3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2023; 20(5):581-594. doi:10.7150/ijms.80920 This issue Cite
Research Paper
SIRT6 ameliorates LPS-induced apoptosis and tight junction injury in ARDS through the ERK1/2 pathway and autophagy
1. Department of Pulmonary and Critical Care Medicine, Zhongshan Hospital, Fudan University, Shanghai 200032, China
2. Department of Respiratory and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, Sichuan 610041, China
3. Department of Respiratory and Critical Care Medicine, Huadong Hospital, Fudan University, Shanghai 200040, China
Abstract
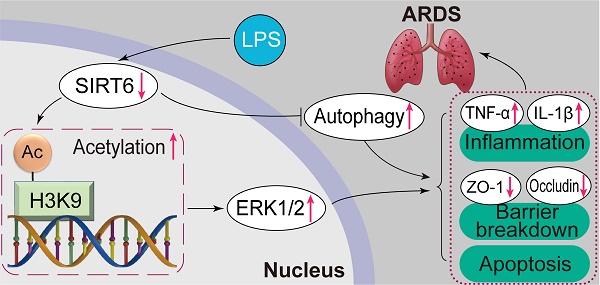
Sirtuin6 (SIRT6) has been demonstrated to be involved in a range of physiological processes and diseases, while its role in acute respiratory distress syndrome (ARDS) remains unclear. Therefore, this study focused on the role and underlying mechanism of SIRT6 in ARDS with the aim of identifying potential therapeutic targets. In this study, we found that SIRT6 was significantly decreased in lipopolysaccharide (LPS)-induced A549 cells and a murine model. In vitro overexpression of SIRT6 restored the expression of tight junction proteins (ZO-1 and occludin) and alleviated cell apoptosis and inflammation, while knockdown of SIRT6 aggravated the loss of tight junction proteins (ZO-1 and occludin) and promoted cell apoptosis and inflammation in LPS-induced A549 cells. Furthermore, the overexpression of SIRT6 enhanced autophagy and inhibited the ERK1/2 pathway, while the knockdown of SIRT6 inhibited autophagy and activated the ERK1/2 pathway. The autophagy activator rapamycin and the ERK1/2 inhibitor PD98059 rescued the effects of SIRT6 knockdown on tight junction proteins, apoptosis, and inflammation. Mechanistically, SIRT6 deacetylated histone 3 at Lys9 to negatively regulate the ERK1/2 pathway. In vivo, the SIRT6-specific inhibitor OSS_128167 also significantly accelerated LPS-induced loss of tight junction proteins, lung inflammation, and apoptosis. Meanwhile, the SIRT6-specific inhibitor OSS_128167 also activated the ERK1/2 pathway and inhibited lung autophagy. These results suggested that SIRT6 could ameliorate the loss of tight junction proteins, inflammation, and apoptosis in LPS-induced ARDS by inhibiting the ERK1/ 2 pathway and enhancing autophagy, indicating that SIRT6 plays a beneficial role in ARDS and might be a potential therapeutic target for ARDS.
Keywords: Acute respiratory distress syndrome (ARDS), Sirtuin6, tight junction, ERK1/2, OSS_128167, autophagy

 Global reach, higher impact
Global reach, higher impact