3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2023; 20(5):682-688. doi:10.7150/ijms.83075 This issue Cite
Research Paper
Dental Caries, Dental Erosion and Periodontal Disease in Children with Inflammatory Bowel Disease
1. Marmara University, Faculty of Dentistry, Department of Paediatric Dentistry, Istanbul, Turkey
2. University of Health Sciences, Sancaktepe Sehit Prof. Dr. Ilhan Varank Training and Research Hospital, Department of Pediatric Gastroenterology, Istanbul, Turkey
Received 2023-1-31; Accepted 2023-3-28; Published 2023-4-9
Abstract
Background: There is reportedly a higher prevalence of dental caries and periodontal disease in adults with inflammatory bowel disease (IBD) than in healthy adults. Similar data for children are lacking in the literature. We aimed to evaluate the prevalence of dental erosion, dental caries, and periodontal disease in children with IBD.
Methods: This was a cross-sectional comparative study. Using the established criteria of the World Health Organization, oral investigations and detailed questionnaires that covered nutritional habits were completed by the same pediatric dentist for 32 patients with IBD, aged 11 to 18 years (15.53 ± 2.00), and 32 healthy controls.
Results: The decayed, missing, and filled tooth index showed no significant difference between the groups (p = 0.072). The frequency of consumption of salad, lemon gum, candy and sweetened milk was significantly higher in the control group (p = 0.041, 0.012, 0.001, and 0.001, respectively) than in the IBD group. No dental erosion was observed in the IBD group. Oral mucosal history determined that 20/32 patients with IBD (62.5%) had at least one oral extraintestinal manifestation. Despite no significant differences in plaque scores between the two groups, the gingival evaluation showed a much higher mean value of gingival index scores in the IBD group than in the control group (p = 0.003).
Conclusion: Although the number of patients included in the study is small, we can conclude that oral extraintestinal manifestations and periodontal disease are more prevalent in paediatric patients with IBD than in healthy populations.
Keywords: oral health, permanent dentition, nutritional habits, oral extraintestinal manifestation, gingival index
Introduction
Many recent studies have explored the relationship between oral health and systemic diseases [1,2]. Inflammatory bowel disease (IBD) is a recurrent immune disease of unidentified etiology that effects many people globally and impairs quality of life [3,4]. The most common symptoms of IBD include abdominal pain, anemia, vomiting, and rectal bleeding [5], with the oral cavity displaying a variety of signs and symptoms of the disease. Between 48% and 80% of the paediatric IBD population reveal with oral extra-intestinal manifestations (EIMs) [4,6], which most frequently include joint disorders, skin diseases, and oral lesions such as aphthous ulcers and periodontal diseases [7].
The prevalence of IBD is rising in developed countries. In addition to its increasing general prevalence, the incidence of IBD is also rising in the paediatric population, affecting both genders [8,9]. Although data are lacking for undeveloped countries, the incidence of IBD is also increasing in the recently developed countries of Asia, the Middle East, and South America, where societies have become more westernized and children are being detected at earlier ages [10,11]. In Turkey, the reported incidence for ulcerative colitis is 2.6 per 100000, and for Crohn's disease, it is 1.4 per 100000 [12].
The etiology of IBD is multifactorial and includes the interaction of genetic, environmental, and microbial factors with immunological reactions [13,14]. Dental caries is a multifactorial disorder relating previous and current caries experience, nutrition, fluoride exposure, presence of cariogenic microorganisms, salivary status, socio-demographic impacts, food texture, high-sugar beverage consumption, and long-term oral medications with xerostomic potential. It is still known as the most prevalent chronic childhood disease in the world. A Turkish National Survey reported that 61% of children aged 12 presented with caries [15].
Dietary habits and nutritional influences are critical environmental factors in the etiology of IBD, dental erosion and caries. The Western diet is characterized by high levels of refined sugar and sugar-containing soft drinks: it has long been suspected of increasing the risk of both types of IBD which are the Crohn's disease and ulcerative colitis [16,17]. According to recent studies, frequent consumption of carbonated/soft drinks is the main nutritional factor related with dental erosion, whereas frequent consumption of yoghurt is a protective factor [18]. Gingivitis is related with biofilm and/or endogenous hormonal variations, medications, systemic disorders, and malnutrition [19]. Plaque-associated gingivitis is the most frequent form of periodontal disorder in children, whereas periodontitis is rare [2].
IBD and periodontal disease are autoimmune-based chronic infections, with different mucosal inflammatory reactions to derestricted local microbiota in a heritable condition, leading to tissue damage. More meta-analyses have found the same conclusion [20]. A large-scale national study by Lin et al. found that patients with periodontal disease had higher risk of subsequent ulcerative colitis than healthy population [21].
The prevalence of dental caries and periodontal disease is informed to be superior in adults with IBD than in healthy control subjects [22]. According to a recently published review by Nijakowski et al. [23], the possibility of oral health problems in patients with IBD cannot be definitely accepted because of the possible association of other factors such as nutrition, environment, and socio-demographics [23].
To the best of our knowledge, data on the incidence of dental erosion in children with IBD are lacking; caries and periodontal disease is only one article [24]. Our study aimed to assess the prevalence of dental erosion, dental caries, and the periodontal diseases in children with IBD and to compare them with the results from healthy population in a dental practice.
Materials and Methods
Study Population
Prior to this study, the minimum sample size was determined based on the study reported by Koutsochristou et al. as 32 with G*power 3.1.9.6 version by taking impact size 1,202, α = 0.05, power (1-β) = 0.90 at a confidence level of 95% [24].
This cross-sectional comparative study comprised 32 children (20 males, 12 females) aged 11 to 18 years (mean age:15.53 ± 2.00) with IBD in the clinical remission period: (the IBD group) who provided the inclusion criteria. The inclusion criteria were being in remission period, being in permanent dentition, not smoking, and consented the child and family to participate in the study. They were followed up in the Department of Paediatric Gastroenterology at the University of Health Sciences, Prof. Dr. Ilhan Varank Training and Research Hospital in Turkey, between June 2021 and June 2022. Thirty-two systemically healthy children (20 males, 12 females) aged 11 to 18 years (mean age: 15.25 ± 1.72) who applied to the Marmara University, Dental Faculty, Paediatric Dentistry clinic for any reason, oral examination were enrolled as the control group. Both the patients and controls came from the Anatolian side of Istanbul. Participants were excluded if they, or their families, refused contribution in this study, were at primary or mixed dentition stage, smoking, systemic disease and had orthodontic or periodontal treatment within the previous year.
All procedures performed in our study including human participants were in accordance with the Declaration of Helsinki. The study was permitted by the Ethics Committee of the University of Health Sciences, Umraniye Training and Research Hospital (Number of ethical liscense: B.10.1.TKH.4.34.H.GP.0.01/135). Written informed consent was taken from parents before participants were included in the study.
Clinical Examination
Only participants in the IBD group were assessed by a paediatric gastroenterologist. According to the IBD guidelines, we have evaluated the patients, if they are in clinical remission with Paediatric Ulcerative Colitis Activity Index (PUCAI) scoring for ulcerative colitis and Paediatric Crohn's Disease Activity Index (PCDAI) scoring for Crohn's Disease. Therefore, these scoring systems include clinical symptoms, physical examination findings and laboratory evaluation. A total score of less than 10 for PUCAI, less than 12.5 for PCDAI indicate the period of remission.
Medical history was obtained for children in both participant groups. Anthropometric measures (weight, height) were taken for both participant groups.
Questionnaires
According to the Oral Health Surveys: Basic Methods published by the World Health Organization (WHO) [25] and the Guideline on Caries-Risk Assessment and Management for Infants, Children, and Adolescents [26] a questionnaire containing thirty three questions was designed to elicit information for each participant regarding age, sex, height, weight, level of mothers' education, mother's age at birth, number of siblings, occupation of parents, smoking status of parents, monthly family income, detailed dietary habits, oral hygiene behaviors, mouth breathing, frequency and reason for dental visits, the year of diagnosis of IBD, types of medications used, and other factors. Questionnaire was administered and completed by dental examiner prior to intraoral examination.
Intraoral Examination
The intraoral examination assessed oral EIMs (including all soft tissues of the mouth); dental erosion; the decayed, missing, and filled tooth (DMFT) index, gingival index system, the plaque control record index (PCR), and the community periodontal index of treatment needs (CPITN). WHO criteria were used to determine DMFT scores [16]. The mean DMFT value for each patient in both groups was calculated.
Dental erosion status was defined with the basic erosive wear examination index. This index is based on the clinical features of the different grades of erosive wear (score 0, no erosion; score 1, initial loss of hard tissue; score 2, distinctive defect, hard tissue loss < 50%; and score 3, hard tissue loss ≥ 50%) [27]. The gingival condition of all participants was also scored according to the gingival index system, which is according to the clinical features of the different grades of gingival inflammation (0, no inflammation; 1, mild inflammation; 2, moderate inflammation-moderate redness, edema and bleeding on pressure; 3, severe inflammation with propensity to spontaneous bleeding and ulceration) [28].
Oral hygiene status was stated using the plaque control record index to evaluate the presence or absence of marginal plaques [29]. Periodontal disease was described using the CPITN after dividing the oral cavity into sextants. For individuals younger than 20 years, only six index teeth are specified. All teeth of each patient were scanned, and the highest score per sextant was noted; with the following range: 0, healthy; 1, bleeding; 2, calculus; 3, probing pocket depth ≥ 4 mm but <6 mm; and 4, probing pocket depth ≥ 6 m [30].
All intraoral examinations were performed by the same clinical experienced paediatric dentist (E.H.). Before initiation of this study, an examiner calibrated this dentist for reliability through two repeated assessments of the same 10 participants in a dental practice in 2-day intervals to decrease intra-examiner error and provide measurements within less than 1 mm of error (gold standard). The kappa scores were 0.82 and 0.88.
Statistical Analysis
Statistical analysis was conducted using IBM SPSS Statistics for Windows Version 22.0 software (IBM Corp., Armonk, NY). Results were contemplated significant at the 5% significance level (p < 0.05).
The conformity of the numerical variables to a normal allocation was tested with the Shapiro-Wilk test. Student's t-test was used to compare normally distributed numerical variables in the two groups, the Mann-Whitney U test was used to compare non-normally distributed numerical variables between the two groups, and the interrelationships between categorical variables were tested using the chi-square test [31].
Results
Study Population
Sixty-four participants aged 11-18 years were comprised in this study, 32 of whom had IBD (Crohn's disease: 11; ulcerative colitis: 21); 32 served as controls.
All participants with IBD included in the study were in remission (Paediatric Ulcerative Colitis Activity Index < 10, Paediatric Crohn's Disease Activity Index < 12.5). Seven patients were treated with mesalazine, one with azathioprine, 15 with mesalazine and azathioprine, four with infliximab, four with mesalazine, azathioprine, and infliximab, and one with azathioprine and infliximab.
The demographic features of the study participants are presented in Table 1A and Table 1B. Compared to the control group, patients in the IBD group showed a significantly lower level of mother's education and a higher number of siblings (p = 0.008 and p = 0.001, respectively).
The nutritional and oral hygiene routines, mouth breathing, frequency of dental visits, and professional topical fluoride application status are presented in Table 2. With regard to nutritional habits, the frequency of consumption of salad, lemon gum, candy, and sweetened milk was significantly higher in the control group (p = 0.041, 0.012, 0.001, and 0.001, respectively) than in the IBD group.
Demographic characteristics of IBD patients and controls
| Patients with IBD | Controls | |||
|---|---|---|---|---|
| n (%) | n (%) | P | ||
| Gender | Male | 20(62.5) | 20(62.5) | 1 |
| Female | 12(37.5) | 12(37.5) | ||
| Mother's education | No school | 1(3.1) | 3(9.4) | 0.008* |
| Primary | 22(68.8) | 9(28.1) | ||
| Secondary | 2(6.3) | 6(18,8) | ||
| High | 6(18.8) | 6(18.8) | ||
| University | 1(3.1) | 8(25) | ||
| Does mother work? | Yes | 6(18.8) | 10(31.3) | 0.248 |
| No | 26(81.3) | 22(68.8) | ||
| Does mother smoking? | Smoking | 4(12.5) | 6(18.8) | 0.491 |
| Non-smoking | 28(87.5) | 26(81.3) | ||
| Does father smoking? | Smoking | 13(40.6) | 15(46.9) | 0.614 |
| Non-smoking | 19(59,4) | 17(53.1) | ||
Monthly family income | <subsistence level | 8(25.0) | 7(21.9) | 0.915 |
| subsistence level | 20(62.5) | 20(62.5) | ||
| >subsistence level | 4(12.5) | 5(15.6) | ||
| Birth time | Term | 30(93.8) | 31(96.9) | |
| Preterm | 2(6.3) | 1(3.1) | ||
*Chi-square tests
Dental Caries and Dental Erosion Assessment
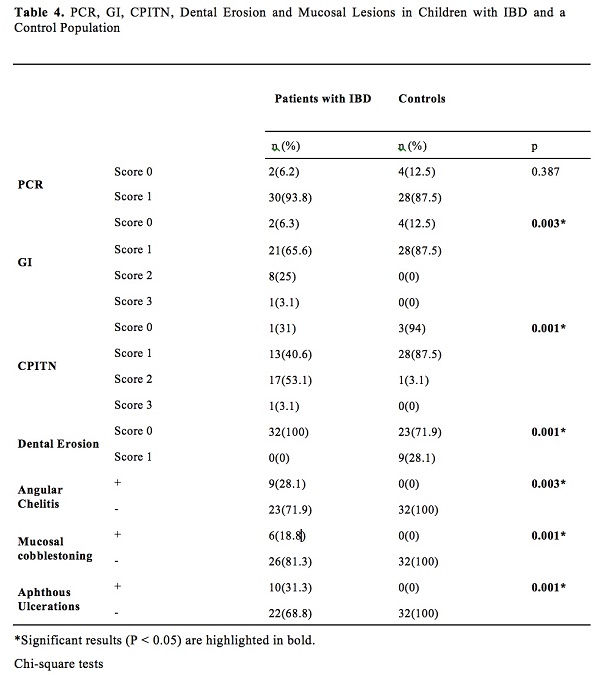
The DMFT index scores are presented in Table 3. Although not significant, the decayed tooth scores were higher in the IBD group (p=0.072, Table 3) than in the control group. Intraoral examinations revealed that 9 of 32 children in the control group (28.1%) had erosive tooth wear, but those in the IBD group had no erosive tooth wear (Table 4).
Oral Mucosal Lesion Assessment
Oral mucosal history determined that 20 of the 32 patients with IBD (62.5%) had at least one EIM. The most common oral lesions were aphthous ulcerations (10 patients, 31.3%), angular cheilitis (9 patients, 28.1%), and mucosal cobblestoning (6 patients, 18.8%). The children in the control group had no oral mucosal lesions on examination (Table 4).
Periodontal Disease Assessment
Although gingivitis index scores were significantly higher (p=0.003, Table 4) in patients with IBD, there was no statistically significant difference between plaque control record scores in participants with IBD and the control group (Table 4).
More than half (53.1%) the patients in the IBD group had gingivitis with calculus (CPITN score: 2), whereas 40.6% had only gingival bleeding (CPITN score: 1), 3.1% (one patient) had probing pocket depth values between 4 and 6 mm (CPITN score: 3), and 3.1% had a healthy periodontium (p=0.001, Table 4).
Discussion
This study evaluated the status of dental caries, dental erosion, dietary and oral hygiene habits, gingival status, and periodontal treatment needs in children with IBD. To our knowledge, this is the first study on dental erosion and second study on oral and periodontal health in children with IBD.
Various studies have reported a significantly higher incidence of dental caries in adults with IBD [32-34]. Koutsochristou et al [24] studied 55 participants with IBD aged 4-18 years and found that dental caries indices were statistically higher in patients with IBD than in healthy control groups. In contrast, in this study DMFT index scores were higher in patients with IBD than in controls, but the difference was not significant (p=0.072). Unlike other studies, we investigated a number of caries risk factors together. We included only participants who were at the permanent dentition stage of development, in contrast to Koutsochristou et al [24] When primary dentition changes to permanent dentition, there is an increase in the diversity of the oral microbiota and a specific set of microorganisms living in the body develops [35]. Similar to our study, study by Grössner-Shreiber et al. [36] reported no significant differences in the prevalence of caries between groups.
Demographic characteristics of IBD patients and controls (continued)
| Patients with IBD (n=32) | Controls (n=32) | ||||
|---|---|---|---|---|---|
| Mean ± SD | Median (%25-%75) | Mean ± SD | Median (%25-%75) | P | |
| Age | 15.53±2.00 | 16.00(14.00-1700) | 15.25±1.72 | 15.00(14.00-17.00) | 0.389ỻ |
| Height (cm) | 165.25±11.33 | 166.00(160.00-171.00) | 162.94±12.19 | 161.50(155.00-172.00) | 0.270ỻ |
| Weight (kg) | 58.03±15.19 | 55.50(49.00-67.00) | 53.09±10.46 | 50.00(46.50-60.00) | 0.135† |
| BMI | 20.95±3.90 | 20.68(18.20-23.18) | 19.80±1.53 | 19.91(18.45-20.85) | 0.129† |
| Time of diagnosis (months ago) | 19.00±15,13 | 14.00(9.00-25.00) | - | - | - |
| Mother's age at birth | 27.16±8.10 | 25.50(20.00-35.00) | 26.22±4.49 | 27.00(23.50-28.50) | 0.925 ỻ |
| Number of siblings | 3.19±1.03 | 3.00(3.00-4.00) | 2.31±0.69 | 2.00(2.00-3.00) | 0.001* ỻ |
†Student t test
ỻ Mann Whitney U test
Comparison Between Children with IBD and Control Population for Dietary and Oral Hygiene Habits, Mouth Breathing, Frequency of Dental Visits and Professional Topical Fluoride Application
| Patients with IBD | Controls | ||||
|---|---|---|---|---|---|
| n (%) | n (%) | P | |||
| Salad | Seldom/Never | 6(18.8) | 4(12.5) | 0.041* | |
| Twice per week | 12(37.5) | 11(34.4) | |||
| Once per day | 12(37.5) | 11(34.4) | |||
| Twice per day | 0 (0) | 6(18.8) | |||
| More than twice per day | 1(3.1) | 0(0) | |||
| More frequent | 1(3.1) | 0(0) | |||
| Lemon gum | Seldom/Never | 26(81.3) | 16(50) | 0.012* | |
| Twice per week | 5(15.6) | 14(43.8) | |||
| Once per day | 0(0) | 2(6.3) | |||
| Twice per day | 1(3.1) | 0(0) | |||
| Candy | Seldom/Never | 29(90.6) | 13(40.6) | 0.001* | |
| Twice per week | 2(6.3) | 15(46.9) | |||
| Once per day | 0(0) | 3(9.4) | |||
| Twice per day | 0(0) | 1(3.1) | |||
| More than twice per day | 1(3.1) | 0(0) | |||
| Sweetened milk | Seldom/Never | 23(71.9) | 7(21.9) | 0.001* | |
| Twice per week | 6(18.8) | 12(37.5) | |||
| Once per day | 2(6.3) | 9(28.1) | |||
| Twice per day | 1(3.1) | 3(9.4) | |||
| More frequent | 0(0) | 1(3.1) | |||
| Orange juice | Seldom/Never | 24 (75) | 15(46.9) | 0.079 | |
| Twice per week | 6(18.8) | 14(43.8) | |||
| Once per day | 2(6.3) | 2(6.3) | |||
| Twice per day | 0(0) | 1(3.1) | |||
| Carbonated soft drink | Seldom/Never | 18(56.3) | 19(59.4) | 0.113 | |
| Twice per week | 13(40.6) | 10(31.3) | |||
| Once per day | 1(3.1) | 0(0) | |||
| Twice per day | 0(0) | 3(9.4) | |||
| Snacks | Seldom/Never | 9(28.1) | 3 (9.4) | 0.011* | |
| Twice per week | 9(28.1) | 7(21.9) | |||
| Once per day | 4(12.5) | 13(40.6) | |||
| Twice per day | 7(21.9) | 2(6.3) | |||
| More frequent | 3(9.4) | 7(21.9) | |||
| Frequency of toothbrushing | Once per day | 9(28.1) | 9(28.1) | 0.957 | |
| Twice or more per day | 13(40.6) | 12(37.5) | |||
| Less than once per week | 10(31,3) | 11(34.4) | |||
| Type of toothbrush | Electric | 4(12.5) | 0(0) | 0.016* | |
| Manual | 28(87.5) | 32(100) | |||
| Toothpaste | With F | 32(100) | 26(81.3) | 0.003* | |
| Without F | 0(0) | 6(18.8) | |||
| Visit of dentist | First visit | 4(12.5) | 1(3.1) | 0.001* | |
| If necessary Regularly | 28(87.5) 0(0) | 23(71.9) 8(25) | |||
| Professional Topical Fluoride | Yes | 0(0) | 8(25) | 0.001* | |
| No | 32(100) | 24(75) | |||
| Mouth Breathing | Yes | 8(25) | 9(28,1) | 0.777 | |
| No | 24(75) | 23(71.9) | |||
*Significant results (P < 0.05) are highlighted in bold.
Chi-square tests
Decayed (D), Missing (M), and Filled (F) Tooth (DMF-T) Index in Children with IBD and a Control Population
| Patients with IBD (n=32) | Controls (n=32) | ||||
|---|---|---|---|---|---|
| Mean ± SD | Median (%25-%75) | Mean ± SD | Median (%25-%75) | p | |
| DT | 8.84±5.74 | 8.00(5.00-9.50) | 6.13±4.62 | 6.00(4.00-9.00) | 0.072 |
| MT | 0.28±0.68 | 0(0 -0) | 0.72±1.40 | 0(0 -1.00) | 0.281 |
| FT | 0.78±1.26 | 0(0 -1.50) | 1.41±1.58 | 1.0(0-2.50) | 0.108 |
| DMFT | 9.88±5.76 | 9.00(7.50-11.00) | 8.25±5.05 | 8.00(5.00-11.00) | 0.338 |
Mann Whitney U test
In our study, we determined that since all patients in the IBD group were in clinical remission, they consumed sugar and sugary food drinks very rarely to prevent disease flare-ups (as advised by the paediatric gastroenterologist). However, the frequency of consumption of sugar and sweetened milk was found to be statistically higher in our control group (p=0.001 and 0.001, respectively) than in the IBD group. Eating habits is one of the most critical risk factors for caries. It is possible that dental caries may not have been found to be significant between the groups due to patients in the IBD group having better eating habits.
PCR, GI, CPITN, Dental Erosion and Mucosal Lesions in Children with IBD and a Control Population
| Patients with IBD | Controls | |||
|---|---|---|---|---|
| n (%) | n (%) | p | ||
| PCR | Score 0 | 2(6.2) | 4(12.5) | 0.387 |
| Score 1 | 30(93.8) | 28(87.5) | ||
| GI | Score 0 | 2(6.3) | 4(12.5) | 0.003* |
| Score 1 | 21(65.6) | 28(87.5) | ||
| Score 2 | 8(25) | 0(0) | ||
| Score 3 | 1(3.1) | 0(0) | ||
| CPITN | Score 0 | 1(31) | 3(94) | 0.001* |
| Score 1 | 13(40.6) | 28(87.5) | ||
| Score 2 | 17(53.1) | 1(3.1) | ||
| Score 3 | 1(3.1) | 0(0) | ||
| Dental Erosion | Score 0 | 32(100) | 23(71.9) | 0.001* |
| Score 1 | 0(0) | 9(28.1) | ||
| Angular Chelitis | + | 9(28.1) | 0(0) | 0.003* |
| - | 23(71.9) | 32(100) | ||
| Mucosal cobblestoning | + | 6(18.8) | 0(0) | 0.001* |
| - | 26(81.3) | 32(100) | ||
| Aphthous Ulcerations | + | 10(31.3) | 0(0) | 0.001* |
| - | 22(68.8) | 32(100) | ||
*Significant results (P < 0.05) are highlighted in bold.
Chi-square tests
We did not detect dental erosion in children in the IBD group. However, 9 out of 32 children in the control group (28.1%) had erosive tooth wear, possibly as a result of their dietary habits, i.e. consuming significantly more acidic foods.
Although the absence of statistically differences in plaque index scores between the two groups in our study, the gingival evaluation showed that the mean gingival index scores in patients with IBD was much higher than that in the control group. This is in agreement with the results of the study by Koutsochristou et al. [24].
CPITN is mainly an evaluation index for the presence or absence of periodontal pockets, calculus, and gingival bleeding [21], and it requires clinical assessment. A little over half (53.1%) the patients in the IBD group had a CPITN score of 2, 3.1% had a score of 3, and 3.1% had a healthy periodontium. Among the participants in the control group, 3.1 % had a CPITN score of 2, and none had a CPITN score of 3. Although there was no disparity in oral hygiene habits between the two groups, a higher rate of periodontal disease was observed in the IBD group. This finding is similar to those of previous studies [6,32,35,37-39]. Poor oral hygiene is likely to have caused the high rate of bleeding on probing in the control group.
A recent study using the full-mouth clinical periodontal assessment in a large group of patients established a relationship between IBD severity and periodontal diseases [40]. According to Kitamoto et al. periodontal disease, aggravates gut inflammation by supplying the gut with both colitogenic pathobionts and pathogenic T cells [41]. Although the researchers considered variations in the host immune response, the role of periodontal microorganisms in the relationship between periodontal disease and IBD remains uncertain.
According to Shazib et al. [4], between 48% and 80% of paediatric population with IBD presented with oral extra-intestinal manifestations, which are often observed in patients with active intestinal diseases [4,42]. Although all participants with IBD in this study were in clinical remission and receiving medical treatment, 62.5% of them had at least one EIM: aphthous ulcerations were the most common (31.3%), followed by angular cheilitis (28.1%) and mucosal cobblestoning (18.8%). As none of these EIMs was too large, painful, or inconvenient for eating and drinking, no treatment was required. Participants in the control group had no intraoral soft tissue lesions. The mean duration of IBD was 19 months in this study.
Oral manifestations in paediatric IBD patients may be related to a longstanding or severe deficiency of specific nutrients or to a medication-induced reaction. Deficiencies in some vitamins can cause intraoral soft tissue lesions. These deficiencies may also be related to anemia. In one study, 15% of IBD patients were found to be zinc deficient [4]. The ability of zinc to modify the crystal growth pathways of calcium phosphates has been exploited to control calculus formation and reduce demineralization of the enamel [43]. In our study, gingivitis, angular chelitis and dental caries in patients with IBD may also be related to deficiency of some vitamins or minerals.
The primary strength of this study is that it is the first study to investigate dental erosion in children with IBD. Additionally, we explored eight categories of risk factors for caries, all at the same time. This is also the first study conducted only on paediatric patients with permanent dentition.
A limitation of this study is that patients in the active phase of IBD were not included. Further limitations were the relatively small sample size and lack of evaluation of salivary biomarkers such as pH, cariogenic microorganisms, calcium, phosphate, and zinc.
Conclusions
Oral EIMs and periodontal disease are more prevalent in paediatric patients with IBD than in healthy populations. Dentists should familiarize themselves with the probable oral manifestations of this disease. Regular intraoral examination may be included in the multidisciplinary management of paediatric patients with IBD, and the risk of dental caries can be reduced by practicing healthy nutritional habits and proper oral hygiene. Instead of evaluating the relationship between oral EIMs and IBD, future longitudinal large-scale studies are needed to determine whether oral EIMs are the cause or not. Preventive dentistry is needed to improve the quality of life of paediatric patients with IBD.
Acknowledgements
Language editing: We would like to thank Editage (www.editage.com) for English language editing.
Data analysis: We would like to thank Dr. Tanyeli Kazaz for data analysis.
Author contributions
- E. Haznedaroğlu and E. Polat conceived and designed the work.
- E. Haznedaroğlu performed the intraoral examinations.
- The acquisition and interpretation of data for the work were performed by E. Haznedaroğlu.
- The data were analyzed by a biostatistician.
- E. Haznedaroğlu drafted the work.
- E. Haznedaroğlu and E. Polat revised it critically for important intellectual content.
- Final approval of the version to be published were done by E. Haznedaroğlu and E. Polat.
E. Haznedaroğlu ve E. Polat are in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bui FQ, Almeida-da-Silva C, Huynh B. et al. Association between periodontal pathogens and systemic disease. Biomed J. 2019;42:27-35
2. Vagdouti T, Tsilingaridis G. Periodontal Diseases in Children and Adolescents Affected by Systemic Disorders - A Literature Review. Int J Oral Dent Health. 2018;4(1):1-10
3. Aydın O, Kabasakal Çetin A, Samur G. Pediatrik İnflamatuvar Bağırsak Hastalıklarında Güncel Beslenme Tedavisi Yaklaşımları. J Curr Pediatr. 2022;20:103-115
4. Shazib MA, Byrd KM, Gulati AS. Diagnosis and Management of Oral Extraintestinal Manifestations of Pediatric Inflammatory Bowel Disease. JPGN. 2022;74:7-12
5. Shivashankar R, Lichtenstein GR. Mimics of inflammatory bowel disease. Inflamm Bowel Dis. 2018;24(11):2315-21
6. Vavricka SR, Schoepfer A, Scharl M. et al. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(8):1982-92
7. She YY, Kong XB, Ge YP. et al. Periodontitis and inflammatory bowel disease: a meta-analysis. BMC Oral Health. 2020;20:1-11
8. Benchimol EI, Mack DR, Nguyen GC. et al. Incidence, outcomes, and health services burden of very early onset inflammatory bowel disease. Gastroenterology. 2014;147:803-13
9. Fatani B, Al-Safadi A. Inflammatory Bowel Disease and Oral Health: A Review of Dental Consideration. Saudi J Oral Dent Res. 2022;7(6):165-168
10. Rubalcava NS, Gadepalli SK. Inflammatory Bowel Disease in Children and Adolescents, Advances in Pediatrics 2021; 68: 121-142
11. Ng SC, Shi HY, Hamidi N. et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet (London, England). 2017;390:2769-78
12. Buran T. İnflamatuar Barsak Hastalığında Epidemiyoloji, Prevalans ve İnsidansı. Turkiye Klinikleri J Gastroenterohepatol- Special Topics. 2017;10:15-7
13. Rufo PA, Bousvaros A. Current Therapy of Inflammatory Bowel Disease in Children. Pediatr Drugs. 2006;8:279-302
14. Sood A, Ahuja V, Kedia S. et al. Diet and inflammatory bowel disease: The Asian Working Group Guidelines. Indian J Gastroenterol. 2019;38:220-46
15. Gokalp S, Dogan BG, Tekcicek M. et al. National survey of oral health status of children and adults in Turkey. Community Dent Health. 2010;27:12-17
16. Park S, Kang Y, Koh H. et al. Increasing incidence of inflammatory bowel disease in children and adolescents: significance of environmental factors. Clin Exp Pediatr. 2020;63:337-44
17. Racine A, Carbonnel F, Chan SS. et al. Dietary Patterns and Risk of Inflammatory Bowel Disease in Europe. Inflamm Bowel Dis. 2016;22:345-354
18. Carvalho TS, Lussi A. Chapter 9: Acidic Beverages and Foods Associated with Dental Erosion and Erosive Tooth Wear. Zohoori FV, Duckworth RM (eds): The Impact of Nutrition and Diet on Oral Health. Monogr Oral Sci Basel, Karger. 2020;28:91-98
19. Murakami S, Mealey BL, Mariotti A. Chapple ILC Dental plaque-induced gingival conditions. J Periodontol. 2018;89(Suppl 1):S17-S27
20. Lorenzo-Pouso AI, Castelo-Baz P, Rodriguez-Zorrilla S. et al. Association between periodontal disease and inflammatory bowel disease: a systematic review and meta-analysis. Acta Odontologica Scandinavica. 2021;79(5):344-353
21. Lin CY, Tseng KS, Liu JM. et al. Increased risk of ulcerative colitis in patients with periodontal disease: a nationwide population based cohort study. IJERPH. 2018;15(11):2602
22. Marruganti C, Discepoli N, Gaeta C. et al. Dental Caries Occurrence in Inflammatory Bowel Disease Patients: A Systematic Review and MetaAnalysis. Caries Research. 2021;55(5):485-495
23. Nijakowski K, Gruszczy´nski D, Surdacka A. Oral Health Status in Patients with Inflammatory Bowel Diseases: A Systematic Review. Int J Environ Res Public Health. 2021;18:115-21
24. Koutsochristou V, Zellos A, Dimakou K. et al. Dental caries and periodontal disease in children and adolescents with inflammatory bowel disease: a case-control study. Inflamm Bowel Dis. 2015;21:1839-46
25. Yeung CA. Oral Health Surveys: Basic Methods, 5th ed. London: British Dental Journal. 2014;42-56:111-114
26. American Academy of Pediatric Dentistry (AAPD). Guideline on caries-risk assessment and management for infants, children, and adolescents. Reference Manual. 2014;37:15-16
27. Bartlett D, Ganss C, Lussi A. Basic erosive wear examination (BEWE): a new scoring system for scientific and clinical needs. Clin Oral Investig. 2008;12(Suppl 1):S65-S8
28. Löe H. The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol. 1967 38(6)Suppl: S610-S6
29. O'Leary TJ, Drake RB, Naylor JE. The plaque control record. J Periodontol. 1972;43:38
30. Cutress TW, Ainamo J, Sardo-Infirri J. The community periodontal index of treatment needs (CPITN) procedure for population groups and individuals. International Dental Journal. 1987;37(4):222-233
31. Ali Z, Bhaskar SB. Basic statistical tools in research and data analysis. Indian J Anaesth. 2016;60(9):662-669
32. Brito F, de Barros FC, Zaltman C. et al. Prevalence of periodontitis and DMFT index in patients with Crohn's disease and ulcerative colitis. J Clin Periodontol. 2008;35(6):555-60
33. Rodrigues E, Laranjeira N, Nunes G. et al. Are cariogenic bacteria the major risk factor to dental caries in patients with ulcerative colitis? Arq Gastroenterol. 2019;56:118-23
34. Zhang L, Gao X, Zhou J. et al. Increased risks of dental caries and periodontal disease in Chinese patients with inflammatory bowel disease. Int Dent J. 2020;70(3):227-36
35. Xiao J, Fiscella KA, Gill SR. Oral microbiome: possible harbinger for children's health. Int J Oral Sci. 2020;12(1):1-13
36. Grössner-Schreiber B, Fetter T, Hedderich J. et al. Prevalence of Dental Caries and Periodontal Disease in Patients with Inflammatory Bowel Disease: A Case-Control Study. J Clin Periodontol. 2006;33:478-484
37. Ślebioda Z, Szponar E, Linke K. Comparative analysis of the oral cavity status in patients with Crohn's disease and ulcerative colitis. J Stomatol. 2011;64(3):382-93
38. Vavricka SR, Manser CN, Hediger S. et al. Periodontitis and gingivitis in inflammatory bowel disease: a case-control study. Inflamm Bowel Dis. 2013;19(13):2768-77
39. Tan CXW, Brand HS, Kalender B. et al. Dental and periodontal disease in patients with inflammatory bowel disease. Clinical Oral Investigations. 2021;25(9):5273-5280
40. Baima G, Muwalla M, Testa G. et al. Periodontitis prevalence and severity in inflammatory bowel disease: A case-control study. J Periodontol. 2022:1-10
41. Kitamoto S, Nagao-Kitamoto H, Jiao Y. et al. The intermucosal connection between the mouth and gut in commensal pathobiont-driven colitis. Cell. 2020;182:447-462
42. Vasovic M, Gajovic N, Brajkovic D. et al. The relationship between the immune system and oral manifestations of inflammatory bowel disease: A review. Cent Eur J Immunol. 2016;41:302-310
43. Mohammed NR, Mneimne M, Hill RG. et al. Physical chemical effects of zinc on in vitro enamel demineralization. J Dent. 2014;42:1096-104
Author contact
![]() Corresponding author: Marmara University, Faculty of Dentistry, Department of Paediatric Dentistry, Recep Tayyip Erdogan Complex Health Campus, Basibuyuk yolu 9/3 34854, Basibuyuk/Maltepe/ Istanbul. Tel: 00902167775088; Fax: 00902167775001; E-mail: ehaznedarogluedu.tr
Corresponding author: Marmara University, Faculty of Dentistry, Department of Paediatric Dentistry, Recep Tayyip Erdogan Complex Health Campus, Basibuyuk yolu 9/3 34854, Basibuyuk/Maltepe/ Istanbul. Tel: 00902167775088; Fax: 00902167775001; E-mail: ehaznedarogluedu.tr

 Global reach, higher impact
Global reach, higher impact