020;8:529544
50. Syapin PJ. Regulation of haeme oxygenase-1 for treatment of neuroinflammation and brain disorders. Br J Pharmacol. 2008;155:623-40
51. Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci U S A. 1997;94:10925-30
52. Huang N, Li W, Wang X, Qi S. MicroRNA-17-5p aggravates lipopolysaccharide-induced injury in nasal epithelial cells by targeting Smad7. BMC Cell Biol. 2018;19:1
53. Estfanous S, Daily KP, Eltobgy M, Deems NP, Anne MNK, Krause K. et al. Elevated Expression of MiR-17 in Microglia of Alzheimer's Disease Patients Abrogates Autophagy-Mediated Amyloid-beta Degradation. Front Immunol. 2021;12:705581
54. Jia J, Feng X, Xu W, Yang S, Zhang Q, Liu X. et al. MiR-17-5p modulates osteoblastic differentiation and cell proliferation by targeting SMAD7 in non-traumatic osteonecrosis. Exp Mol Med. 2014;46:e107
55. Torres-Querol C, Torres P, Vidal N, Portero-Otin M, Arque G, Purroy F. Acute ischemic stroke triggers a cellular senescence-associated secretory phenotype. Sci Rep. 2021;11:15752
56. Lessard F, Igelmann S, Trahan C, Huot G, Saint-Germain E, Mignacca L. et al. Senescence-associated ribosome biogenesis defects contributes to cell cycle arrest through the Rb pathway. Nat Cell Biol. 2018;20:789-99
57. Kumari R, Jat P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front Cell Dev Biol. 2021;9:645593
58. Pan K, Chen Y, Roth M, Wang W, Wang S, Yee AS. et al. HBP1-mediated transcriptional regulation of DNA methyltransferase 1 and its impact on cell senescence. Mol Cell Biol. 2013;33:887-903
59. Yang K, Hitomi M, Stacey DW. Variations in cyclin D1 levels through the cell cycle determine the proliferative fate of a cell. Cell Div. 2006;1:32
60. Fairchild CLA, Cheema SK, Wong J, Hino K, Simo S, La Torre A. Let-7 regulates cell cycle dynamics in the developing cerebral cortex and retina. Sci Rep. 2019;9:15336
61. Su JL, Chen PS, Johansson G, Kuo ML. Function and regulation of let-7 family microRNAs. Microrna. 2012;1:34-9
62. Hatakeyama M, Ninomiya I, Kanazawa M. Angiogenesis and neuronal remodeling after ischemic stroke. Neural Regen Res. 2020;15:16-9
63. Houlton J, Abumaria N, Hinkley SFR, Clarkson AN. Therapeutic Potential of Neurotrophins for Repair After Brain Injury: A Helping Hand From Biomaterials. Front Neurosci. 2019;13:790
64. Guo S, Mangal R, Dandu C, Geng X, Ding Y. Role of Forkhead Box Protein O1 (FoxO1) in Stroke: A Literature Review. Aging Dis. 2022;13:521-33
Author contact
![]() Corresponding authors: Prof. Lijie Ren, Department of Neurology, The First Affiliated Hospital of Shenzhen University, Shenzhen Second People's Hospital, Shenzhen, China, 518000, Phone:86-755- 83366388; Email: rlj2020szu.edu.cn. Prof. Gang Lu, CUHK-SDU Joint Laboratory on Reproductive Genetics, School of Biomedical Sciences, The Chinese University of Hong Kong, Hong Kong SAR, China, Phone: 852-39435739, Email: lugangedu.hk.
Corresponding authors: Prof. Lijie Ren, Department of Neurology, The First Affiliated Hospital of Shenzhen University, Shenzhen Second People's Hospital, Shenzhen, China, 518000, Phone:86-755- 83366388; Email: rlj2020szu.edu.cn. Prof. Gang Lu, CUHK-SDU Joint Laboratory on Reproductive Genetics, School of Biomedical Sciences, The Chinese University of Hong Kong, Hong Kong SAR, China, Phone: 852-39435739, Email: lugangedu.hk.

 Global reach, higher impact
Global reach, higher impact