3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(10):1586-1595. doi:10.7150/ijms.59018 This issue Cite
Research Paper
Protective effect of microorganism biotransformation-produced resveratrol on the high fat diet-induced hyperlipidemia, hepatic steatosis and synaptic impairment in hamsters
1. Division of Cardiology, Department of internal medicine, Chung-Shan Medical University Hospital Taichung 40201.
2. Institute of Medicine, School of Medicine, Chung Shan Medical University, Taichung 40201.
3. Graduate Institute of Bio-industry Management, College of Agriculture and Nature Resources, National Chung Hsing University, Taichung 40227.
4. Department of Pharmacy, Chung Shan Medical University Hospital, Taichung 40201.
5. Department of Occupational Safety and Health, College of Health Care and Management, Chung Shan Medical University, Taichung 40201.
6. Department of Physiology, School of Medicine, Chung Shan Medical University, Taichung 40201.
7. Department of Early Childhood Educare, College of Health Sciences, TransWorld University, Douliu City, Yunlin County 64063.
8. Department of Medical Research, Chung Shan Medical University Hospital, Taichung 40201, Taiwan.
*Dr. Hung-Chih Ting and Dr. Ching-Han Yu have the same contribution to this paper.
Received 2021-2-4; Accepted 2022-8-12; Published 2022-9-11
Abstract
Background: Resveratrol, a natural antioxidant polyphenol, has the functions of anti-inflammation, anti-cancer, liver protection and cardioprotection. Microorganism biotransformation-produced resveratrol (MBR) product shows higher purity than the natural source of resveratrol and costs less than the chemically synthesized resveratrol. The aim of the present study was to investigate the protective effects of MBR in hamsters treated with a high-fat diet (HFD).
Methods: MBR was obtained by the fermentative process of piceid. Hamsters were randomly divided into four groups: HFD plus oral administration of MBR 0 (C), 5 (L), 20 (M) or 50 mg/kg (H), respectively. After six-week of treatment, hamsters were sacrificed, and tissues were collected for further analysis.
Results: MBR at these three dosages did not influence the appetite or growth of the hamsters. Liver enzymes, blood glucose, total cholesterol, triglyceride, and liver weight were significantly reduced in the MBR groups than in the control group. Additionally, high-density lipoprotein-cholesterol (HDL-C) was also elevated in all MBR groups. On the other hand, serum low-density lipoprotein-cholesterol (LDL-C) was decreased in the MBR groups. Triglyceride (TG) in liver tissue and fatty liver level were lower in group H. Memory-associated proteins, phosphorylation of calmodulin-dependent protein kinase II (p-CaMK II) and synaptophysin (SYP), were increased in the brains of MBR groups.
Conclusion: The high yield- and short procedure-produced MBR has the potential to protect animals fed with HFD from hyperlipidemia, hepatic steatosis, hyperglycemia, and synaptic impairment, which might be beneficial for patients with these types of diseases.
Keywords: hepatic steatosis, hyperlipidemia, microorganism biotransformation-produced resveratrol, synaptic impairment.
Introduction
Hyperlipidemia, such as higher levels of total cholesterol (TC), triglyceride (TG), and low-density lipoprotein cholesterol (LDL-C) and lower levels of high-density lipoprotein cholesterol (HDL-C), leads to multiple metabolic diseases. According to statistical data from the Ministry of Health and Welfare, Taiwan, there are five metabolic-related diseases on the list of the top 10 leading causes of death in 2021, including heart, cerebrovascular, and hypertensive diseases, diabetes mellitus, chronic-hepatic diseases and hepatic cirrhosis. In addition to Taiwan, diseases related to hyperlipidemia are also an important issue worldwide [1, 2]. The mechanisms of how blood lipids result in these diseases are related to chronic inflammation and oxidative stress [3].
Cognitive impairment is one of the symptoms resulted from hyperlipidemia. Although brain cholesterol is involved in synapse development, dendrite differentiation and axonal elongation, people with familial hypercholesterolemia reveal higher incidence of memory deficits. On the other hand, patients with long-term statin treatment show better performance in episodic memory than control group [4, 5]. Oxidized-LDL stimulates microvascular endothelial cells to secret inflammatory mediators which destroyed tight-junction structure of blood-brain barrier (BBB) [5]. In addition, beta-amyloid peptides (Aβ) accumulation and the subsequent neuroinflammation, cerebral microhemorrhages, and cognitive decline may result from hypercholesterolemia [6].
Resveratrol is the non-flavonoid polyphenolic compound in grapes, peanuts, berries, and Polygonum cuspidatum (buffalo pea) [7, 8]. Researchers are investigating its anti-inflammatory [9], antiapoptotic [10], antimicrobial [11], anticancer [12], antilipidemic [13], and osteogenic properties [14]. The main function of resveratrol is to work as an antioxidant. The mechanism works to elevate the amount and activity of antioxidant enzymes and to reduce the lipoperoxidation [15]. Accordingly, resveratrol prevents hepatic steatosis via decreasing reactive oxygen species (ROS) and regulating autophagy pathway in animal models [16, 17]. Sirtuin-1 (SIRT1), a nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase, is a key mediator of resveratrol for its antioxidant function. SIRT1 and its downstream transcription factor, forkhead box O1 (FoxO1), modulate autophagy induced by resveratrol [18]. In addition, fasting blood glucose (FBG) and insulin resistance were significantly lower in patients taking resveratrol as a supplement [19]. Furthermore, resveratrol protects brain function by lowering oxidative stress [20].
Natural sources of resveratrol may contain phytochemical impurities whereas chemically synthesized resveratrol may contain undesirable impurities. [21]. Moreover, chemical synthesis requires expensive precursor and complicated multiple reactions and cleanup steps [22]. In comparison, a fermentative process using yeasts such as Aspergillus niger or Penicillium oxalium to ferment precursor- or resveratrol-containing solutions provides higher yields than traditional methods. P. cuspidatum containing about 1~2% piceid (glycosylated resveratrol) is a applicable precursor for biotransformative producing of resveratrol [23]. Dekkera bruxellensis, a yeast, presents highest β-glucosidase activity than other strains of microbes in our collaborator's previous study [24]. Therefore, we used D. bruxellensis to ferment the root powder of P. cuspidatum. The fermentative product was called microorganism biotransformation-produced resveratrol (MBR). Syrian hamsters fed with high-fat diet (HFD) have been widely used as the animal model to examine the effects of dietary fibers, polyphenols, and other plant components on hyperlipidemia [25-27]. The hypothesis of this study was that the MBR prepared from this study also revealed the bio-activities of pure resveratrol. Hence, three dosages of MBR were employed in this study to examine its effects on blood lipids, hepatic function, blood glucose, and synaptic function in hamsters fed with HFD.
Materials and methods
Materials
The yeast, D. bruxellensis was obtained from the Bioresource Collection and Research Center (BCRC 920084; Hsinchu, Taiwan). Yeasts were maintained in the yeast extract peptone dextrose medium (YPD broth) (BD, Sparks, MD, USA). Standard compounds of resveratrol and piceid were products of Sigma-Aldrich (St. Louis, MO, USA). Dried P. cuspidatum root was purchased from the local market (Taiwan) and ground into powder.
Preparation of MBR
D. bruxellensis yeast was seeded at 5% in the YPD broth and shaken in a mechanical shaker at 150 rpm at 25°C for 3 days. Afterward, powdered P. cuspidatum root (2% w/v) and pre-incubated yeast (5% v/v) were added into the fermentation tank containing 20 mM acetic acid buffer solution (pH 6). The broth was incubated on the shaker at 150 rpm at 25°C with 1 vessel volume per minute (VVM) aeration for 0 to 64 hours. At each time point, the freeze-dried broth was weighed and extracted with 80% alcohol (1:20 w/v). After centrifugation, the liquid was filtered through a 0.45 µm membrane and was further concentrated by rotary evaporation (Sunway, Taipei, Taiwan). The concentrate was extracted with ethyl acetate twice. The ethyl acetate extract was again placed in the rotary evaporator. The concentrated powder was placed in the chemical hood until completely dry. The dry powder was then dissolved in 80% alcohol for the detection of resveratrol and piceid by high-performance liquid chromatography (HPLC). The column C18 (4.6 x 250 nm, 5 μm, Waters, Milford, MA, USA) at 40℃ was used with the eluting solvent of 1% acetic acid (A) and acetonitrile (B) at the flow rate of 1.5 ml/min. At the beginning, the proportion of eluting solvent was 95% A: 5% B. The proportion of solvent B was gradually increased to 55% after 25 min, and then continuously elevated to 100% from 25 to 31 minutes. From 31 to 33 minutes, 100% B was used. During the final 33 to 45 minutes, the proportion gradually turned back to 95% A: 5% B. The injected sample size was 20 μl. The wavelength of detected UV light was 307 nm. Resveratrol were detected at retention time of 18.3 minutes. [24].
Animals and experimental groups
Six-week-old male Syrian hamsters, housed in a 12-h day/night schedule at 25° ± 2°C, were obtained from the National Laboratory Animal Center (Taipei, Taiwan). All animals could ad libitum access to the HFD (5TJN, TestDiet, St. Louis, MO, USA) and water. There are 40% of total calories from fat in this HFD. All animal experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Chung Shan Medical University in Taiwan (No. 1613). Animals were divided randomly into four experimental groups of 10 animals each, including vehicle group (control [C], HFD only), low-dose group (L, HFD + 5 mg/kg MBR), medium-dose group (M, HFD + 20 mg/kg MBR) and high dose group (H, HFD + 50 mg/kg MBR). The amount of MBR fed was as suggested in a previous study [28]. MBR was given to each hamster through gavage feeding every day for six weeks. This HFD-fed animal model which shows the increasing of body weight and hepatic steatosis in comparison to the standard chow diet-fed group has been established in our colleagues' previous study [29]. Body weight, HFD and fluid intake were recorded once a week for six weeks. During the experiment, once the hamster showed distress, weakness or decreasing 15% of body weight, the animal was euthanized by pentobarbital sodium salt (intraperitoneal injection, 100 mg/kg) according to the American veterinary medical association (AVMA) guidelines for the euthanasia of animals: 2013 edition. At the end of this experiment, hamsters were fasted overnight and euthanized. The blood and organs were collected for further studies [30].
Blood chemistry evaluation
Aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum glucose, TC, TG, and HDL-C were determined by the biochemistry analyzer (Spotchem, Arkray, Kyoto, Japan). LDL-C was calculated by the following equation: LDL-C = TC - (HDL-C + TG/5) [31].
Histological evaluation
At the end of this experiment, bilateral removal of epididymal white adipose tissue (EWAT) and the whole liver were performed. After weighting, part of liver tissue was homogenized to detect the hepatic TC and TG. Histopathological analysis was performed by Research Center for Animal Medicine (RCAM) of National Chung-Hsing University, Taichung, Taiwan. In brief, liver, heart, kidney, and pancreas were fixed in 10% formalin solution (pH 6.8-7.2) at 4ºC. Tissue sections were prepared by standard procedures and then stained with hematoxylin and eosin (H&E). The sections were analyzed by light microscopy. The criteria used for grading hepatic steatosis were described in the previous study [32].
Immunoblotting analysis
Brain tissues were collected and divided into the cerebrum and cerebellum. Tissues were homogenized in a grinder in radioimmunoprecipitation assay (RIPA) buffer on ice for 30 minutes. After centrifugation at 10,000 ×g for 15 minutes of tissue lysate, the protein concentration of supernatant was determined by protein assay dye (Bio-Rad, Hercules, CA, USA). The proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membranes (PerkinElmer, Waltham, MA, USA). After blocking, the membranes were incubated with primary antibodies, which including connexin 36 (Cx36) and phosphorylated Ca2+/calmodulin-dependent protein kinase (p-CaMKII, Santa Cruz Biotechnology, Santa Cruz, CA, USA), synaptophysin (SYP, Proteintech, Rosemont, IL, USA), and β-actin (Sigma), at 4°C for 16 h. After washing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology). The membranes were developed using enhanced chemiluminescence reagents (Perkin Elmer, Waltham, MA, USA).
Statistical analysis
All data were given as the mean ± standard error (SE). Statistical analysis of results in Figure 2 was performed by two-way analysis of variance (ANOVA) using Duncan post hoc. In Figure 3~6 and Table 1, one-way ANOVA with post hoc Dunnett's test was used to analysis the results. A value of P<0.05 was considered statistically significant (Sigma-Stat 2.0, Jandel Scientific, San Rafael, CA, USA).
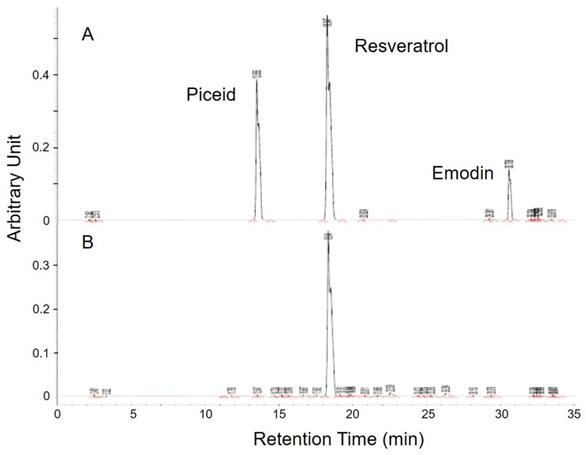
HPLC analysis of MBR. (A) Three standards were appeared at the retention time of 13.6, 18.3 and 30.7 minutes. (B) Among these three similar structures, resveratrol was significantly detected in the sample.
Diffuse of microvesicular fatty infiltration of liver in resveratrol-treated hamsters on a high-fat diet.
| Organ | Histophathological Finding | Groups | |||
|---|---|---|---|---|---|
| C | L | M | H | ||
| Liver | Fatty infiltration with microvesicles, portal area, diffuse, slight to moderate/severe1 | 3.6±0.72 | 3.1±0.6 | 3.1±0.6 | 2.7±0.7* |
1Degree of lesions was graded from one to five depending on severity: 1 = minimal (< 1%); 2 = slight (1-25%); 3 = moderate (26-50%); 4 = moderate/severe (51-75%); 5 = severe/high (76-100%).
2The final numerical score was calculated by dividing the sum of the number per grade of affected hamsters by the total number of examined hamsters.
* Statistically significant difference between control and treated groups at P<0.05.
Results
Purity of resveratrol in MBR
There were plenty of glycosides in the P. cuspidatum roots, which were an ideal base for the biotransformation-produced resveratrol. After screening for the activity of glucosidase in different strains, D. bruxellensis was selected to do the biotransformation of piceid to resveratrol. In the study of our collaborative team, the content of resveratrol reached the maximum level after 48 hours of incubation [24]. The extraction of fermentative product was diluted and detected by HPLC to examine the concentration of piceid, resveratrol and emodin, which are the main phenolic compounds extracted from P. cuspidatum roots, according to the integration of area under curve in comparison with standards (Figure 1). The standard piceid, resveratrol and emodin appeared at the retention time of 13.6, 18.3 and 30.7 minutes. The average percentage of piceid, resveratrol and emodin in MBR sample was 0.3%, 67% and 0.03%, respectively. Therefore, the resveratrol concentration in MBR used in the animal study would be 3.35 (group L), 13.4 (group M) and 33.5 (group H) mg/kg/day.
Lipid-lowering effect of MBR
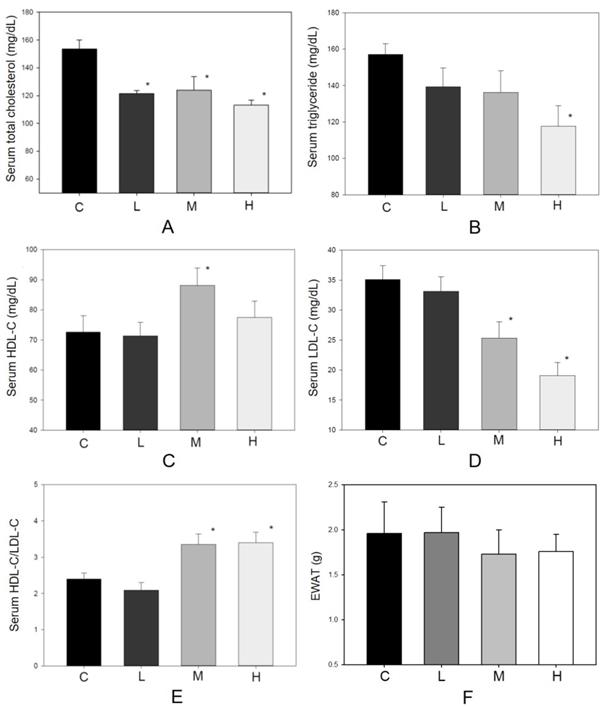
As an antioxidant, resveratrol shows a protective effect in lowering blood lipid concentrations [13, 17]. During the experiment, no hamster showed significant adverse effects such as loss of body weight or reducing food intake (Figure 2). There were 10 hamsters in each group in the following analysis. Figure 3 shows the serum lipid levels including TC, TG, LDL-C, HDL-C, and HDL-C/LDL-C. In group L (5 mg/kg MBR), the lipid-lowering effect could only be found in the result of serum TC compared to the group C (control group)(Figure 3A). On the other hand, TC, LDL-C, and HDL-C/LDL-C in groups M and H significantly differed from that of group C (Figure 3A, D, and E). TG was significantly downregulated only in group H (Figure 3B). The weight of EWAT was used to represent the fat mass in the animal [33]. The mean level of EWAT in groups M and H was 10% lower in comparison with group C. However, there was no significant difference between each group (Figure 3F).
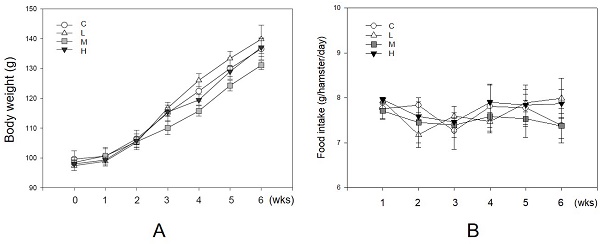
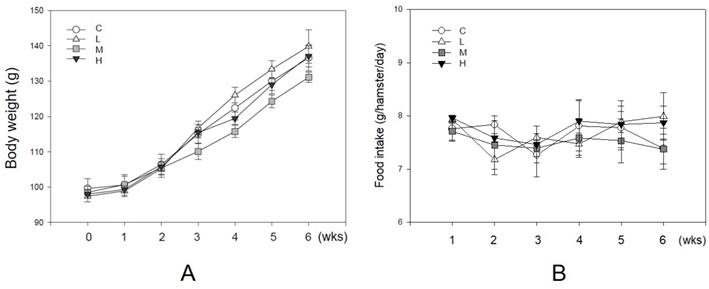
Unaffecting of MBR on the body weight and food intake of hamsters during the experiment. Hamsters were separated into four groups and fed with a HFD (control group, C), HFD + resveratrol 5 mg/kg (low dose group, L), HFD + resveratrol 20 mg/kg (medium dose group, M) and HFD + resveratrol 50 mg/kg (high dose group, H), respectively for 6 weeks. Body weight and food intake were recorded every week. (A) Body weight and (B) Food intake.
Effect of MBR on lipid levels of hamsters fed with HFD. Hamsters were fed with different diet for 6 weeks. After sacrificing, serum was collected and analyzed for (A) total cholesterol, (B) triglyceride, (C) HDL-C, (D) LDL-C and (E) HDL-C/LDL-C. (F) The weights of epididymal white adipose tissue (EWAT) were measured. Data are expressed as the mean ± standard error (SE). *P<0.05 vs. group C.
Anti-hepatic steatosis effect of MBR
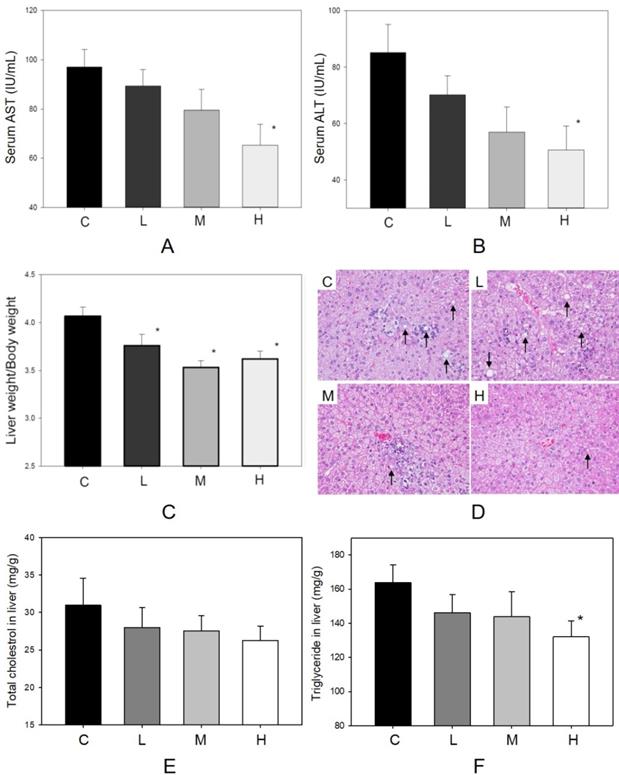
The factors influencing hepatic function and histopathological alteration of the liver were determined in four groups. In Figure 2A, body weight increased during the 6-week treatment period. The dosages of MBR treatment did not affect the appetite of hamsters (Figure 2B). In addition, administration of MBR resulted in a decrease in levels of serum AST and ALT compared to the control group, especially in group H (Figure 4A and B). MBR treatment also decreased the liver weight/body weight ratio in groups L, M and H in comparison to the group C (Figure 4C). Figure 4D and Table 1 show the results of the histopathological examination of the liver tissue. Fatty infiltration with micro- and macrovesicles in hepatocytes and mononuclear cell infiltration were found at different levels in the four groups. The severity was graded as 3.6 ± 0.7 in group C, 3.1 ± 0.6 in group L, 3.1 ± 0.6 in group M, and 2.7 ± 0.7 in group H. The significant lowering of severity could be found in group H (Table 1). TC and TG data of liver tissue were examined to represent the hepatic lipid accumulation (Figure 4E and F). The result showed that there were descending trends of both TC and TG in liver tissue in MBR-fed groups. The significant 19% reduction can be found in the result of TG in group H (Figure 4F).
Protective effect of MBR on liver function of hamsters fed with HFD. Hamsters were separated into 4 groups and fed with HFD for 6 weeks. At the end of experiment, serum was gathered to examine the (A) AST and (B) ALT. Liver was collected to measure the (C) liver weight/body weight, to detect by (D) H&E stain, and to observe the (E) total cholesterol and (F) triglyceride in homogenized liver. Data are expressed as the mean ± SE. *P<0.05 vs. group C.
Effect of MBR on blood glucose level in hamsters fed with HFD. After feeding for 6 weeks, serum was collected to measure the glucose level. Data are expressed as the mean ± SE. *P<0.05 vs. group C.
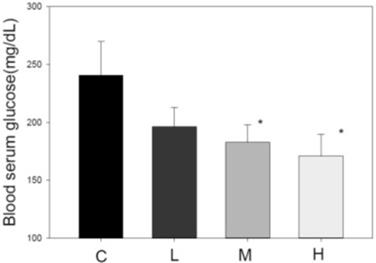
Blood glucose-lowering effect of MBR
Another index of metabolic syndrome is high blood glucose. HFD-fed animal model also revealed elevated blood glucose [34]. After administration of MBR for 6 weeks, serum was collected for FBG examination. In HFD-treated group C, the glucose level was higher than that in the three MBR-treated groups. MBR supplementation in groups M and H significantly lowered glucose levels (Figure 5).
Effect of MBR on synaptic function
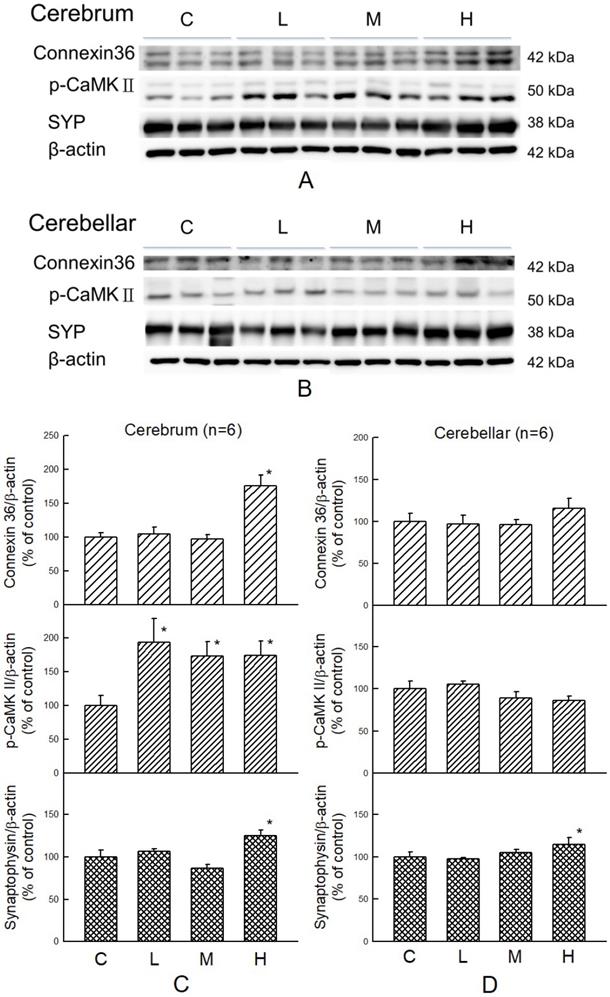
Cognitive-related proteins were analyzed in the cerebrum and cerebellum of HFD-treated hamsters with or without co-administration of MBR. In the cerebrum, which mainly controls the cognitive process, p-CaMKII, a kinase which is related to synaptic strength, was elevated for all three dosage groups of MBR. Cx36, a gap junction protein, and SYP, a presynaptic protein, were significantly increased only in group H for 74% and 26% in comparison with group C, respectively. On the other hand, in the cerebellum, which is less relevant to cognitive function, only SYP were significantly elevated for 18% in group H compared with group C (Figure 6).
Discussion
Resveratrol usually shows as glycosylated form, also called piceid, in natural sources. Piceid could not present as many bioactivities as resveratrol. On the other hand, the product of bioengineered resveratrol needs complicated processes to remove the unexpected by-products [35]. Therefore, to identify a microbe with efficient β-glucosidase and optimal fermentative formula may be a better way to obtain resveratrol in the industry. In the study of our collaborative team, the fermentative biotransformation of P. cuspidatum to MBR was optimum at about 48 hrs. Using 4-Nitrophenyl-β-D-glucopyranoside (pNPG) as a substrate to test the activity of β-glucosidase, D. bruxellensis showed high yield rate than other strains [24]. During the analytic process of HPLC, the only minor percentage of piceid (0.3%) and emodin (0.03%) could be found in the sample (Figure 1). This result suggested that the effect of MBR was not from the piceid or emodin. The substances in the other 33% could not be detected by the HPLC method which we used in this study. During the fermentation process of yeast, saccharides were reduced to produce sugar alcohols [36]. In comparison with sucrose, blood glucose and insulin levels are affecting less by sugar alcohols. This is the main mechanism of sugar alcohols to show the anti-diabetic and lipid-lowering effects [37]. However, the sugar content in the diet used in this study was not replaced by MBR. Hence, protective effects revealed in the results were not from the by-product, sugar alcohols, in MBR. In addition, the lipid-lowering effect of pure compound of resveratrol and purified resveratrol has been reported in recent studies [38, 39]. Therefore, we suggested that the results presented in this manuscript were mainly resulted from the resveratrol of MBR. Even though, the effects of by-product could not be totally ruled out since the direct result of by-product was not examined in this manuscript.
In the results, MBR ameliorated hyperlipidemia, hepatic steatosis, and hyperglycemia, and revealed neuroprotective effect in the HFD-treated hamsters. According to the references, a formula designed by pharmacologists to estimate dosages between species was using a conversion factor (km) based on the body weight/surface area of a species [40, 41]. Therefore, the dose used in Species 1 is equal to (km Species 2/km Species 1) times of the dose in Species 2. In this study, MBR doses of 5, 20 and 50 mg/kg in hamster might be comparable to 0.42, 1.66 and 4.16 mg/kg in human, respectively. Although the results on hamster might not reflect directly on human, these amounts of MBR might be applicable in clinical usage.
Experimental studies indicate that HFD consumption by animals is a well-established model to study hepatic steatosis, insulin resistance [29], as well as impaired neurogenesis [42]. Therefore, HFD-treated hamsters were used to examine the protective effect of MBR on the above disease-related factors. The decrease of TC and TG and increase of HDL-C/LDL-C were observed in the serum of HFD-treated hamsters supplemented with MBR (Figure 3A to E). However, EWAT weight which was usually used to represent the fat mass of the animal was not significantly reduced in MBR-fed groups (Figure 3F). According to the literature, the reduction of EWAT weight is not significant, but the size of adipocyte is [43]. Therefore, MBR might also decrease the lipid accumulation in adipocyte in the current animal model. The downregulation of the lipids may result from decreasing fat accumulation and lipogenesis via modulation the transcription and activity of 5-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase by resveratrol [44, 45].
Effect of MBR on the expressions of cognitive-associated proteins in the brain of hamsters fed with HFD. After 6-week treatment, hamsters were sacrificed. (A and C) Cerebrum and (B and D) cerebellum were collected and analyzed by Western blot to detect the expressions of synaptic-related proteins, Cx36, p-CaMKII, and SYP. β-actin was used as a loading control. Data are expressed as the mean ± SE. *P<0.05 vs. group C. (A and B) Western blot; (C and D) Quantification data.
Metabolic syndrome is characterized not only by hyperlipidemia but also by hepatic steatosis. Dyslipidemia results in fatty infiltration and chronic inflammation of the liver. This effect may further extend to steatohepatitis [29]. In group C, these indexes also appeared in the liver tissue sections (Table 1). In addition to reducing serum AST and ALT level, MBR treatment also ameliorated several hepatic lipid accumulated indexes, including hepatic fat droplets and hepatic TG level (Figure 4D and F). Resveratrol improves fatty liver disease and prevents hepatic steatosis via decreasing ROS and regulating the autophagy pathway [16, 46]. Resveratrol and caloric restriction induced autophagy stimulated by the SIRT1-FoxO1 pathway has been identified as a critical protective mechanism of nonalcoholic fatty liver disease (NAFLD). Autophagy related genes are activated by the transcription complex of FoxO1 and other factors to remove of TG and lipid droplets in hepatic cells [16]. Accordingly, MBR used in this study might also activate autophagy pathway in HFD-fed animal model to improve hepatic steatosis.
HFD induces obesity and dyslipidemia, which are risk factors associated with insulin resistance and hyperglycemia [19]. The MBR produced in the present study displayed the ability to reduce hyperglycemia (Figure 5). It is reported that resveratrol reduces insulin resistance, fasting blood glucose, and inflammatory biomarkers [19]. The mechanisms include increasing liver glycogen accumulation and elevating the expression, activity, and translocation of glucose transporter 4 (GLUT4)[47]. In addition, mitochondrial biogenesis elevates the glucose uptake in skeletal muscle. SIRT1 and its substrate peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) stimulate the mitochondrial biogenesis which may be the glucose lowering pathway of resveratrol [48]. Therefore, both GLUT4 and mitochondria in skeletal muscle might be the targets of MBR on lowering blood glucose.
Hyperlipidemia gives rise to oxidative stress by increasing lipid peroxidation. This lipotoxic effect of HFD treatment can damage human brain and cognitive functions [49]. Additionally, lipid profile disorder also boosts the risk of vascular dementia, the second common cause of dementia after stroke [50]. Resveratrol protects brain function by lowering oxidative stress and activates extracellular signal-regulated kinase (ERK) - cAMP response element-binding protein (CREB) pathway to preserve hippocampal CA1 neurons in ischemic reperfusion-treated rats [20, 51]. CaMKII is necessary for synaptic function and behavioral memory formation [52]. Cx36 is highly expressed in the nervous system and is an essential protein to form gap junctions, which facilitate the signal transport between neurons [53]. SYP is the synaptic protein in the presynaptic element, and it can be used as a specific synaptic marker in the central nervous system. The amount of SYP may represent the quantity and quality of synapses [54]. These three types of protein were upregulated in the MBR-treated groups, especially in group H. These results suggest that MBR might protect brain tissue from cognition impairment resulting from HFD via elevation of signal transmission between neurons.
Conclusion
In conclusion, the MBR obtained by biotransformation, which is a high yield method, might have protective effects for hyperlipidemia, hepatic steatosis, hyperglycemia and synaptic impairment.
Acknowledgements
The authors thank Hsiao-Ping Kuo, Jinn-Tsyy Lai, and Shyue-Tsong Huang from the Bioresources Collection and Research Center, Food Industry Research and Development Institute for their technical support.
Funding
This work is supported by the grants of Zhen-Ho Advanced Technology Co., Ltd (E106N0150) and Chung Shan Medical University (CSMU-INT-105-07), Taiwan, R.O.C. The funders had no role in study design, in the collection, analysis and interpretation of data, and in the writing of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Karr S. Epidemiology and management of hyperlipidemia. Am J Manag Care. 2017;23:S139-48
2. Zheng J, Shi L, Xu W, Zhao N, Liang F, Zhou J. et al. Impact of hyperlipidemia and atrial fibrillation on the efficacy of endovascular treatment for acute ischemic stroke: A meta-analysis. Oncotarget. 2017;8:72972-84
3. Polonskaya YV, Shramko VS, Morozov SV, Chernyak EI, Chernyavsky AM, Ragino YI. Balance of fatty acids and their correlations with parameters of lipid metabolism and markers of inflammation in men with coronary atherosclerosis. Bull Exp Biol Med. 2017;164:33-5
4. Suárez Bagnasco M. Psychological issues and cognitive impairment in adults with familial hypercholesterolemia. Fam Pract. 2017;34:520-4
5. Li R, Wang TJ, Lyu PY, Liu Y, Chen WH, Fan MY. et al. Effects of plasma lipids and statins on cognitive function. Chin Med J (Engl). 2018;131:471-6
6. Kuang ZM. Effect of combined antihypertensive and lipid-lowering therapies on cognitive function: A new treatment strategy? Cardiol Res Pract. 2020;2020:1484357
7. Jeong YJ, Woo SG, An CH, Jeong HJ, Hong YS, Kim YM. et al. Metabolic engineering for resveratrol derivative biosynthesis in escherichia coli. Mol Cells. 2015;38:318-26
8. Kuo HP, Wang R, Lin YS, Lai JT, Lo YC, Huang ST. Pilot scale repeated fed-batch fermentation processes of the wine yeast dekkera bruxellensis for mass production of resveratrol from polygonum cuspidatum. Bioresour Technol. 2017;243:986-93
9. Zhang X, Wu Q, Zhang Q, Lu Y, Liu J, Li W. et al. Resveratrol attenuates early brain injury after experimental subarachnoid hemorrhage via inhibition of nlrp3 inflammasome activation. Front Neurosci. 2017;11:611
10. Hao Q, Xiao X, Zhen J, Feng J, Song C, Jiang B. et al. Resveratrol attenuates acute kidney injury by inhibiting death receptormediated apoptotic pathways in a cisplatininduced rat model. Mol Med Rep. 2016;14:3683-9
11. Bostanghadiri N, Pormohammad A, Chirani AS, Pouriran R, Erfanimanesh S, Hashemi A. Comprehensive review on the antimicrobial potency of the plant polyphenol resveratrol. Biomed Pharmacother. 2017;95:1588-95
12. Carter LG, D'Orazio JA, Pearson KJ. Resveratrol and cancer: Focus on in vivo evidence. Endocr Relat Cancer. 2014;21:R209-25
13. Momtazi-Borojeni AA, Katsiki N, Pirro M, Banach M, Rasadi KA, Sahebkar A. Dietary natural products as emerging lipoprotein(a)-lowering agents. J Cell Physiol. 2019;234:12581-94
14. Mobasheri A, Shakibaei M. Osteogenic effects of resveratrol in vitro: Potential for the prevention and treatment of osteoporosis. Ann N Y Acad Sci. 2013;1290:59-66
15. Borriello A, Cucciolla V, Della Ragione F, Galletti P. Dietary polyphenols: Focus on resveratrol, a promising agent in the prevention of cardiovascular diseases and control of glucose homeostasis. Nutr Metab Cardiovasc Dis. 2010;20:618-25
16. Ding S, Jiang J, Zhang G, Bu Y, Zhang G, Zhao X. Resveratrol and caloric restriction prevent hepatic steatosis by regulating sirt1-autophagy pathway and alleviating endoplasmic reticulum stress in high-fat diet-fed rats. PloS one. 2017;12:e0183541
17. Tanko Y, Jimoh A, Ahmed A, Mohammed A, Ayo JO. Resveratrol protects rabbits against cholesterol diet-induced hyperlipidaemia. Niger J Physiol Sci. 2016;31:71-5
18. Wu Q, Hu Y, Jiang M, Wang F, Gong G. Effect of autophagy regulated by sirt1/foxo1 pathway on the release of factors promoting thrombosis from vascular endothelial cells. Int J Mol Sci. 2019 20
19. Zare Javid A, Hormoznejad R, Yousefimanesh HA, Zakerkish M, Haghighi-Zadeh MH, Dehghan P. et al. The impact of resveratrol supplementation on blood glucose, insulin, insulin resistance, triglyceride, and periodontal markers in type 2 diabetic patients with chronic periodontitis. Phytother Res. 2017;31:108-14
20. Wang R, Liu YY, Liu XY, Jia SW, Zhao J, Cui D. et al. Resveratrol protects neurons and the myocardium by reducing oxidative stress and ameliorating mitochondria damage in a cerebral ischemia rat model. Cell Physiol Biochem. 2014;34:854-64
21. Becker JV, Armstrong GO, van der Merwe MJ, Lambrechts MG, Vivier MA, Pretorius IS. Metabolic engineering of saccharomyces cerevisiae for the synthesis of the wine-related antioxidant resveratrol. FEMS Yeast Res. 2003;4:79-85
22. Santos AC, Veiga F, Ribeiro AJ. New delivery systems to improve the bioavailability of resveratrol. Expert Opin Drug Deliv. 2011;8:973-90
23. Jin S, Luo M, Wang W, Zhao CJ, Gu CB, Li CY. et al. Biotransformation of polydatin to resveratrol in polygonum cuspidatum roots by highly immobilized edible aspergillus niger and yeast. Bioresour Technol. 2013;136:766-70
24. Kuo HP, Wang R, Huang CY, Lai JT, Lo YC, Huang ST. Characterization of an extracellular beta-glucosidase from dekkera bruxellensis for resveratrol production. J Food Drug Anal. 2018;26:163-71
25. Zhao Y, Liu J, Hao W, Zhu H, Liang N, He Z. et al. Structure-specific effects of short-chain fatty acids on plasma cholesterol concentration in male syrian hamsters. J Agric Food Chem. 2017;65:10984-92
26. Yang TH, Yao HT, Chiang MT. Red algae (gelidium amansii) hot-water extract ameliorates lipid metabolism in hamsters fed a high-fat diet. J Food Drug Anal. 2017;25:931-8
27. Zhao MJ, Wang SS, Jiang Y, Wang Y, Shen H, Xu P. et al. Hypolipidemic effect of xh601 on hamsters of hyperlipidemia and its potential mechanism. Lipids Health Dis. 2017;16:85
28. Ramprasath VR, Jones PJ. Anti-atherogenic effects of resveratrol. Eur J Clin Nutr. 2010;64:660-8
29. Wu CC, Weng WL, Lai WL, Tsai HP, Liu WH, Lee MH. et al. Effect of lactobacillus plantarum strain k21 on high-fat diet-fed obese mice. Evid Based Complement Alternat Med. 2015;2015:391767
30. Reaven GM, Chen YD, Jeppesen J, Maheux P, Krauss RM. Insulin resistance and hyperinsulinemia in individuals with small, dense low density lipoprotein particles. J Clin Invest. 1993;92:141-6
31. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499-502
32. Shackelford C, Long G, Wolf J, Okerberg C, Herbert R. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol Pathol. 2002;30:93-6
33. Mauer MM, Bartness TJ. Temporal changes in fat pad mass and cellularity after lipectomy in siberian hamsters. Physiol Behav. 1997;62:1029-36
34. Gong L, Guo S, Zou Z. Resveratrol ameliorates metabolic disorders and insulin resistance in high-fat diet-fed mice. Life Sci. 2020;242:117212
35. Sydor T, Schaffer S, Boles E. Considerable increase in resveratrol production by recombinant industrial yeast strains with use of rich medium. Appl Environ Microbiol. 2010;76:3361-3
36. Zhang G, Lin Y, He P, Li L, Wang Q, Ma Y. Characterization of the sugar alcohol-producing yeast pichia anomala. J Ind Microbiol Biotechnol. 2014;41:41-8
37. Zoidl GR, Spray DC. The roles of calmodulin and camkii in cx36 plasticity. Int J Mol Sci. 2021 22
38. Li Z, Zhang Z, Ke L, Sun Y, Li W, Feng X. et al. Resveratrol promotes white adipocytes browning and improves metabolic disorders in sirt1-dependent manner in mice. FASEB J. 2020;34:4527-39
39. Zhang YJ, Zhao H, Dong L, Zhen YF, Xing HY, Ma HJ. et al. Resveratrol ameliorates high-fat diet-induced insulin resistance and fatty acid oxidation via atm-ampk axis in skeletal muscle. Eur Rev Med Pharmacol Sci. 2019;23:9117-25
40. Boxenbaum H, DiLea C. First-time-in-human dose selection: Allometric thoughts and perspectives. J Clin Pharmacol. 1995;35:957-66
41. Ings RM. Interspecies scaling and comparisons in drug development and toxicokinetics. Xenobiotica. 1990;20:1201-31
42. Wang Q, Yuan J, Yu Z, Lin L, Jiang Y, Cao Z. et al. Fgf21 attenuates high-fat diet-induced cognitive impairment via metabolic regulation and anti-inflammation of obese mice. Mol Neurobiol. 2018;55:4702-17
43. Im JY, Ki HH, Xin M, Kwon SU, Kim YH, Kim DK. et al. Anti-obesity effect of triticum aestivum sprout extract in high-fat-diet-induced obese mice. Biosci Biotechnol Biochem. 2015;79:1133-40
44. Villanueva JA, Sokalska A, Cress AB, Ortega I, Bruner-Tran KL, Osteen KG. et al. Resveratrol potentiates effect of simvastatin on inhibition of mevalonate pathway in human endometrial stromal cells. J Clin Endocrinol Metab. 2013;98:E455-62
45. Cho IJ, Ahn JY, Kim S, Choi MS, Ha TY. Resveratrol attenuates the expression of hmg-coa reductase mrna in hamsters. Biochem Biophys Res Commun. 2008;367:190-4
46. Ma Z, Zhang Y, Li Q, Xu M, Bai J, Wu S. Resveratrol improves alcoholic fatty liver disease by downregulating hif-1alpha expression and mitochondrial ros production. PloS one. 2017;12:e0183426
47. Szkudelska K, Szkudelski T. Resveratrol, obesity and diabetes. Eur J Pharmacol. 2010;635:1-8
48. Tang BL. Sirt1 and the mitochondria. Mol Cells. 2016;39:87-95
49. Charradi K, Mahmoudi M, Bedhiafi T, Kadri S, Elkahoui S, Limam F. et al. Dietary supplementation of grape seed and skin flour mitigates brain oxidative damage induced by a high-fat diet in rat: Gender dependency. Biomed Pharmacother. 2017;87:519-26
50. Appleton JP, Scutt P, Sprigg N, Bath PM. Hypercholesterolaemia and vascular dementia. Clin Sci (Lond). 2017;131:1561-78
51. Li Z, Fang F, Wang Y, Wang L. Resveratrol protects ca1 neurons against focal cerebral ischemic reperfusion-induced damage via the erk-creb signaling pathway in rats. Pharmacol Biochem Behav. 2016;146-147:21-7
52. Lisman J, Schulman H, Cline H. The molecular basis of camkii function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175-90
53. Corsini S, Tortora M, Rauti R, Nistri A. Nicotine protects rat hypoglossal motoneurons from excitotoxic death via downregulation of connexin 36. Cell Death Dis. 2017;8:e2881
54. Chen Y, Cui Z, Wang L, Liu H, Fan W, Deng J. et al. The impairment of learning and memory and synaptic loss in mouse after chronic nitrite exposure. Environ Toxicol. 2016;31:1720-30
Author contact
![]() Corresponding author: Ching-Han Yu: chyuedu.tw; Department of Physiology, School of Medicine, Chung Shan Medical University, No. 110, Section 1, Jianguo North Road, Taichung 40201, Taiwan; Tel: +886-4-24730022 ext 11655; Fax: +886-4-23248155.
Corresponding author: Ching-Han Yu: chyuedu.tw; Department of Physiology, School of Medicine, Chung Shan Medical University, No. 110, Section 1, Jianguo North Road, Taichung 40201, Taiwan; Tel: +886-4-24730022 ext 11655; Fax: +886-4-23248155.
Email: chyuedu.tw

 Global reach, higher impact
Global reach, higher impact