3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(7):1155-1162. doi:10.7150/ijms.74161 This issue Cite
Review
Treatment of ischemic stroke with modified mesenchymal stem cells
Department of Neurovascular Surgery, First Hospital of Jilin University, 1xinmin Avenue Changchun130021, Jilin Province, China.
Received 2022-4-19; Accepted 2022-6-15; Published 2022-6-27
Abstract
Ischemic stroke is one of the leading causes of death and disability. Ischemia triggers a cascade of events leading to cell death and cerebral infarction. Mesenchymal stem cell (MSC) therapy is a promising treatment modality to promote the development of nerve and blood vessels and improve nerve function. However, MSCs have a limited therapeutic effect in the harsh microenvironment of ischemic brain tissue. Modified MSC therapy shows better therapeutic effect under different pathological conditions, and is expected to be translated into clinical practice. In this article, we review the latest advances in the development of modified MSCs for the treatment of cerebral ischemia. In particular, we summarize the targets involved in migration, homing, antioxidant stress, anti-inflammatory, nerve and vascular regeneration, providing new ideas for clinical transformation.
Keywords: Mesenchymal stem cells, Modification, Ischemic stroke, treatment
Introduction
Stroke is the second leading cause of death in the world after ischemic heart disease [1]. Ischemic stroke (IS) accounts for 87% of all stroke patients, and its incidence rate is still rising [2, 3]. IS is also a leading cause of disability. In China, IS accounts for approximately 70% of all cases of stroke [4]. An estimated three quarters of patients with ischemic stroke drop out of the labor force, and two fifths of these patients develop severe disability [5]. Therefore, prevention and treatment of ischemic stroke is a key research imperative.
Currently, there is a paucity of effective treatments for IS. Tissue plasminogen activator (T-PA) is administered intravenously to unblock the blocked blood vessels. However, the time window for T-PA treatment is short (≤4.5 hours) and there is a risk of secondary intracerebral hemorrhage [6]. Mechanical thrombectomy (MT) can extend the treatment window to 24 hours, but this particular procedure can only be performed in a limited number of qualified hospitals and requires strict screening for indications and contraindications; therefore, only a few patients are able to receive MT treatment [7]. Therefore, it is imperative to develop a new treatment method for IS.
Mesenchymal stem cells (MSCs) are multipotent cells that can specialize into several cell types from different lineages. Intravenously administered MSCs can migrate to the site of tissue damage and promote angiogenesis, growth, and differentiation of local progenitor cells [8-12]. Some studies have shown no significant risk of host immune response to allogeneic transplantation of MSCs [13-15]. In addition, MSCs are easy to isolate and culture from different tissues such as cord blood, bone marrow, and adipose tissue. These attributes make MSCs the main source of cell therapy for many diseases.
MSC therapy has been shown to promote post-stroke functional recovery and neurological outcomes [16, 17]. In addition, clinical trials of MSC therapy for the treatment of IS have demonstrated its safety and feasibility [18-22]. However, there are several barriers that limit its use and therapeutic effectiveness. For example, in the harsh microenvironment of stroke (inflammation storm, oxidative stress), isolated MSCs gradually lose their homing ability to the lesion [23]. In clinical trials, although MSC therapy was shown to be safe and confer some therapeutic effects, the effects were not significant [24-26]. Attempts have been made to develop novel MSC-based methods for the treatment of IS, such as genetically-modified MSCs, and use of preconditioning, electrical stimulation, and ultrasonic stimulation. A large number of studies published in recent years have demonstrated the improved ability of MSCs in the treatment of IS by using genetic modification or preconditioning of MCS in combination with physical therapy [23, 27-30]. This paper provides an overview of the recent advances in this field.
Application of modified MSCs in the treatment of ischemic stroke
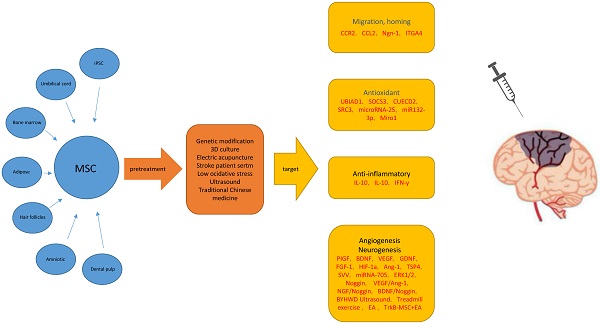
There are several therapeutic challenges in the use of MSC therapy for treatment of IS. The key issues include whether MSCs and their exosomes (microvesicles) can migrate to the target organ and play a role, their ability to survive for a long time in the ischemic and hypoxic brain tissue, and their ability to successfully transform into functional nerve cells in the damaged area (Figure 1).
Migration and Homing
It is generally believed that the homing ability of MSCs at the site of target lesions and their implantation determines the therapeutic effect of MSC therapy [31, 32]. However, the decreased expression of some chemokine receptors (such as CCR2 and CXCR4) during the continuous passage of MSC was found to affect the homing ability of MSCs to target lesions [33-39]. In order to improve the homing ability of MSCs, it was found that the expression of some chemokines (CCR1, CCR2, CXCR4) was increased after gene modification or pretreatment of MSCs [37, 40]. At the same time, the interaction of CCL2/CCR2 and SDF-1/CXCR4 was shown to significantly improve the homing and migration of modified MSCs during acute ischemic attack [41-43]. This was also shown to significantly improve the neurological function [23, 44-50].
Secondly, in the experimental middle cerebral artery occlusion (MCAO) model, only a small part of intravenously injected MSCs entered the ischemic brain tissue, and most of them were trapped in the lung and spleen [33, 51], which also affected the homing of MSCs. Compared with intravenous administration, MSCs were found to more readily migrate to the damaged brain tissue after arterial injection; in addition, genetically-modified MSCs were found to survive longer in the ischemic brain tissue [40] and reduce vascular embolization [43]. Exosomes and microvesicles of MSCs are small in size and more readily pass through the lung tissue; in addition, these contain many molecules that may have therapeutic effects on stroke [52, 53]. However, extractable exosomes and microvesicles require large amounts of MSCs for therapeutic purposes. MSC culture methods can be modified, such as by using microcarriers and hollow fiber bioreactors to culture MSCs in a 3D environment, so that they can be massively amplified [54]. This can serve the purpose of treatment.
Antioxidant
During brain ischemia and hypoxia, the adverse microenvironment induced by excessive oxidative stress leads to the death of a large number of transplanted MSCs, which further hinders the therapeutic effect of MSC therapy [33, 55, 56]. Oxidative stress results from the excessive production of reactive oxygen species (ROS), which triggers many cellular and molecular events, leading to the oxidation of proteins and lipids and ultimately to neuronal death [57-59]. Mitochondria are the main organelles responsible for ROS production [60]. Therefore, oxidant/antioxidant imbalance and mitochondrial dysfunction are the basic triggers of neuronal injury in IS. Studies have shown that some target genes (UBIAD1, SOCS-3, CUEDC2, SRC3) or specific miRNAs (microrNA-25, Mir-132-3p) can target specific antioxidant enzymes [61] or activate the PI3K/Akt/eNOS pathway [62]. This can increase the ratio of antioxidant enzyme to oxidase and inhibit oxidative stress reaction [28, 29, 63, 64], thus enabling MSCs or their exosomes to obtain a stronger antioxidant effect. In addition, when neurons and astrocytes are exposed to excessive ROS, mitochondria more efficiently transfer from mesenchymal stem cells to the damaged cells [65, 66]. Interestingly, mitochondrial movement from MSCs to damaged brain regions during oxidative stress was enhanced through genetic modification of MSCs [67]. At the same time, different types of MSCs have different adaptability in the harsh environment of oxidative stress. For example, umbilical cord derived mesenchymal stem cells showed more adaptability [68].
Anti-inflammatory
In the acute stage of cerebral ischemia, the progression of cerebral infarction and the formation of cerebral edema are closely related to the strong inflammatory response. It is characterized by rapid microglial activation, production of pro-inflammatory mediators, and infiltration of inflammatory cells into injured brain tissue [69, 70]. The anti-inflammatory effects of MSCs are characterized by down-regulation of secretion of anti-inflammatory molecules by pro-inflammatory cytokines [71], prevention of leukocyte infiltration [72], and promotion of polarization of the M2 phenotype of microglia [73]. Inhibition of inflammation can stabilize blood brain barrier (BBB) function and inhibit neuronal apoptosis.
As mentioned above, MSCs play an anti-inflammatory role by secreting IL-6 and reducing the pro-inflammatory factor TNF-α. This signaling pathway may be related to the inhibition of NF-κB by MSCs [74]. Meanwhile, the immunomodulatory cytokine IL-23/IL-17 of MSCs play a role in ischemic stroke [75].
MSCs can induce pro-inflammatory M1 microglia to differentiate into anti-inflammatory M2 microglia after IS. It has been reported that MSCs cause low expression of microglia activation markers (ED1 and Iba) and astrocyte proliferation markers (GFAP) [76]. These results suggest that the immunomodulatory effect of MSCs may be related to the inhibition of microglia and astrocytes residing in the brain, which may be related to the non-phosphorylation of STAT3 in the atypical JAK-STAT signaling pathway [77].
MSCs can reduce the release of neutrophil matrix metalloproteinase-9 (MMP-9), maintain the integrity of the blood-brain barrier, and reduce the infiltration of inflammatory cells in brain parenchyma [78]. In addition, MSCs reduce Monocyte chemotactic protein-1 (McP-1) production by secreting the anti-inflammatory cytokine TGF-β, thereby blocking the migration of CD68 + immune cells to ischemic regions [79].
Overexpression of anti-inflammatory factors enhanced the anti-inflammatory effect of MSCs, leading to enhanced neuroprotective function. In addition, IL-10 overexpressed MSCs can delay the time window for MSC therapy without affecting serum IL-10 levels, which may reduce the risk of systemic IL-10-induced adverse reactions such as anemia, thrombocytopenia, and immunosuppression [27, 80-82]. At the same time, MSCs activated by interferon gamma showed a better effect in the treatment of acute IS, resulting in a significant reduction of CD68 + monocytes and microglia [83] (Table 1).
Possible main mechanisms for improving the therapeutic effect of mesenchymal stem cells in ischemic stroke models. Abbreviations: MSC: Mesenchymal Stem Cells; ROS: Reactive Oxygen Species; t-PA: tissue plasminogen activator; BMSCs: bone marrow mesenchymal stem cells; UMSCs: umbilical cord stromal cells; ADSC: Adipogenic stromal cells; MCAO: Middle cerebral artery occlusion; BBB: blood brain barrier.
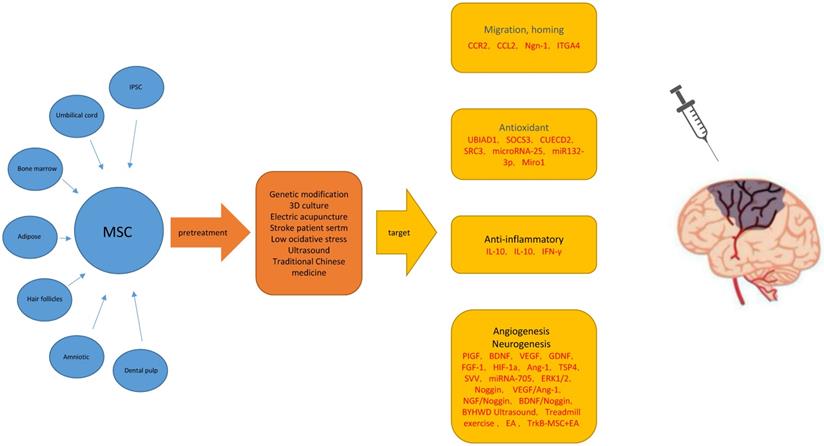
Target genes that promote migration, homing, antioxidant, anti-inflammatory ability of modified mesenchymal stem cells, MSC source, infusion mode, infusion time, and cell number
| Transplantation | Number of transplanted cells | Transplantation of time | Source of MSC | target | ||
|---|---|---|---|---|---|---|
| Homing and migration | [23] | IV | 2X10 6 | 24 after MCAO | hBM-MSC | CCR2 |
| [50] | IV | 1x10 6 | 1 and 4 days after MCAO | hUC-MSC | CCL2 | |
| [40] | IA | 1x10 6 | 3 days after MCAO | hBM-MSC | Ngn-1 | |
| [43] | IA | 5X10 5 | 24 h after MCAO | rBM-MSC | ITGA4 | |
| Antioxidant stress | [63] | IV | 5X10 6 | 24 h after MCAO | OM-MSC | UBIAD1 |
| [28] | IV | 2X10 6 | 3 h after MCAO | rBM-MSC | SOCS3 | |
| [29] | ICV | 2X10 6 | 24 h after MCAO | rBM-MSC | CUECD2 | |
| [64] | rBM-MSCs | SRC3 | ||||
| [61] | Intrathecal Injection | 20ug | 1day before Ischemia | BM-MSCs | microRNA-25 | |
| [62] | IV | 1 × 1010 particles/100 μL in PBS | 90min after MCAO | BM-MSCs | miR-132-3p | |
| [67] | IV | 3 × 106 cells/kg | - | hMMSC | Miro1 | |
| Anti-inflammatory | [82] | IV | 2X10 6 | 3 h after MCAO | rBM-MSC | IL-10 |
| [27] | IV | 1x10 6 | 0 or3 h after MCAO | hBM-MSC | IL-10 | |
| [83] | IV | 5×106 cells/kg | 3 h after MCAO | BM-MSC | IFN-γ | |
| Neurogenesis, Angiogenesis | [96] | ICV | 1x106/2ul | 5d after MCAO | BM-MSC | TrkB-MSC+EA |
| [90] | IV | 1x10 7 | 3 h after MCAO | hBM-MSC | PIGF | |
| [87] | ICV | 5X10 5 | 24 h after MCAO | hBM-MSC | BDNF | |
| [91] | ICV | 1x10 6 | 24 h after MCAO | rBM-MSC | VEGF | |
| [88] | IV | 1x10 7 | 3 h after MCAO | hBM-MSC | GDNF | |
| [86] | IV | 2X10 6 | 30 min after MCAO | AD-MSC | FGF-1 | |
| [30] | IV | 1x10 6 | 6 h after MCAO | hBM-MSC | Ang-1 | |
| [93] | IA | 2x10 5 | 24 h after MCAO | mBM-MSC | miRNA-705 | |
| [92] | IV | 5X10 6 | 6 h after MCAO | rBM-MSC | Noggin |
Neurogenesis and Angiogenesis
It is generally believed that the paracrine effect of MSCs plays a role in endogenous neural differentiation and proliferation [83]. Although endogenous neural stem cells (NSCs) do exhibit an acute response to IS, such as increased cell proliferation and cell migration, only 10%-20% of these cells survive long-term, and only a few of these surviving cells can mature into functional cells. However, most of them develop into thorny new striatal projection neurons or calretinin-positive interneurons [84]. This hinders the differentiation of MSCs into functional nerve cells after transplantation. Neural factors are known to play an important role in neurogenesis and vasculogenesis. MSCs genetically modified with neural factors can significantly increase the content of FGF, BDNF, VEGF, NGF, PIGF, and GDNF in the ischemic brain tissue, and promote the differentiation and proliferation of NSCs in the SVZ region during treatment. In addition to the generation of blood vessels, it promotes the generation of mature nerve cells and improves nerve function [85-92]. Some targeted miRNAs also play an important role in functional neurogenesis [93, 94]. MSCs overexpressing mirNA-133B were shown to regulate CTGF expression in astrocytes and RhoA expression in the IBZ region, promoting neurite remodeling and improving functional recovery in MCAO rats [94]. In addition to the chemical methods, use of physical methods may also play an important role. In a study, use of ultrasound probe to stimulate the ischemic brain tissue was shown to increase the expression of P-ERK and P-CREb and significantly promote neurogenesis [95]. In addition, the combination of chemical and physical methods significantly improved the therapeutic effect of modified MSCs on neurogenesis and angiogenesis. Studies have shown that the combination of overexpression of TRKB in MSCs and electroacupuncture stimulation may result in successful transdifferentiation of transplanted MSCS into functional nerve cells [96].
In conclusion, modification of MSCs by sensitive targets has shown a more significant effect in the treatment of IS stroke than MSCs alone.
Clinical research status of modified mesenchymal stem cells
Clinical trials have demonstrated the safety of MSC therapy in IS. MSCs derived from allofat were found to be safe for treatment in the acute phase of ischemia [97], and a Phase IIa clinical trial (NCT01678534) has been completed. Other clinical trials of MSC allografts are also being recruited (NCT04811651) (NCT05008588) (NCT04280003) (NCT03384433) (NCT04434768) (NCT04590118) (NCT02580019) (NCT04093336). In addition, the safety of hypoxic-treated allograft BM-MSCs has been demonstrated in the treatment of chronic stroke. In addition, there was significant improvement in behavioral endpoints [98] (NCT01297413). This evidence makes us look forward to the transformation of MSCs.
Some experimental studies suggest the feasibility of use of modified MSCs for the treatment of IS. In particular, genetically modified MSCs have shown promising therapeutic effects, but not much work has been done in clinical transformation. The key challenges to clinical transformation include the cytotoxicity of vectors such as lentiviruses, adenoviruses or retroviruses, and carcinogenicity and immunogenicity of viral DNA integration into the host genome. However, clinical studies in the context of other diseases have demonstrated the safety of the treatment process and good results have been achieved. For example, in a clinical study of neuroblastoma [99], autologous MSCs injected with ICoVIR-5 (a novel oncolytic adenovirus) for the treatment of metastatic neuroblastoma was found to be safe and effective. In addition, the use of transgenic autologous MSCs has been shown to improve the targeting of tumor cells in the treatment of gastrointestinal tumors [100]. In addition to neoplastic diseases, there have been some clinical trials of inducing MSCs to secrete target proteins in degenerative diseases of the nervous system. Brain-Storm Cell Therapeutics concluded a phase I/IIa clinical trial in patients with amyotrophic lateral sclerosis (ALS) using autologous MSCs induced to express neurotrophic factor (NurOwn) with mild and transient adverse effects reported. Strikingly, treated ALS patients demonstrated slowed disease progression following the conclusion of the Phase IIa trial with improvements in breathing and reduced motor decline compared to pre-treatment level [101] (NCT01051882) (NCT01777646). In addition, clinical studies evaluating MSC/BDNF in a dose-dependent manner to demonstrate the safety of transgenic MSCs for striate injection transplantation in patients with HD are being observed [102] (NCT01937923). In a 2-year 1/2A study, Gary K Steinberg and his team implanted modified bone marrow MSCs (SB623) into chronic ischemic brain tissue using transient transfection of human Notch-1 intracellular domain. They concluded that SB623 cell implantation in patients with stable chronic stroke is safe and accompanied by improved clinical outcomes [103] (NCT01287936).
In conclusion, allogeneic MSC therapy for IS appears safe and feasible. The safety and effectiveness of genetically-modified MSCs has been demonstrated in clinical trials. The available evidence suggests a promising outlook of the use of various gene targets or preconditioning of modified allogeneic MSCs for the treatment of IS at all stages.
Outlook
Advances in the field of biotechnology have helped improve the treatment of a wide range of diseases. In recent years, the development of crisPR-Cas9 and other gene technologies has made rapid progress in the treatment of metabolic diseases and cancers. Modification of MSCs appears a particularly promising approach as a therapeutic modality. In conclusion, modification of MSCs for ischemic stroke to enhance their targeting ability is a feasible and highly applicable research direction for future clinical transformation.
Conclusion
Ischemic stroke is a disease characterized by high morbidity, disability, and mortality. Complex pathological changes occurring in the damaged brain tissue including inflammatory storm, oxidative stress, and nerve cell apoptosis lead to severe neurological dysfunction. MSC therapy is a promising treatment for IS. However, the harsh ischemic and hypoxic microenvironment limits the effectiveness of this treatment modality. Modification of MSCs to improve their therapeutic ability represents a feasible and applicable research direction in clinical transformation.
Acknowledgements
Informed consent
Informed consent was obtained from all individual participants included in the study.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Mathers CD, Boerma T, Ma Fat D. Global and regional causes of death. Br Med Bull. 2009;92:7-32
2. Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke. 2011;42:2351-5
3. Gan Y, Wu J, Zhang S, Li L, Yin X, Gong Y. et al. Prevalence and risk factors associated with stroke in middle-aged and older Chinese: A community-based cross-sectional study. Sci Rep. 2017;7:9501
4. Moreau F, Patel S, Lauzon ML, McCreary CR, Goyal M, Frayne R. et al. Cavitation after acute symptomatic lacunar stroke depends on time, location, and MRI sequence. Stroke. 2012;43:1837-42
5. Uyeki TM, Mehta AK, Davey RT Jr, Liddell AM, Wolf T, Vetter P. et al. Clinical Management of Ebola Virus Disease in the United States and Europe. N Engl J Med. 2016;374:636-46
6. Del Zoppo GJ, Saver JL, Jauch EC, Adams HP Jr. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40:2945-8
7. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P. et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378:11-21
8. Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9-20
9. Bittira B, Shum-Tim D, Al-Khaldi A, Chiu RC. Mobilization and homing of bone marrow stromal cells in myocardial infarction. Eur J Cardiothorac Surg. 2003;24:393-8
10. Wu GD, Nolta JA, Jin YS, Barr ML, Yu H, Starnes VA. et al. Migration of mesenchymal stem cells to heart allografts during chronic rejection. Transplantation. 2003;75:679-85
11. Hung SC, Pochampally RR, Chen SC, Hsu SC, Prockop DJ. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells. 2007;25:2363-70
12. Shyu KG, Wang BW, Hung HF, Chang CC, Shih DT. Mesenchymal stem cells are superior to angiogenic growth factor genes for improving myocardial performance in the mouse model of acute myocardial infarction. J Biomed Sci. 2006;13:47-58
13. Shuaib A, Motan D, Bhattacharya P, McNabb A, Skerry TM, Lacroix D. Heterogeneity in The Mechanical Properties of Integrins Determines Mechanotransduction Dynamics in Bone Osteoblasts. Sci Rep. 2019;9:13113
14. Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC. et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559
15. Kimbrel EA, Lanza R. Next-generation stem cells - ushering in a new era of cell-based therapies. Nat Rev Drug Discov. 2020;19:463-79
16. Liu Z, Li Y, Zhang RL, Cui Y, Chopp M. Bone marrow stromal cells promote skilled motor recovery and enhance contralesional axonal connections after ischemic stroke in adult mice. Stroke. 2011;42:740-4
17. Eckert MA, Vu Q, Xie K, Yu J, Liao W, Cramer SC. et al. Evidence for high translational potential of mesenchymal stromal cell therapy to improve recovery from ischemic stroke. J Cereb Blood Flow Metab. 2013;33:1322-34
18. Chung JW, Chang WH, Bang OY, Moon GJ, Kim SJ, Kim SK. et al. Efficacy and Safety of Intravenous Mesenchymal Stem Cells for Ischemic Stroke. Neurology. 2021;96:e1012-e23
19. Hess DC, Wechsler LR, Clark WM, Savitz SI, Ford GA, Chiu D. et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16:360-8
20. Bhasin A, Srivastava MV, Kumaran SS, Mohanty S, Bhatia R, Bose S. et al. Autologous mesenchymal stem cells in chronic stroke. Cerebrovasc Dis Extra. 2011;1:93-104
21. Jaillard A, Hommel M, Moisan A, Zeffiro TA, Favre-Wiki IM, Barbieux-Guillot M. et al. Autologous Mesenchymal Stem Cells Improve Motor Recovery in Subacute Ischemic Stroke: a Randomized Clinical Trial. Transl Stroke Res. 2020;11:910-23
22. de Celis-Ruiz E, Fuentes B, Alonso de Leciñana M, Gutiérrez-Fernández M, Borobia AM, Gutiérrez-Zúñiga R. et al. Final Results of Allogeneic Adipose Tissue-Derived Mesenchymal Stem Cells in Acute Ischemic Stroke (AMASCIS): A Phase II, Randomized, Double-Blind, Placebo-Controlled, Single-Center, Pilot Clinical Trial. Cell Transplant. 2022;31:9636897221083863
23. Huang Y, Wang J, Cai J, Qiu Y, Zheng H, Lai X. et al. Targeted homing of CCR2-overexpressing mesenchymal stromal cells to ischemic brain enhances post-stroke recovery partially through PRDX4-mediated blood-brain barrier preservation. Theranostics. 2018;8:5929-44
24. Moniche F, Escudero I, Zapata-Arriaza E, Usero-Ruiz M, Prieto-León M, de la Torre J. et al. Intra-arterial bone marrow mononuclear cells (BM-MNCs) transplantation in acute ischemic stroke (IBIS trial): protocol of a phase II, randomized, dose-finding, controlled multicenter trial. Int J Stroke. 2015;10:1149-52
25. Rosado-de-Castro PH, Schmidt Fda R, Battistella V, Lopes de Souza SA, Gutfilen B, Goldenberg RC. et al. Biodistribution of bone marrow mononuclear cells after intra-arterial or intravenous transplantation in subacute stroke patients. Regen Med. 2013;8:145-55
26. Le VC, Nguyen NH, Le SH. Intra-arterial infusion of autologous bone marrow mononuclear cells combined with intravenous injection of cerebrolysin in the treatment of middle cerebral artery ischemic stroke: Case report. SAGE Open Med Case Rep. 2021;9:2050313x211002313
27. Nakajima M, Nito C, Sowa K, Suda S, Nishiyama Y, Nakamura-Takahashi A. et al. Mesenchymal Stem Cells Overexpressing Interleukin-10 Promote Neuroprotection in Experimental Acute Ischemic Stroke. Mol Ther Methods Clin Dev. 2017;6:102-11
28. Zhou B, Liu HY, Zhu BL. Protective Role of SOCS3 Modified Bone Marrow Mesenchymal Stem Cells in Hypoxia-Induced Injury of PC12 Cells. J Mol Neurosci. 2019;67:400-10
29. Huang Y, Xiao X, Xiao H, Hu Z, Tan F. CUEDC2 ablation enhances the efficacy of mesenchymal stem cells in ameliorating cerebral ischemia/reperfusion insult. Aging (Albany NY). 2021;13:4335-56
30. Onda T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:329-40
31. Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N. et al. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010;19:667-79
32. Sohni A, Verfaillie CM. Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013;2013:130763
33. Gervois P, Wolfs E, Ratajczak J, Dillen Y, Vangansewinkel T, Hilkens P. et al. Stem Cell-Based Therapies for Ischemic Stroke: Preclinical Results and the Potential of Imaging-Assisted Evaluation of Donor Cell Fate and Mechanisms of Brain Regeneration. Med Res Rev. 2016;36:1080-126
34. Yavagal DR, Lin B, Raval AP, Garza PS, Dong C, Zhao W. et al. Efficacy and dose-dependent safety of intra-arterial delivery of mesenchymal stem cells in a rodent stroke model. PLoS One. 2014;9:e93735
35. Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160-70
36. Wynn RF, Hart CA, Corradi-Perini C, O'Neill L, Evans CA, Wraith JE. et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643-5
37. Li L, Chu L, Fang Y, Yang Y, Qu T, Zhang J. et al. Preconditioning of bone marrow-derived mesenchymal stromal cells by tetramethylpyrazine enhances cell migration and improves functional recovery after focal cerebral ischemia in rats. Stem Cell Res Ther. 2017;8:112
38. Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005;105:3793-801
39. Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J. et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254-64
40. Kim GH, Subash M, Yoon JS, Jo D, Han J, Hong JM. et al. Neurogenin-1 Overexpression Increases the Therapeutic Effects of Mesenchymal Stem Cells through Enhanced Engraftment in an Ischemic Rat Brain. Int J Stem Cells. 2020;13:127-41
41. Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796-808
42. Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181-98
43. Cui LL, Nitzsche F, Pryazhnikov E, Tibeykina M, Tolppanen L, Rytkönen J. et al. Integrin α4 Overexpression on Rat Mesenchymal Stem Cells Enhances Transmigration and Reduces Cerebral Embolism After Intracarotid Injection. Stroke. 2017;48:2895-900
44. Mohammadian M, Abasi E, Akbarzadeh A. Mesenchymal stem cell-based gene therapy: A promising therapeutic strategy. Artif Cells Nanomed Biotechnol. 2016;44:1206-11
45. Porada CD, Almeida-Porada G. Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. Adv Drug Deliv Rev. 2010;62:1156-66
46. Andres RH, Choi R, Pendharkar AV, Gaeta X, Wang N, Nathan JK. et al. The CCR2/CCL2 interaction mediates the transendothelial recruitment of intravascularly delivered neural stem cells to the ischemic brain. Stroke. 2011;42:2923-31
47. Jiang L, Newman M, Saporta S, Chen N, Sanberg C, Sanberg PR. et al. MIP-1alpha and MCP-1 Induce Migration of Human Umbilical Cord Blood Cells in Models of Stroke. Curr Neurovasc Res. 2008;5:118-24
48. Lee SH, Jin KS, Bang OY, Kim BJ, Park SJ, Lee NH. et al. Differential Migration of Mesenchymal Stem Cells to Ischemic Regions after Middle Cerebral Artery Occlusion in Rats. PLoS One. 2015;10:e0134920
49. Wang L, Li Y, Chen X, Chen J, Gautam SC, Xu Y. et al. MCP-1, MIP-1, IL-8 and ischemic cerebral tissue enhance human bone marrow stromal cell migration in interface culture. Hematology. 2002;7:113-7
50. Lee S, Kim OJ, Lee KO, Jung H, Oh SH, Kim NK. Enhancing the Therapeutic Potential of CCL2-Overexpressing Mesenchymal Stem Cells in Acute Stroke. Int J Mol Sci. 2020 21
51. Acosta SA, Tajiri N, Hoover J, Kaneko Y, Borlongan CV. Intravenous Bone Marrow Stem Cell Grafts Preferentially Migrate to Spleen and Abrogate Chronic Inflammation in Stroke. Stroke. 2015;46:2616-27
52. Moon GJ, Sung JH, Kim DH, Kim EH, Cho YH, Son JP. et al. Application of Mesenchymal Stem Cell-Derived Extracellular Vesicles for Stroke: Biodistribution and MicroRNA Study. Transl Stroke Res. 2019;10:509-21
53. Katsuda T, Ochiya T. Molecular signatures of mesenchymal stem cell-derived extracellular vesicle-mediated tissue repair. Stem Cell Res Ther. 2015;6:212
54. Phan J, Kumar P, Hao D, Gao K, Farmer D, Wang A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J Extracell Vesicles. 2018;7:1522236
55. Coppin L, Sokal E, Stéphenne X. Thrombogenic Risk Induced by Intravascular Mesenchymal Stem Cell Therapy: Current Status and Future Perspectives. Cells. 2019 8
56. Lalu MM, Montroy J, Dowlatshahi D, Hutton B, Juneau P, Wesch N. et al. From the Lab to Patients: a Systematic Review and Meta-Analysis of Mesenchymal Stem Cell Therapy for Stroke. Transl Stroke Res. 2020;11:345-64
57. Janardhan V, Qureshi AI. Mechanisms of ischemic brain injury. Curr Cardiol Rep. 2004;6:117-23
58. Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015;6:524-51
59. Sugawara T, Chan PH. Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid Redox Signal. 2003;5:597-607
60. Marchi S, Giorgi C, Suski JM, Agnoletto C, Bononi A, Bonora M. et al. Mitochondria-ros crosstalk in the control of cell death and aging. J Signal Transduct. 2012;2012:329635
61. Zhao L, Jiang X, Shi J, Gao S, Zhu Y, Gu T. et al. Exosomes derived from bone marrow mesenchymal stem cells overexpressing microRNA-25 protect spinal cords against transient ischemia. J Thorac Cardiovasc Surg. 2019;157:508-17
62. Pan Q, Kuang X, Cai S, Wang X, Du D, Wang J. et al. miR-132-3p priming enhances the effects of mesenchymal stromal cell-derived exosomes on ameliorating brain ischemic injury. Stem Cell Res Ther. 2020;11:260
63. Liu J, Huang Y, He J, Zhuo Y, Chen W, Ge L. et al. Olfactory Mucosa Mesenchymal Stem Cells Ameliorate Cerebral Ischemic/Reperfusion Injury Through Modulation of UBIAD1 Expression. Front Cell Neurosci. 2020;14:580206
64. Lei Q, Deng M, Liu J, He J, Lan Z, Hu Z. et al. SRC3 Promotes the Protective Effects of Bone Marrow Mesenchymal Stem Cell Transplantation on Cerebral Ischemia in a Mouse Model. ACS Chem Neurosci. 2022;13:112-9
65. Debattisti V, Gerencser AA, Saotome M, Das S, Hajnóczky G. ROS Control Mitochondrial Motility through p38 and the Motor Adaptor Miro/Trak. Cell Rep. 2017;21:1667-80
66. Liao PC, Tandarich LC, Hollenbeck PJ. ROS regulation of axonal mitochondrial transport is mediated by Ca2+ and JNK in Drosophila. PLoS One. 2017;12:e0178105
67. Babenko VA, Silachev DN, Popkov VA, Zorova LD, Pevzner IB, Plotnikov EY. et al. Miro1 Enhances Mitochondria Transfer from Multipotent Mesenchymal Stem Cells (MMSC) to Neural Cells and Improves the Efficacy of Cell Recovery. Molecules. 2018 23
68. Russo E, Lee JY, Nguyen H, Corrao S, Anzalone R, La Rocca G. et al. Energy Metabolism Analysis of Three Different Mesenchymal Stem Cell Populations of Umbilical Cord Under Normal and Pathologic Conditions. Stem Cell Rev Rep. 2020;16:585-95
69. Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87:779-89
70. Kleinig TJ, Vink R. Suppression of inflammation in ischemic and hemorrhagic stroke: therapeutic options. Curr Opin Neurol. 2009;22:294-301
71. Chi L, Huang Y, Mao Y, Wu K, Zhang L, Nan G. Tail Vein Infusion of Adipose-Derived Mesenchymal Stem Cell Alleviated Inflammatory Response and Improved Blood Brain Barrier Condition by Suppressing Endoplasmic Reticulum Stress in a Middle Cerebral Artery Occlusion Rat Model. Med Sci Monit. 2018;24:3946-57
72. Chung TN, Kim JH, Choi BY, Chung SP, Kwon SW, Suh SW. Adipose-derived mesenchymal stem cells reduce neuronal death after transient global cerebral ischemia through prevention of blood-brain barrier disruption and endothelial damage. Stem Cells Transl Med. 2015;4:178-85
73. Yoshida Y, Takagi T, Kuramoto Y, Tatebayashi K, Shirakawa M, Yamahara K. et al. Intravenous Administration of Human Amniotic Mesenchymal Stem Cells in the Subacute Phase of Cerebral Infarction in a Mouse Model Ameliorates Neurological Disturbance by Suppressing Blood Brain Barrier Disruption and Apoptosis via Immunomodulation. Cell Transplant. 2021;30:9636897211024183
74. Liu N, Chen R, Du H, Wang J, Zhang Y, Wen J. Expression of IL-10 and TNF-alpha in rats with cerebral infarction after transplantation with mesenchymal stem cells. Cell Mol Immunol. 2009;6:207-13
75. Ma S, Zhong D, Chen H, Zheng Y, Sun Y, Luo J. et al. The immunomodulatory effect of bone marrow stromal cells (BMSCs) on interleukin (IL)-23/IL-17-mediated ischemic stroke in mice. J Neuroimmunol. 2013;257:28-35
76. Oh SH, Choi C, Noh JE, Lee N, Jeong YW, Jeon I. et al. Interleukin-1 receptor antagonist-mediated neuroprotection by umbilical cord-derived mesenchymal stromal cells following transplantation into a rodent stroke model. Exp Mol Med. 2018;50:1-12
77. McGuckin CP, Jurga M, Miller AM, Sarnowska A, Wiedner M, Boyle NT. et al. Ischemic brain injury: a consortium analysis of key factors involved in mesenchymal stem cell-mediated inflammatory reduction. Arch Biochem Biophys. 2013;534:88-97
78. Tang G, Liu Y, Zhang Z, Lu Y, Wang Y, Huang J. et al. Mesenchymal stem cells maintain blood-brain barrier integrity by inhibiting aquaporin-4 upregulation after cerebral ischemia. Stem Cells. 2014;32:3150-62
79. Yoo SW, Chang DY, Lee HS, Kim GH, Park JS, Ryu BY. et al. Immune following suppression mesenchymal stem cell transplantation in the ischemic brain is mediated by TGF-β. Neurobiol Dis. 2013;58:249-57
80. Liang QJ, Jiang M, Wang XH, Le LL, Xiang M, Sun N. et al. Pre-existing interleukin 10 in cerebral arteries attenuates subsequent brain injury caused by ischemia/reperfusion. IUBMB Life. 2015;67:710-9
81. Zhou Z, Peng X, Insolera R, Fink DJ, Mata M. Interleukin-10 provides direct trophic support to neurons. J Neurochem. 2009;110:1617-27
82. Lü C, Liu Q, Zeng X. [Effects of interleukin 10 gene modified bone marrow mesenchymal stem cells on expression of inflammatory cytokines and neuronal apoptosis in rats after cerebral ischemia reperfusion injury]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017;31:240-5
83. Tobin MK, Stephen TKL, Lopez KL, Pergande MR, Bartholomew AM, Cologna SM. et al. Activated Mesenchymal Stem Cells Induce Recovery Following Stroke Via Regulation of Inflammation and Oligodendrogenesis. J Am Heart Assoc. 2020;9:e013583
84. Salehi MS, Safari A, Pandamooz S, Jurek B, Hooshmandi E, Owjfard M. et al. The Beneficial Potential of Genetically Modified Stem Cells in the Treatment of Stroke: a Review. Stem Cell Rev Rep. 2022;18:412-40
85. Chen B, Zhang F, Li QY, Gong A, Lan Q. Protective Effect of Ad-VEGF-Bone Mesenchymal Stem Cells on Cerebral Infarction. Turk Neurosurg. 2016;26:8-15
86. Ghazavi H, Hoseini SJ, Ebrahimzadeh-Bideskan A, Mashkani B, Mehri S, Ghorbani A. et al. Fibroblast Growth Factor Type 1 (FGF1)-Overexpressed Adipose-Derived Mesenchaymal Stem Cells (AD-MSC(FGF1)) Induce Neuroprotection and Functional Recovery in a Rat Stroke Model. Stem Cell Rev Rep. 2017;13:670-85
87. Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Kobune M, Hirai S. et al. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther. 2004;9:189-97
88. Horita Y, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. J Neurosci Res. 2006;84:1495-504
89. Ding J, Cheng Y, Gao S, Chen J. Effects of nerve growth factor and Noggin-modified bone marrow stromal cells on stroke in rats. J Neurosci Res. 2011;89:222-30
90. Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H. et al. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain. 2006;129:2734-45
91. Zong X, Wu S, Li F, Lv L, Han D, Zhao N. et al. Transplantation of VEGF-mediated bone marrow mesenchymal stem cells promotes functional improvement in a rat acute cerebral infarction model. Brain Res. 2017;1676:9-18
92. Chen C, Cheng Y, Chen J. Transfection of Noggin in bone marrow stromal cells (BMSCs) enhances BMSC-induced functional outcome after stroke in rats. J Neurosci Res. 2011;89:1194-202
93. Ji M, Wang W, Li S, Hu W. Implantation of bone mesenchymal stem cells overexpressing miRNA-705 mitigated ischemic brain injury. Mol Med Rep. 2017;16:8323-8
94. Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y. et al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737-46
95. Cho SE, Kim YM, Jeong JS, Seo YK. The effect of ultrasound for increasing neural differentiation in hBM-MSCs and inducing neurogenesis in ischemic stroke model. Life Sci. 2016;165:35-42
96. Ahn SM, Kim YR, Shin YI, Ha KT, Lee SY, Shin HK. et al. Therapeutic Potential of a Combination of Electroacupuncture and TrkB-Expressing Mesenchymal Stem Cells for Ischemic Stroke. Mol Neurobiol. 2019;56:157-73
97. Díez-Tejedor E, Gutiérrez-Fernández M, Martínez-Sánchez P, Rodríguez-Frutos B, Ruiz-Ares G, Lara ML. et al. Reparative therapy for acute ischemic stroke with allogeneic mesenchymal stem cells from adipose tissue: a safety assessment: a phase II randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. J Stroke Cerebrovasc Dis. 2014;23:2694-700
98. Levy ML, Crawford JR, Dib N, Verkh L, Tankovich N, Cramer SC. Phase I/II Study of Safety and Preliminary Efficacy of Intravenous Allogeneic Mesenchymal Stem Cells in Chronic Stroke. Stroke. 2019;50:2835-41
99. García-Castro J, Alemany R, Cascalló M, Martínez-Quintanilla J, Arriero Mdel M, Lassaletta A. et al. Treatment of metastatic neuroblastoma with systemic oncolytic virotherapy delivered by autologous mesenchymal stem cells: an exploratory study. Cancer Gene Ther. 2010;17:476-83
100. von Einem JC, Peter S, Günther C, Volk HD, Grütz G, Salat C. et al. Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells - TREAT-ME-1 - a phase I, first in human, first in class trial. Oncotarget. 2017;8:80156-66
101. Petrou P, Gothelf Y, Argov Z, Gotkine M, Levy YS, Kassis I. et al. Safety and Clinical Effects of Mesenchymal Stem Cells Secreting Neurotrophic Factor Transplantation in Patients With Amyotrophic Lateral Sclerosis: Results of Phase 1/2 and 2a Clinical Trials. JAMA Neurol. 2016;73:337-44
102. Deng P, Torrest A, Pollock K, Dahlenburg H, Annett G, Nolta JA. et al. Clinical trial perspective for adult and juvenile Huntington's disease using genetically-engineered mesenchymal stem cells. Neural Regen Res. 2016;11:702-5
103. Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Kim AS, Johnson JN. et al. Two-year safety and clinical outcomes in chronic ischemic stroke patients after implantation of modified bone marrow-derived mesenchymal stem cells (SB623): a phase 1/2a study. J Neurosurg. 2018:1-11
Author contact
![]() Corresponding author: Liangfu Zhou, Department of Neurovascular Surgery, First Hospital of Jilin University, 1xinmin Avenue Changchun130021, Jilin Province, China. E-mail: lfzhouccom.
Corresponding author: Liangfu Zhou, Department of Neurovascular Surgery, First Hospital of Jilin University, 1xinmin Avenue Changchun130021, Jilin Province, China. E-mail: lfzhouccom.

 Global reach, higher impact
Global reach, higher impact