3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2023; 20(2):225-231. doi:10.7150/ijms.80262 This issue Cite
Review
Research progress of PBX1 in developmental and regenerative medicine
1. Department of Neurovascular Surgery, First Hospital of Jilin University, 1 Xinmin Avenue Changchun 130021, Jilin Province, China.
2. Department of Toxicology, School of Public Health, Jilin University, Changchun 130021, Jilin Province, China.
* Hao Chen and Zhuyuan Yu have contributed equally to this work.
Received 2022-10-28; Accepted 2023-1-10; Published 2023-1-22
Abstract
Pre-B-cell leukemia transcription factor 1 (PBX1) proteins are a subfamily of evolutionarily conserved atypical homeodomain transcription factors belonging to the superfamily of triple amino acid loop extension homeodomain proteins. PBX family members play crucial roles in the regulation of various pathophysiological processes. This article reviews the research progress on PBX1 in terms of structure, developmental function, and regenerative medicine. The potential mechanisms of development and research targets in regenerative medicine are also summarized. It also suggests a possible link between PBX1 in the two domains, which is expected to open up a new field for future exploration of cell homeostasis, as well as the regulation of endogenous danger signals. This would provide a new target for the study of diseases in various systems.
Keywords: Pbx1, Development, Regenerative medicine, Transcription factors
Introduction
Congenital genetic defects, autoimmune diseases, diabetes, neurodegenerative disorders, and trauma all involve the loss or dysfunction of healthy human tissue and contribute greatly to human disability. Some improvements in clinical management of symptoms and slowing of disease progression have been made for some of these conditions, but restorative and regenerative therapies are still severely lacking. Stem cells possess the unique ability to self-renew and differentiate and play vital roles in embryonic development and tissue and organ regeneration; as such, they are attractive candidates for cell therapy and regenerative medicine [1-4]. Stem cells maintain their unique properties through several mechanisms including pluripotent transcription factors [5], signaling pathways [6], specific microRNAs [7], long non-coding RNAs [8], alternative pre-messenger RNA splicing [9], histone modification [10], and cell cycle progression [11]. These mechanisms play key roles in embryonic development and tissue and organ regeneration.
Pre-B-cell leukemia transcription factor 1 (PBX1) is a member of the triple amino acid loop extension family of homologous transcription factors [12]. It is well known for its function in lymphocytic leukemia [13] and several cancers [14-20]. PBX1 also plays an important role in regulating stem cell developmental gene expression [21], maintaining stem cell stemness and self-renewal [22, 23], and regulating cellular oxidative stress and apoptosis during development [24]. It also plays a role in anti-aging, tissue and organ homeostasis, and tissue and organ regeneration [25-27]. Thus, PBX1 is a promising therapeutic target and biomarker in developmental biology and regenerative medicine.
In this review, we summarize the structure of PBX1, its regulatory mechanisms involved in the development of various tissues and organs, and research targets of PBX1 in regenerative medicine. A possible relationship between PBX1 in these two fields is proposed, which is expected to open up a new perspective for exploring cell homeostasis and tissue and organ reconstruction in the future.
PBX1 structure, development, and function in regenerative medicine
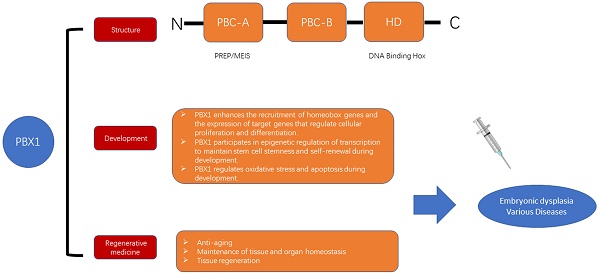
PBX1 structure
PBX1 is approximately 430 amino acid residues long, missing 78 amino acid residues in the N-terminal domain and a 30-residue stretch in the C-terminal domain, and contains a 60-residue-long homeodomain (HD) that mediates its binding to DNA or other proteins [28]. The HD and C termini of PBX proteins can also mediate their interaction with Hox/HOM-C proteins, thus playing a role in transcription [29, 30]. PBX proteins comprise two highly conserved protein-protein interaction domains, including PBC-A and PBC-B [31]. The PBC-A domain of PBX members can mediate interactions with PREP/MEIS proteins to form a transcription regulatory complex that plays a role in development [32]. The PBC-A domain also mediates the interaction of PBX1 with CRM1, which is required for the nuclear export of PBX1, while the PBC-B domain in PBX1 can be specifically phosphorylated by PKA, leading to PBX1 accumulation in the nucleus [33]. In addition, NMHCB competes with the PBC-B region of PBX1 for interaction, leading to cytoplasmic accumulation of PBX1 [34].
Development
PBX1 plays an important role in tissue and organ development by enhancing the recruitment of homeobox genes, enhancing the expression of developmental homeobox genes, and promoting cell proliferation and differentiation through epigenetic regulation. Moreover, genes regulating oxidative stress and apoptosis also promote cell development (FURGE1).
PBX1 enhances the recruitment of homeobox genes and the expression of target genes that regulate cellular proliferation and differentiation
In fact, PBX1 is a key transcriptional regulator of the development of multiple tissues and organs. Its tight binding to homeobox genes facilitates their transcriptional regulation. In the nervous system, PBX1 and its family members are important transcriptional regulators of neurons [35-38]. For instance, Hoxc5 affects many aspects of motor neuron (MN) development and function [39-41]. In D2 MN cell cultures, at shared binding sites, it showed higher binding to Pbx1, and the possible mechanism is that PBX1a enhances the recruitment of Meis1 to increase Hoxc5 expression [42]. Another study indicated that PBX1 interactions with HOX proteins and DNA are dispensable for the RA-induced ability of ES cells to express neural genes [43]. In the adult mammalian olfactory bulb, the transcription factor PBX1 controls neurogenesis in progenitor cells and the survival of migrating neuroblasts. Furthermore, PBX1 acts downstream or in parallel with other transcription factors such as Dlx2, Pax6, Etv1, Meis2, and Couptf1 that are required for dopaminergic specification of neurons. During midbrain dopaminergic neuron development, PBX1a is present in developing mDA and type 2 neuroblasts and improves the differentiation of neural progenitor cells towards mDA fate. The molecular mechanism by which PBX1 regulates mDA development may be that PBX1 controls the specification of mDA neurons by directly activating Pitx3 and repressing lateral fate genes, such as Onecut2. Moreover, by combining with recruited development-related genes, it promotes the expression of target genes and promotes the development of tissues and organs. For instance, early expression of PBX1a promotes the expression of neuronal genes, such as Phox2b, Cntn2, Ntng2, Olig1, Isl1, Nrp2, Ngfr, Nav2, Slit1, Igf2, Dlk1, and Meox1 [17, 21, 42-46].
In addition to neuronal differentiation, PBX1 is crucial for the proper differentiation of stem cells and tissue development in other organs. Binding of PBX1 to Meis1 and Rnux1 during the morphogenesis of the diaphragm and lung branches is also crucial, and its reduced expression disrupts lung mesenchymal cell proliferation and proper airway branch development [47]. PBX1 is also an important epigenetic regulator of the balance between pulmonary vasoconstriction and vasodilation. Studies have shown that, several genes that promote VSM contraction (endothelin-1 [Edn1], angiotensinogen [Agt], and smooth muscle myosin [Myh11]) were upregulated, whereas genes that promote VSM relaxation (natriuretic peptide C [Nppc] and adenylate cyclase 8 [Adcy8]) were downregulated in PBX1 mutants. Interestingly, both Edn1 and Agt gene promoters contain conserved PBX binding consensus elements: binding elements within the transcriptional regulatory regions of the genome [48]. Hox11 is a direct in vivo target of PBX1 and autoregulates its own promoter with PBX1 during spleen ontogeny. PBX1 and Hox11 genetically interact in spleen formation, and the loss of either is associated with a similar reduction in progenitor cell proliferation and failed expansion of the splenic anlage [49].
Research targets and underlying mechanisms of each system in development.
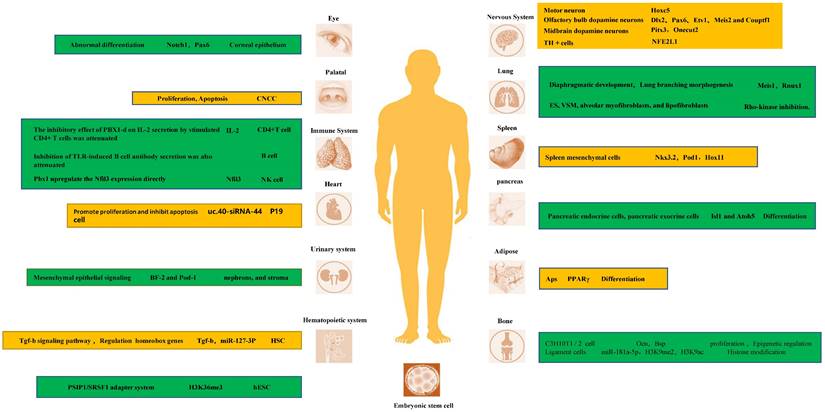
PBX1 participates in epigenetic regulation of transcription to maintain stem cell stemness and self-renewal during development
PBX is also involved in transcription regulation through histone modifications [50]. MiR-181a-5p exerts its ossification function by downregulating the expression of PBX1. PBX1 has also been found to be an important negative regulator of ligament cell ossification. This molecular mechanism is partly due to PBX1- mediated modifications of H3K9me2 and H3K9ac in the promoter regions of genes related to ossification, such as OSX and OCN [51, 52]. Moreover, in a study on hematopoietic stem cells, it was found that MiR-127-3p is absent in HSC obtained from PBX1-cKO mice, which display a profound self-renewal defect. These results suggest that PBX1 and its homeobox partners regulate the expression of miR-127-3p [53]. In a study of ES cells, PBX1 was one of the genes whose Alternative splicing (ANS) was positively correlated with H3K36me3. They found that the transcription of two isoforms of PBX1, PBX1a and PBX1b, is regulated by H3K36me3 during hESC differentiation. Their protein isoforms competitively bind NANOG, and the binding of PBX1b abolishes the binding of PBX1a, further attenuating the activity of the core pluripotency regulatory network composed of Yamanaka factors [54]. PBX1 splicing is dynamically regulated during olfactory bulb neurogenesis, with all dopaminergic cells expressing PBX1a but not PBX1b. Moreover, almost all circulating progenitor cells of the SEZ express PBX1b and lack the subtype expression of PBX1a [55]. More research is needed to fully delineate the unique roles between these two different splice isoforms.
PBX1 regulates oxidative stress and apoptosis during development
Nfe2l1 is expressed during tyrosine hydroxylase (TH) neuron development at E12.5 [24]. It has been proposed that PBX1 directly controls the expression of the antioxidant transcription factor Nfe2l1, which counteracts oxidative stress, mitochondrial dysfunction, and proteasome damage [56-58]. This promotes the survival of mDAn. Characterization of the cranial neural crest cell (CNCC) mutants revealed reduced proliferation of palatal progenitor cells, disturbed skeletal differentiation, and heterotopic ossification. This suggests a role of PBX1 in coordinating CNCC-dependent hypomorphological and skeletal differentiation [59]. Knockdown of uc40-siRNA44 increases the number of cardiac stem cells during P19 cell differentiation. It promotes the proliferation of P19 cells and inhibits apoptosis induced by serum starvation, which may play a role in regulating PBX1 [60].
PBX1 plays a critical role in the development of various tissues and organs. In addition, some sensitive targets have been identified in these studies. It also provides a direction for the treatment of clinical diseases. Each PBX1 isoform has different effects on development. Therefore, further studies on the modification and phenotype of PBX1 isoform switching are required.
Regenerative medicine
Anti-aging: Inhibiting oxidative stress and reducing DNA damage to slow down stem cell aging
PBX1 is important for maintaining stem cell function by alleviating oxidative stress and preventing cellular apoptosis. In a hair follicle stem cell (HFSCS) study, PBX1 overexpression attenuated HFMSC senescence and apoptosis by alleviating reactive oxygen species (ROS) - mediated DNA damage without enhancing DNA repair. It has been found that PBX1 is an upstream regulator of PARP1 [25]. Further studies on HF-MSCs showed that PBX1 overexpression alleviated senescence and apoptosis of HF-MSC accompanied by up-regulation of SIRT1, down-regulation of PARP1, and increased intracellular NAD and ATP levels. SIRT1 knockdown enhanced cellular senescence and apoptosis, accompanied by increased ROS accumulation, aggravated DNA damage, and decreased intracellular NAD and ATP levels. PBX1 overexpression rescued HF-MSCs senescence and apoptosis induced by SIRT1 knockdown. PBX1 rescued ATP and NAD depletion mediated by PARP1 overexpression, accompanied by increased SIRT1 expression. This highlights a critical role of the PBX1-SIRT1-PARP1 axis in alleviating senescence and apoptosis in HF-MSCs [61].
PBX1 promotes stem cell reprogramming of hF-MSC into HF-iPSCs through epigenetic upregulation of NANOG. Specifically, PBX1 increased the efficiency of reprogramming by activating NANOG's promoter to up-regulate NANOG transcription and promote the expression of endogenous SOX2 and OCT4 [62].
Maintenance of tissue and organ homeostasis
PBX1 regulates stem cell proliferation and cell cycle to maintain cell homeostasis
The proliferation of normal cells is tightly controlled and influenced by many signaling pathways to maintain homeostasis [63]. PBX1 is usually associated with tumor growth by promoting proliferation and regulating cell cycle [19, 64- 67]. However, many studies have shown that PBX1 also plays a crucial role in the proliferation of non-tumor stem cells [26, 62, 68, 69]. Lichtenauer et al. demonstrated that PBX1, in addition to its role in genitourinary development and adrenocortical cells, is also required for the maintenance of adult adrenal growth, and in vitro studies have revealed that PBX1 and Sf-1 synergistically stimulates Mc2-r promoter activity. [26]. In a study on hair follicle mesenchymal stem cells, Jiang et al. showed that PBX homeobox 1 enhances hair follicle mesenchymal stem cell proliferation through the AKT/glycogen synthase kinase signaling pathway and suppression of apoptosis. Moreover, ectopic expression of NANOG significantly upregulates PBX1 and results in decreased expression of p16 and p21 [62].
PBX1 regulates the immune response during apoptosis to maintain cell homeostasis
PBX1 is a physiologically critical mediator of IL-10 gene transcription induced by apoptotic cells. In studies on the induction of IL-10 production, CD36 was found to be physiologically important, but not the only receptor for IL-10 induction by apoptotic cells. Further studies showed that PBX1 had little effect on basal IL-10 transcription in the absence of apoptotic cell stimulation. apoptotic cell-induced IL-10 production was significantly reduced. Therefore, the transcriptional potential of PBX1 highly depends on cell fate status and apoptotic cell signaling. Further research showed that PBX1b (PBX1a is not expressed in macrophages) is a major physiological and selective transcriptional mediator of apoptotic cell-induced IL-10 gene expression through ACRE [70].
Tissue regeneration
Nerve regeneration
PBX1 plays important roles in neurogenesis, the generation of new adult neurons from neural stem cells. In the nervous system, PBX1 plays an important role in the generation, survival, and terminal differentiation of adult V-SVZ neurons, and the deletion of transient amplifying progenitors (TAPs) can guide them from neurogenic to oligodendrogliogenic fate. Deletion of differentiating dopaminergic neurons disturbs their maturation and the expression of characteristic dopamine pathway genes [55, 71]. Of note, PBX1 is important for the differentiation of neural progenitor cells towards a mDA fate [24]. In fact, the neuron-specific gene doublecortin (Dcx) and the dopaminergic neuron marker gene tyrosine hydroxylase (Th) are known to be direct PBX1 target genes [72]. Neuroblasts leave the SVZ and migrate into the olfactory bulb, where they eventually differentiate into different types of interneurons that are constantly replaced in existing circuits as part of lifelong remodeling of the mammalian olfactory system. Many transcription factors, including DLX2 and PAX6, bias progenitor cells toward a general neuronal fate and promote their subsequent maturation into specific types of interneurons [73]. However, PBX1 can elicit the antioxidant effect of NFE2L1 on TH+ neurons, and lentiviral overexpression of PBX1 in human NES cells differentiated into mDAn significantly reduced the number of TH+ and aCASP3+ cells in cultures treated with 100 µmol H2O2 [24, 74].
Role of Pbx1 in regenerative medicine.
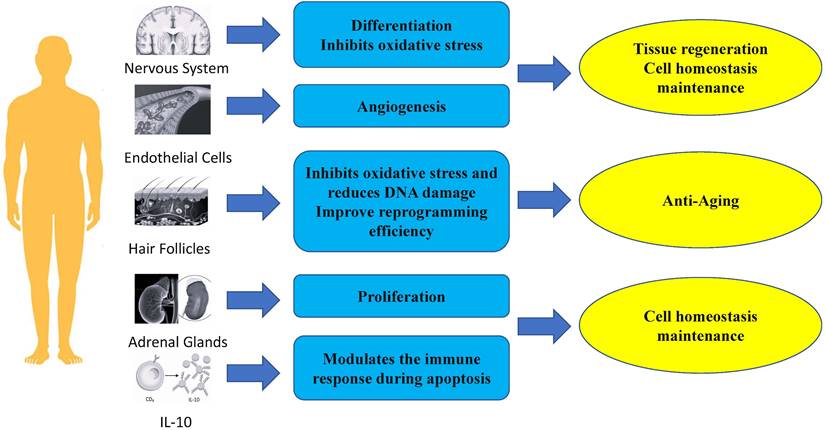
Angiogenesis
PBX1 also plays important roles in angiogenesis. In adult tissues, angiogenesis occurs transiently and in a highly regulated manner in events such as wound healing, tissue remodeling, and the female reproductive cycle [63]. Of note, PBX1b is required for proangiogenic Hox DNA binding and transcriptional activity in endothelial cells. It was observed that endothelial cells (EC) predominantly express the Pbx1b isoform. Nuclear extracts of angiogenic EC expressed higher levels of active PBX1 on PBX1/Hox consensus DNA oligos and formed complexes more efficiently than nuclear extracts of quiescent ECs did. Furthermore, In the absence of PBX1, Hox D3 fails to induce the expression of integrin avb3, a key angiogenic mediator, and endothelial cells fail to migrate and form new blood vessels as a result [27]. Thus, PBX1 may serve as an important therapeutic target or biomarker for diseases requiring angiogenic modulation, such as stroke and cancer.
In summary, PBX1 plays an important role in regenerative medicine, such as in anti-aging, maintenance of tissue homeostasis, and regeneration of tissues and organs (Fig. 2). Therefore, future clinical research on cell therapy and regenerative medicine with PBX1 modulation is warranted (FURGE2).
Outlook
PBX1 lies at the intersection between cellular homeostasis and the regulation of responses to endogenous danger signals. In particular, PBX1 is a cellular factor that shows promise in the treatment of embryonic dysplasia, autoimmune diseases, diabetes, Alzheimer's disease, Parkinson's disease, trauma and other diseases, and there are potential targets for developmental and regenerative medicine research mechanisms. PBX1 is likely to become a hot spot in the treatment of other clinical diseases.
PBX1 has also been implicated in some neoplastic diseases, so there may be a risk of tumorigenesis in research on embryonic development and regenerative medicine. But further research is needed to be confirmed.
Conclusion
PBX1 is a relatively new transcription factor that governs diverse cellular responses during normal development and tissue homeostasis. It possesses target site binding in closed chromatin, the ability to increase DNA access to other proteins, and is actively involved in cell fate specification. The mechanistic features of PBX1 in developmental and regenerative medicine may establish a link between the two pathways. PBX1 is a promising research target for future exploration of cellular homeostasis as well as the regulation of endogenous danger signals. It brings hope as a target therapy for a variety of diseases.
Abbreviations
PBX1: Pre-B-cell leukemia transcription factor 1; HD: homeodomain; MN: motor neuron; ANS: Alternative splicing; TH: tyrosine hydroxylase; CNCC: Characterization of the cranial neural crest cell; ROS: reactive oxygen species; TAPs: transient amplifying progenitors; EC: endothelial cells.
Acknowledgements
Funding
This study was supported by Jilin Province Health Science and Technology Improvement Plan (2020J054) and Jilin Province Science and Technology Development Plan (20200801022GH, 20210204159YY).
Author contributions
Kan Xu and Jinyu Liu developed the concept of the project and wrote the manuscript. Hao Chen, Zhuyuan Yu, Ye Niu and Litian Wang were involved in the manuscript writing, including discussion of content and writing, and editing of the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science (New York, NY). 2014;346:1248012
2. Miyajima A, Tanaka M, Itoh T. Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell stem cell. 2014;14:561-74
3. Zhang Y, Mignone J, MacLellan WR. Cardiac Regeneration and Stem Cells. Physiological reviews. 2015;95:1189-204
4. Graveley BR. Splicing up pluripotency. Cell. 2011;147:22-4
5. Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP. et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947-56
6. Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X. et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nature protocols. 2013;8:162-75
7. Tay Y, Zhang J, Thomson AM, Lim B, Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. 2008;455:1124-8
8. Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nature reviews Genetics. 2014;15:7-21
9. Gabut M, Samavarchi-Tehrani P, Wang X, Slobodeniuc V, O'Hanlon D, Sung HK. et al. An alternative splicing switch regulates embryonic stem cell pluripotency and reprogramming. Cell. 2011;147:132-46
10. Xie W, Schultz MD, Lister R, Hou Z, Rajagopal N, Ray P. et al. Epigenomic analysis of multilineage differentiation of human embryonic stem cells. Cell. 2013;153:1134-48
11. Vallier L. Cell Cycle Rules Pluripotency. Cell stem cell. 2015;17:131-2
12. Chang CP, Jacobs Y, Nakamura T, Jenkins NA, Copeland NG, Cleary ML. Meis proteins are major in vivo DNA binding partners for wild-type but not chimeric Pbx proteins. Molecular and cellular biology. 1997;17:5679-87
13. Kamps MP, Baltimore D. E2A-Pbx1, the t(1;19) translocation protein of human pre-B-cell acute lymphocytic leukemia, causes acute myeloid leukemia in mice. Molecular and cellular biology. 1993;13:351-7
14. Magnani L, Patten DK, Nguyen VT, Hong SP, Steel JH, Patel N. et al. The pioneer factor PBX1 is a novel driver of metastatic progression in ERα-positive breast cancer. Oncotarget. 2015;6:21878-91
15. Feng Y, Li L, Zhang X, Zhang Y, Liang Y, Lv J. et al. Hematopoietic pre-B cell leukemia transcription factor interacting protein is overexpressed in gastric cancer and promotes gastric cancer cell proliferation, migration, and invasion. Cancer science. 2015;106:1313-22
16. Li W, Huang K, Guo H, Cui G, Zhao S. Inhibition of non-small-cell lung cancer cell proliferation by Pbx1. Chinese journal of cancer research = Chung-kuo yen cheng yen chiu. 2014;26:573-8
17. Thiaville MM, Stoeck A, Chen L, Wu RC, Magnani L, Oidtman J. et al. Identification of PBX1 target genes in cancer cells by global mapping of PBX1 binding sites. PloS one. 2012;7:e36054
18. Magnani L, Ballantyne EB, Zhang X, Lupien M. PBX1 genomic pioneer function drives ERα signaling underlying progression in breast cancer. PLoS genetics. 2011;7:e1002368
19. Park JT, Shih Ie M, Wang TL. Identification of Pbx1, a potential oncogene, as a Notch3 target gene in ovarian cancer. Cancer research. 2008;68:8852-60
20. Qiu Y, Tomita Y, Zhang B, Nakamichi I, Morii E, Aozasa K. Pre-B-cell leukemia transcription factor 1 regulates expression of valosin-containing protein, a gene involved in cancer growth. The American journal of pathology. 2007;170:152-9
21. Kim SK, Selleri L, Lee JS, Zhang AY, Gu X, Jacobs Y. et al. Pbx1 inactivation disrupts pancreas development and in Ipf1-deficient mice promotes diabetes mellitus. Nature genetics. 2002;30:430-5
22. Ficara F, Murphy MJ, Lin M, Cleary ML. Pbx1 regulates self-renewal of long-term hematopoietic stem cells by maintaining their quiescence. Cell stem cell. 2008;2:484-96
23. Xu B, Cai L, Butler JM, Chen D, Lu X, Allison DF. et al. The Chromatin Remodeler BPTF Activates a Stemness Gene-Expression Program Essential for the Maintenance of Adult Hematopoietic Stem Cells. Stem cell reports. 2018;10:675-83
24. Villaescusa JC, Li B, Toledo EM, Rivetti di Val Cervo P, Yang S, Stott SR. et al. A PBX1 transcriptional network controls dopaminergic neuron development and is impaired in Parkinson's disease. The EMBO journal. 2016;35:1963-78
25. Wang Y, Sui Y, Lian A, Han X, Liu F, Zuo K. et al. PBX1 Attenuates Hair Follicle-Derived Mesenchymal Stem Cell Senescence and Apoptosis by Alleviating Reactive Oxygen Species-Mediated DNA Damage Instead of Enhancing DNA Damage Repair. Frontiers in cell and developmental biology. 2021;9:739868
26. Lichtenauer UD, Duchniewicz M, Kolanczyk M, Hoeflich A, Hahner S, Else T. et al. Pre-B-cell transcription factor 1 and steroidogenic factor 1 synergistically regulate adrenocortical growth and steroidogenesis. Endocrinology. 2007;148:693-704
27. Charboneau A, East L, Mulholland N, Rohde M, Boudreau N. Pbx1 is required for Hox D3-mediated angiogenesis. Angiogenesis. 2005;8:289-96
28. Peltenburg LT, Murre C. Specific residues in the Pbx homeodomain differentially modulate the DNA-binding activity of Hox and Engrailed proteins. Development (Cambridge, England). 1997;124:1089-98
29. Ladam F, Sagerström CG. Hox regulation of transcription: more complex(es). Developmental dynamics: an official publication of the American Association of Anatomists. 2014;243:4-15
30. Knoepfler PS, Lu Q, Kamps MP. Pbx-1 Hox heterodimers bind DNA on inseparable half-sites that permit intrinsic DNA binding specificity of the Hox partner at nucleotides 3' to a TAAT motif. Nucleic acids research. 1996;24:2288-94
31. Longobardi E, Penkov D, Mateos D, De Florian G, Torres M, Blasi F. Biochemistry of the tale transcription factors PREP, MEIS, and PBX in vertebrates. Developmental dynamics: an official publication of the American Association of Anatomists. 2014;243:59-75
32. Moens CB, Selleri L. Hox cofactors in vertebrate development. Developmental biology. 2006;291:193-206
33. Kilstrup-Nielsen C, Alessio M, Zappavigna V. PBX1 nuclear export is regulated independently of PBX-MEINOX interaction by PKA phosphorylation of the PBC-B domain. The EMBO journal. 2003;22:89-99
34. Huang H, Paliouras M, Rambaldi I, Lasko P, Featherstone M. Nonmuscle myosin promotes cytoplasmic localization of PBX. Molecular and cellular biology. 2003;23:3636-45
35. Maeda R, Ishimura A, Mood K, Park EK, Buchberg AM, Daar IO. Xpbx1b and Xmeis1b play a collaborative role in hindbrain and neural crest gene expression in Xenopus embryos. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5448-53
36. Vitobello A, Ferretti E, Lampe X, Vilain N, Ducret S, Ori M. et al. Hox and Pbx factors control retinoic acid synthesis during hindbrain segmentation. Developmental cell. 2011;20:469-82
37. Sgadò P, Ferretti E, Grbec D, Bozzi Y, Simon HH. The atypical homeoprotein Pbx1a participates in the axonal pathfinding of mesencephalic dopaminergic neurons. Neural development. 2012;7:24
38. Schulte D, Frank D. TALE transcription factors during early development of the vertebrate brain and eye. Developmental dynamics: an official publication of the American Association of Anatomists. 2014;243:99-116
39. Liu JP, Laufer E, Jessell TM. Assigning the positional identity of spinal motor neurons: rostrocaudal patterning of Hox-c expression by FGFs, Gdf11, and retinoids. Neuron. 2001;32:997-1012
40. Dasen JS, Liu JP, Jessell TM. Motor neuron columnar fate imposed by sequential phases of Hox-c activity. Nature. 2003;425:926-33
41. Philippidou P, Walsh CM, Aubin J, Jeannotte L, Dasen JS. Sustained Hox5 gene activity is required for respiratory motor neuron development. Nature neuroscience. 2012;15:1636-44
42. Linares AJ, Lin CH, Damianov A, Adams KL, Novitch BG, Black DL. The splicing regulator PTBP1 controls the activity of the transcription factor Pbx1 during neuronal differentiation. eLife. 2015;4:e09268
43. Jürgens AS, Kolanczyk M, Moebest DC, Zemojtel T, Lichtenauer U, Duchniewicz M. et al. PBX1 is dispensable for neural commitment of RA-treated murine ES cells. In vitro cellular & developmental biology Animal. 2009;45:252-63
44. Kappen C, Yaworsky PJ, Muller YL, Salbaum JM. Transgenic studies on homeobox genes in nervous system development: spina bifida in Isl1 transgenic mice. Transgenic research. 2013;22:343-58
45. Pattyn A, Hirsch M, Goridis C, Brunet JF. Control of hindbrain motor neuron differentiation by the homeobox gene Phox2b. Development (Cambridge, England). 2000;127:1349-58
46. Samad OA, Geisen MJ, Caronia G, Varlet I, Zappavigna V, Ericson J. et al. Integration of anteroposterior and dorsoventral regulation of Phox2b transcription in cranial motoneuron progenitors by homeodomain proteins. Development (Cambridge, England). 2004;131:4071-83
47. Takahashi T, Friedmacher F, Zimmer J, Puri P. Pbx1, Meis1, and Runx1 Expression Is Decreased in the Diaphragmatic and Pulmonary Mesenchyme of Rats with Nitrofen-Induced Congenital Diaphragmatic Hernia. European journal of pediatric surgery: official journal of Austrian Association of Pediatric Surgery [et al] = Zeitschrift fur Kinderchirurgie. 2021;31:120-5
48. McCulley DJ, Wienhold MD, Hines EA, Hacker TA, Rogers A, Pewowaruk RJ. et al. PBX transcription factors drive pulmonary vascular adaptation to birth. The Journal of clinical investigation. 2018;128:655-67
49. Brendolan A, Ferretti E, Salsi V, Moses K, Quaggin S, Blasi F. et al. A Pbx1-dependent genetic and transcriptional network regulates spleen ontogeny. Development (Cambridge, England). 2005;132:3113-26
50. Grebbin BM, Schulte D. PBX1 as Pioneer Factor: A Case Still Open. Frontiers in cell and developmental biology. 2017;5:9
51. Liu N, Zhang Z, Li L, Shen X, Sun B, Wang R. et al. MicroRNA-181 regulates the development of Ossification of Posterior longitudinal ligament via Epigenetic Modulation by targeting PBX1. Theranostics. 2020;10:7492-509
52. Gordon JA, Hassan MQ, Koss M, Montecino M, Selleri L, van Wijnen AJ. et al. Epigenetic regulation of early osteogenesis and mineralized tissue formation by a HOXA10-PBX1-associated complex. Cells, tissues, organs. 2011;194:146-50
53. Crisafulli L, Muggeo S, Uva P, Wang Y, Iwasaki M, Locatelli S. et al. MicroRNA-127-3p controls murine hematopoietic stem cell maintenance by limiting differentiation. Haematologica. 2019;104:1744-55
54. Xu Y, Zhao W, Olson SD, Prabhakara KS, Zhou X. Alternative splicing links histone modifications to stem cell fate decision. Genome biology. 2018;19:133
55. Remesal L, Roger-Baynat I, Chirivella L, Maicas M, Brocal-Ruiz R, Pérez-Villalba A. et al. PBX1 acts as terminal selector for olfactory bulb dopaminergic neurons. Development (Cambridge, England). 2020 147
56. Hirotsu Y, Hataya N, Katsuoka F, Yamamoto M. NF-E2-related factor 1 (Nrf1) serves as a novel regulator of hepatic lipid metabolism through regulation of the Lipin1 and PGC-1β genes. Molecular and cellular biology. 2012;32:2760-70
57. Furuya N, Ikeda S, Sato S, Soma S, Ezaki J, Oliva Trejo JA. et al. PARK2/Parkin-mediated mitochondrial clearance contributes to proteasome activation during slow-twitch muscle atrophy via NFE2L1 nuclear translocation. Autophagy. 2014;10:631-41
58. Bugno M, Daniel M, Chepelev NL, Willmore WG. Changing gears in Nrf1 research, from mechanisms of regulation to its role in disease and prevention. Biochimica et biophysica acta. 2015;1849:1260-76
59. Welsh IC, Hart J, Brown JM, Hansen K, Rocha Marques M, Aho RJ. et al. Pbx loss in cranial neural crest, unlike in epithelium, results in cleft palate only and a broader midface. Journal of anatomy. 2018;233:222-42
60. Wu R, Xue P, Wan Y, Wang S, Gu M. LncRNA-uc.40 silence promotes P19 embryonic cells differentiation to cardiomyocyte via the PBX1 gene. In vitro cellular & developmental biology Animal. 2018;54:600-9
61. Wang Y, Sui Y, Niu Y, Liu D, Xu Q, Liu F. et al. PBX1-SIRT1 Positive Feedback Loop Attenuates ROS-Mediated HF-MSC Senescence and Apoptosis. Stem cell reviews and reports. 2022
62. Jiang Y, Liu F, Zou F, Zhang Y, Wang B, Zhang Y. et al. PBX homeobox 1 enhances hair follicle mesenchymal stem cell proliferation and reprogramming through activation of the AKT/glycogen synthase kinase signaling pathway and suppression of apoptosis. Stem cell research & therapy. 2019;10:268
63. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-74
64. Han T, Xu D, Zhu J, Li J, Liu L, Deng Y. Identification of a robust signature for clinical outcomes and immunotherapy response in gastric cancer: based on N6-methyladenosine related long noncoding RNAs. Cancer Cell Int. 2021;21:432
65. Wei X, Yu L, Li Y. PBX1 promotes the cell proliferation via JAK2/STAT3 signaling in clear cell renal carcinoma. Biochem Biophys Res Commun. 2018;500:650-7
66. Wang J, Shidfar A, Ivancic D, Ranjan M, Liu L, Choi MR. et al. Overexpression of lipid metabolism genes and PBX1 in the contralateral breasts of women with estrogen receptor-negative breast cancer. Int J Cancer. 2017;140:2484-97
67. Ao X, Ding W, Ge H, Zhang Y, Ding D, Liu Y. PBX1 is a valuable prognostic biomarker for patients with breast cancer. Exp Ther Med. 2020;20:385-94
68. Liu F, Shi J, Zhang Y, Lian A, Han X, Zuo K. et al. NANOG Attenuates Hair Follicle-Derived Mesenchymal Stem Cell Senescence by Upregulating PBX1 and Activating AKT Signaling. Oxid Med Cell Longev. 2019;2019:4286213
69. Zhou Y, Fu B, Xu X, Zhang J, Tong X, Wang Y. et al. PBX1 expression in uterine natural killer cells drives fetal growth. Science translational medicine. 2020 12
70. Chung EY, Liu J, Homma Y, Zhang Y, Brendolan A, Saggese M. et al. Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells is mediated by homeodomain proteins Pbx1 and Prep-1. Immunity. 2007;27:952-64
71. Grebbin BM, Hau AC, Groß A, Anders-Maurer M, Schramm J, Koss M. et al. Pbx1 is required for adult subventricular zone neurogenesis. Development (Cambridge, England). 2016;143:2281-91
72. Hau AC, Mommaerts E, Laub V, Müller T, Dittmar G, Schulte D. Transcriptional cooperation of PBX1 and PAX6 in adult neural progenitor cells. Scientific reports. 2021;11:21013
73. Brill MS, Snapyan M, Wohlfrom H, Ninkovic J, Jawerka M, Mastick GS. et al. A dlx2- and pax6-dependent transcriptional code for periglomerular neuron specification in the adult olfactory bulb. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:6439-52
74. Hack MA, Saghatelyan A, de Chevigny A, Pfeifer A, Ashery-Padan R, Lledo PM. et al. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nature neuroscience. 2005;8:865-72
Author contact
![]() Corresponding authors: Jinyu Liu (Electronic address: jy_liuedu.cn), Kan Xu (Electronic address: xukanedu.cn).
Corresponding authors: Jinyu Liu (Electronic address: jy_liuedu.cn), Kan Xu (Electronic address: xukanedu.cn).

 Global reach, higher impact
Global reach, higher impact