3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(9):1460-1472. doi:10.7150/ijms.74533 This issue Cite
Research Paper
Targeting IRE1α-JNK-c-Jun/AP-1-sEH Signaling Pathway Improves Myocardial and Coronary Endothelial Function Following Global Myocardial Ischemia/Reperfusion
1. The Institute of Cardiovascular Diseases & Department of Cardiovascular Surgery, TEDA International Cardiovascular Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College & Tianjin University, Tianjin, China.
2. Department of Physiology, Hebei Medical University, Shijiazhuang, Hebei, China.
3. University of Health and Rehabilitation Sciences, Qingdao, Shandong, China.
4. Drug Research and Development Center, Wannan Medical College, Wuhu, Anhui, China.
5. Department of Surgery, Oregon Health and Science University, Portland, Oregon, USA.
Abstract
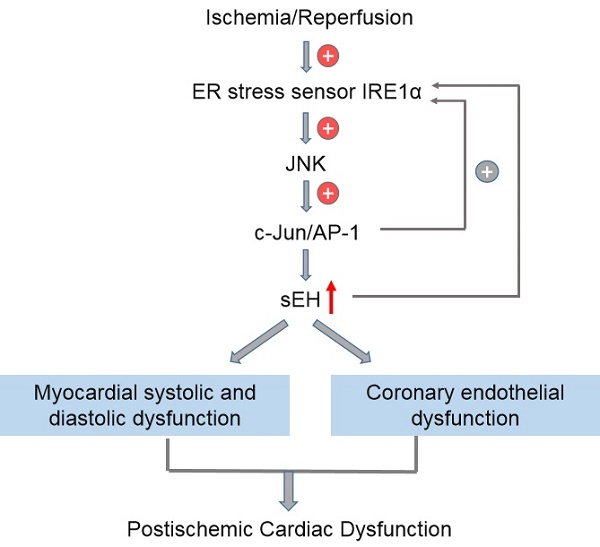
Objectives: Endoplasmic reticulum (ER) stress and soluble epoxide hydrolase (sEH) upregulation/activation have been implicated in myocardial ischemia/reperfusion (I/R) injury. We previously reported that ER stress mediates angiotensin II-induced sEH upregulation in coronary endothelium, whether and how ER stress regulates sEH expression to affect postischemic cardiac function remain unexplored. This study aimed to unravel the signaling linkage between ER stress and sEH in an ex vivo model of myocardial I/R injury.
Methods: Hearts from male Wistar-Kyoto rats were mounted on a Langendorff apparatus and randomly allocated to 7 groups, including control, I/R (30-min ischemia and 60-min reperfusion), and I/R groups pretreated with one of the following inhibitors: 4-PBA (targeting: ER stress), GSK2850163 (IRE1α), SP600125 (JNK), SR11302 (AP-1), and DCU (sEH). The inhibitor was administered for 15 min before ischemia with a peristaltic pump. Hemodynamic parameters including left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP), and maximal velocity of contraction (+dp/dtmax) and relaxation (-dp/dtmax) of the left ventricle were continuously recorded using an intraventricular balloon. Endothelial dilator function of the left anterior descending artery was studied in a wire myograph upon completion of reperfusion. The expression of ER stress molecules, JNK, c-Jun, and sEH was determined by western-blot.
Results: I/R decreased LVSP (105.5±6.4 vs. 146.9±13.4 mmHg), and increased LVEDP (71.4±3.0 vs. 6.0±2.7 mmHg), with a resultant decreased LVDP (34.1±9.2 vs. 140.9±13.1 mmHg). I/R attenuated +dp/dtmax (651.7±142.1 vs. 2806.6±480.6 mmHg/s) and -dp/dtmax (-580.0±109.6 vs. -2118.0±244.9 mmHg/s) (all ps<0.001). The I/R-induced cardiac dysfunction could be alleviated by 4-PBA (LVSP 119.5±15.6 mmHg, p<0.01; LVEDP 21.2±4.2 mmHg, LVDP 98.3±12.0 mmHg, +dp/dtmax 2166.7±208.4 mmHg/s, and -dp/dtmax -1350.9±99.8 mmHg/s, all ps<0.001), GSK2850163 (LVSP 113.4±10.9 mmHg, p<0.01; LVEDP 37.1±3.1 mmHg, LVDP 76.3±13.9 mmHg, +dp/dtmax 1586.5±263.3 mmHg/s, -dp/dtmax -1127.7±159.9 mmHg/s, all ps<0.001), SP600125 (LVSP 113.9±5.6 mmHg, LVDP 40.5±3.3 mmHg, +dp/dtmax 970.1±89.8 mmHg/s, all ps<0.01), SR11302 (LVSP 97.9±7.5 mmHg, p<0.01; LVEDP 52.7±8.6mmHg, p<0.001; LVDP 45.2±9.8mmHg, p<0.05; +dp/dtmax 1231.5±196.6 mmHg/s, p<0.01; -dp/dtmax -658.3±68.9 mmHg/s, p<0.05), or DCU (LVSP 109.9±4.1 mmHg, p<0.01; LVEDP 11.7±1.8 mmHg, LVDP 98.2±4.9 mmHg, +dp/dtmax 1869.8±121.9 mmHg/s, and -dp/dtmax -1492.3±30.8 mmHg/s, all ps<0.001). The relaxant response of the coronary artery to acetylcholine was decreased after I/R in terms of both magnitude and sensitivity (p<0.001). All inhibitors improved acetylcholine-induced relaxation. Global I/R increased sEH expression and induced ER stress in both myocardium and coronary artery. Inhibition of ER stress or IRE1α downregulated I/R-induced sEH expression and inhibited JNK and c-Jun phosphorylation. Both JNK and AP-1 inhibitors lowered sEH level in myocardium and coronary artery in I/R-injured hearts.
Conclusions: This study deciphered the molecular linkage between ER stress and sEH regulation in global I/R insult by uncovering a novel signaling axis of IRE1α-JNK-c-Jun/AP-1-sEH, which provided basis for future research on the therapeutic potential of targeting the IRE1α-JNK-c-Jun/AP-1-sEH axis for ischemic myocardial injury.
Keywords: Cardiac function, Endoplasmic reticulum stress, Endothelial function, Ischemia/Reperfusion injury, Soluble epoxide hydrolase

 Global reach, higher impact
Global reach, higher impact