3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(6):993-1002. doi:10.7150/ijms.73012 This issue Cite
Review
SIRT1: A Novel Protective Molecule in Pre-eclampsia
1. Shanghai Key Laboratory of Female Reproductive Endocrine Related Diseases, Shanghai 200011, China.
2. Department of Obstetrics and Gynecology, Obstetrics and Gynecology Hospital of Fudan University, Shanghai 200011, China.
Received 2022-3-18; Accepted 2022-5-20; Published 2022-5-29
Abstract
Pre-eclampsia is a severe pregnant complication, mainly characterized by insufficient trophoblast invasion, impaired uterine spiral artery remodeling, placental hypoxia and ischemia, and endothelial dysfunction. However, the potential mechanisms of pre-eclampsia remain unclear. SIRT1 is a NAD+-dependent deacetylase, involving in multiple biological processes, including energy metabolism, oxidative stress, inflammatory response, and cellular autophagy. Several studies showed that SIRT1 might play a vital role in the pathogenesis of pre-eclampsia. In this review, we aim to integrate the latest research on SIRT1 and pre-eclampsia to explore the comprehensive mechanisms of SIRT1 in pre-eclampsia. More specifically, SIRT1 might affect placental development and trophoblast invasion through autophagy and senescence in pre-eclampsia, and SIRT1 protects vascular endothelial cells from oxidative stress, inflammatory response, autophagy, and senescence. Furthermore, SIRT1 deficiency mice showed typical pre-eclampsia-like performances, which can be reversed via direct SIRT1 supplement or SIRT1 agonist treatment. Additionally, resveratrol, a SIRT1 agonist, attenuates vascular endothelial injury and placental dysfunction, and exerts protective effect on decreasing blood pressure. In this review, we provide new insights into the development of pre-eclampsia, which can establish a theoretical basis for prevention and treatment for pre-eclampsia. Besides, we also propose questions that still need to be further addressed in order to elucidate the comprehensive molecular mechanisms of pre-eclampsia in the future.
Keywords: pre-eclampsia (PE), SIRT1, trophoblasts, endothelial cells (ECs), resveratrol
Introduction
Pre-eclampsia (PE) is a hypertensive disorder of pregnancy (HDP), characterized by new-onset hypertension and proteinuria at 20-week of pregnancy. It affects 2%-8% pregnancy women worldwide, causing severe fetal and maternal morbidity and mortality [1-3]. Although the comprehensive mechanisms of pre-eclampsia remain unknown, the current mainstream view is the two-stage model of disease [4-6]. Stage1 mainly manifests as impaired placentation due to inadequate trophoblastic invasion of maternal spiral arteries, which leads to reduced placental perfusion and release of numerous secreted factors causing vascular endothelial dysfunction and multiorgan failure, which is called stage2. Recently, the effects of SIRT1 on the biological functions of trophoblasts and endothelial cells have gradually emerged, and the expression of SIRT1 is lower in serum samples and placental tissues of pre-eclampsia patients. Therefore, we inferred that SIRT1 might play a significant role in the pathogenesis of pre-eclampsia.
SIRT1, a NAD+-dependent deacetylase, mediates various biological functions including oxidative stress, aging, inflammatory response and autophagy via deacetylating multiple substrates, such as NF-κB (nuclear factor-kappaB), FOXOs (forkhead box O), and PPARγ (peroxisome proliferator-activated receptor γ) [7-11]. For example, it is reported that SIRT1 promotes the deacetylation of Nrf2 (nuclear factor-erythroid 2 (NF-E2)-related factor2), and increases its transcriptional activity, thereby promoting the expression of downstream two-phase detoxification NQO1 (NADPH quinone oxidoreductase 1) and HO-1 (heme oxygenase-1), and exerting anti-oxidative stress effect in vascular endothelial cells [12-15]. In addition, SIRT1 deacetylates and activates eNOs (neuronal nitricoxide synthase) to produce more nitric oxide (NO), which can dilate blood vessels [16]. In recent years, the research of SIRT1 in pre-eclampsia has progressed. SIRT1 deficiency attenuates the invasion, migration and proliferation of trophoblasts, thereby participating in the development of pre-eclampsia. Our recent study showed that SIRT1 knockdown mice exhibited significantly pre-eclampsia-like symptoms, suggesting that SIRT1 might be a novel protective biomarker in pre-eclampsia [17].
In this review, we mainly explored the role of SIRT1 in pre-eclampsia from the following four aspects. 1) SIRT1 affects the biological functions of trophoblasts; 2) SIRT1 protects vascular endothelial cells; 3) SIRT1 attenuates the performances of pre-eclampsia in animal models; 4) the effect of SIRT1 agonist resveratrol in pre-eclampsia.
2. SIRT1 affects the biological functions of trophoblasts
2.1. SIRT1 affects placental development and differentiation
Trophoblastic dysfunction is a typical feature of pre-eclampsia, resulting in uterine spiral artery remodeling disorder. It is reported that SIRT1 is critical in trophoblast differentiation and placental development [18-21]. SIRT1 is lower in placentas and serum samples of pre-eclampsia patients, and is mainly expressed in the nuclei of trophoblasts including syncytiotrophoblasts and cytotrophoblasts in placental tissues [22, 23]. SIRT1 possibly involves in trophoblastic maintenance and differentiation by mediating SMAD2/3, STAT3 or PPARγ pathways [24-27]. Arul Nambi Rajan et al. [22] found that placentas of SIRT1-null mice were small and showed abnormalities in both labyrinthine layer and junctional zone, and SIRT1-null trophoblast stem cell (TSC) showed blunted differentiation. Specifically, the RNA levels of PPARγ were decreased, and the protein levels of SMAD2, SMAD3 and STAT3 were downregulated in differentiated SIRT1-null TSC. Studies reported that STAT3 was associated with the differentiation of trophoblast giant cells and syncytiotrophoblasts and might be deacetylated and inhibited by SIRT1 [25, 28, 29]. Additionally, the potential role of PPARγ in trophoblast differentiation and placental development is also highlighted [24, 30], and the activity of PPARγ can be deacetylated and regulated by SIRT1 through recruiting cofactors, such as NCoR1 (nuclear receptor corepressor 1), SMRT (silencing mediator of retinoic acid and thyroid hormone) and Prdm16 (PR domain-containing protein 16) [31, 32]. The above-mentioned pathways are shown in Figure 1. Furthermore, our previous research also demonstrated that the placental labyrinthine layer was significantly narrow in SIRT1+/- mice and the invasive ability was relatively lower in SIRT1 knockdown trophoblasts [17]. This evidence indicated that SIRT1 plays a significant role in placental development and differentiation.
SIRT1 affects the biological functions of trophoblasts.
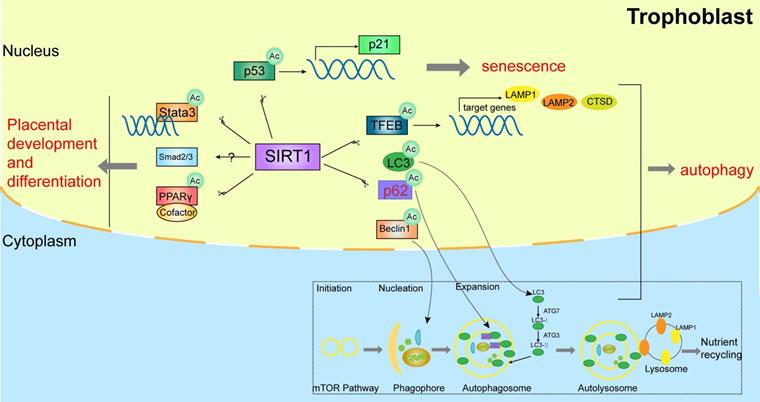
2.2. SIRT1 affects trophoblast autophagy
Autophagy is a cellular homeostasis pathway targeted aggregated proteins and damaged organelles for lysosomal degradation [33-37]. Importantly, autophagy protects the placentas against pathogens and stress. There are impaired trophoblast autophagy and increased protein accumulation in the placentas of patients with pre-eclampsia [38]. Studies showed that SIRT1 prevents H2O2-induced oxidative stress and apoptosis by mediating autophagy in trophoblasts [39]. Mechanistically, some evidence on the autophagic machinery demonstrated that SIRT1 participates in autophagy via deacetylating TFEB (transcription factor EB), LC3 (microtubule associated protein 1 light chain 3), Beclin-1, p62, ATG5 (autophagy-related gene 5), ATG7 (autophagy-related gene 7), and ATG8 (autophagy-related gene 8) in a NAD+-dependent manner [40, 41].
Autophagy-lysosomal biogenesis is tightly regulated by TFEB, which can be deacetylated by SIRT1 and activate the expression of several downstream autophagy-associated genes, such as LAMP1 (lysosomal associated membrane protein 1), LAMP2 (lysosomal associated membrane protein 2) and CTSD (cathepsin D) [42, 43]. Furthermore, the initial stage markers of autophagy activation in pre-eclampsia, such as LC3-II, Beclin-1, and SQSTM1 (sequestosome 1) [44-46], were also significantly altered and could be regulated by SIRT1[47-49]. The above-mentioned pathways are shown in Figure 1. This evidence demonstrated that SIRT1 might exert a potential role in trophoblastic autophagy by deacetylating multiple substrates.
2.3. SIRT1 affects placental senescence
Premature placental senescence is a critical characteristic of pre-eclampsia, with senescence-associated secretory phenotype and increased expression of p53 and p21, which are markers of cellular senescence. SIRT1 is also a specific marker of senescence, and SIRT1 deficiency leads to premature senescence of placentas during placentation [50-53]. Interestingly, Xiong et al. found that SIRT1 deficiency promotes the acetylation of P53, elevates the expression level of P21, and impairs trophoblast invasion and migration in advanced maternal age (AMA) pregnancy women, indicating that SIRT1 might involve in the pathogenesis of pre-eclampsia by inducing placental senescence [50].
2.4. The functions of other sirtuins proteins in trophoblasts
There are seven orthologs (SIRT1-7) of sirtuins family in mammals [54]. All sirtuins deacetylate multiple target proteins using NAD+ as co-substrate and participate in cellular oxidative stress, energy metabolism, and inflammatory response and so on [55]. Several reports revealed that sirtuins play a significant role in the development and differentiation of trophoblasts, as shown in Table 1. SIRT2, one member of the sirtuins family, localizes in placental syncytiotrophoblasts and is downregulated in the placentas of patients with pre-eclampsia. It could inhibit proliferation, migration and invasion, and induce necrosis of placental trophoblast cells [56, 57]. Additionally, it is reported that SIRT3 affects the migration, invasion, tube formation and necroptosis of trophoblasts and is implicated in the pathogenesis of pre-eclampsia [58]. Furthermore, studies showed that SIRT4 might trigger senescence of trophoblasts [59-61]. This evidence further confirms our hypothesis that SIRT1 might participate in the pathogenesis of pre-eclampsia by regulating trophoblastic invasion, migration and proliferation.
3. SIRT1 protects vascular endothelial cells
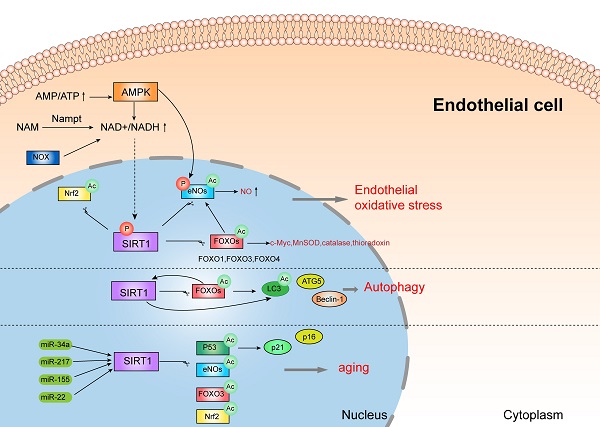
The dysfunction of endothelial cells is one of the typical features in pre-eclampsia, causing by multiple factors, including oxidative stress, inflammatory response and autophagy and so on. SIRT1, a member of sirtuins family, exerts anti-oxidant, anti-inflammatory, and anti-aging effect. Some research showed that SIRT1 expression is lower in serum samples of pre-eclampsia women, and also decreased in human umbilical vein endothelial cells (HUVECs) incubated with pre-eclamptic serum [63]. It is reported that SIRT1 can protect HUVECs from death in pre-eclampsia patients, therefore blocking the development of pre-eclampsia [64]. Mechanistically, SIRT1 might protect endothelial cells from oxidative stress, inflammatory response, senescence and autophagy by various pathways, as shown in Figure 2.
3.1. SIRT1 protects vascular endothelial cells from oxidative stress and inflammatory response
Oxidative stress and inflammation are closely related pathophysiological process and are both involved in the pathogenesis of pre-eclampsia. Oxidative stress is manifested as an overload of reactive oxygen species (ROS), which always result in inflammatory response and endothelial dysfunction. In pre-eclampsia, mitochondrial function is destroyed and reactive oxygen species (ROS, mainly superoxide anions) are excessively produced, triggering oxidative stress and systemic inflammation [65-68]. In vitro model of PE, inhibition of SIRT1 decreases antioxidant activity, and lowers the level of intracellular NO and supernatant nitrite [69, 70]. Additionally, SIRT1 also acts as a necessary role in antagonizing oxidative stress and inflammation in the pathogenesis of diabetic vasculopathy [71-73], which is also a critical etiological factor for pre-eclampsia. For instance, the downregulation of SIRT1 induced by hyperglycemia causes vascular dysfunction, while upregulation of SIRT1 attenuates oxidative stress-induced endothelial senescence in diabetic mice [74, 75].
The effect of sirtuins family in trophoblasts
| Source | Sirtuins | Expression | Location | Effect in trophoblasts | Mechanisms |
|---|---|---|---|---|---|
| Arul Nambi Rajan et al. Lappas et al. Barak et al. Borg et al. Erlebacher et al. Tang et al. | SIRT1 [22-27] | Downregulated in placentas and serum samples from PE, significantly lower after adjusting for gestational age (WB, qPCR, IHC) | Placental syncytiotrophoblasts and cytotrophoblasts (IHC) | SIRT1promotes development, differentiation, migration, invasion, and angiogenesis, while inhibits apoptosis, and senescence of trophoblasts. Furthermore, SIRT1 exerts anti-inflammatory effects and anti-oxidative stress in trophoblasts | SMAD2/3, STAT3 or PPARγ pathways; triggering p53 deacetylation; medicating autophagy |
| Yu et al. Hannan et al. | SIRT2 [56, 57] | Downregulated in placentas from PE, but no significance after adjusting for gestational age (microarray, WB, qPCR, IHC) | Placental syncytiotrophoblasts, scattered interstitial cells, the endothelial cells lining, and the vessel walls of the placental villi (IHC) | SIRT2 deficiency inhibits proliferation, migration and invasion, while promotes apoptosis and necroptosis of trophoblasts | Triggering p65 deacetylation |
| Yu et al. | SIRT3 [58] | Downregulated in placentas from PE, but no significance after adjusting for gestational age (WB, qPCR, IHC) | Placental syncytiotrophoblasts and cytotrophoblasts (IHC) | SIRT3 deficiency inhibits proliferation, migration, invasion and tube formation, while promotes cell death and necroptosis of trophoblasts | —— |
| Castex et al. Sandvoß et al. Bartho et al. | SIRT4 [59-61] | Upregulated in HUVECs from HELLP, but no difference in placentas of FGR | —— | SIRT4 triggers senescence of trophoblasts | Induced by inactivation of LSD1 |
| Lim et al. | SIRT6 [62] | Downregulated in fetal membranes from preterm labor | Placental chorionic trophoblasts and decidua tissues, fetal membranes, and amnion epithelium | —— | —— |
SIRT1 protects vascular endothelial cells.
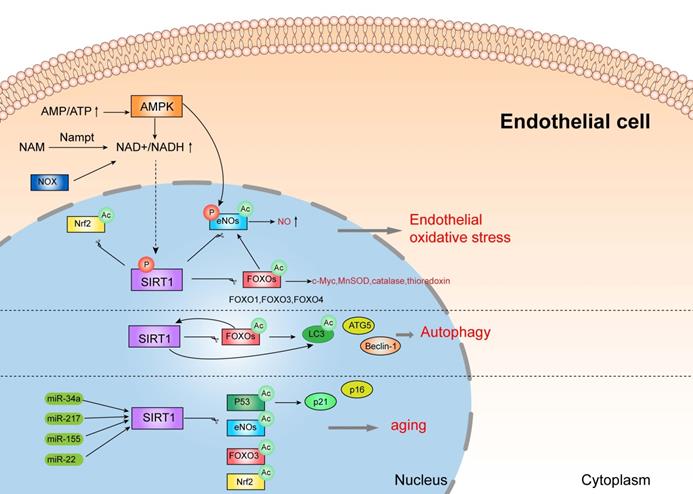
Notably, SIRT1 attenuates oxidative stress and inflammation to regulate vascular endothelial functions through several important signal mediators, such as AMPK, NOXs, eNOs, and FOXOs [76]. There is a complex crosstalk network between AMPK and SIRT1. Studies showed that SIRT1 can stimulate AMPK via the modulation of upstream AMPK kinase such as liver kinase B1(LKB1) [76, 77], suppressing the production of ROS and inflammation response in HUVECs, while AMPK influences SIRT1 deacetylation activity by increasing cellular NAD+ levels or directly phosphorylating SIRT1. Furthermore, increased activity of NOX (NADPH oxidase) may also enhance NAD+ content to elevate SIRT1 levels in endothelial cells [78]. In addition, SIRT1 deacetylates FOXOs and stimulates FOXO-dependent antioxidant [such as catalase (CAT), manganese superoxide dismutase (MnSOD) and thioredoxin] expression to eliminate ROS in endothelial cells, and prevent endothelial dysfunction [78-80]. It is documented that the activation of SIRT1 stimulates the expression of c-Myc by promoting the degradation of FOXO1 to prevent endothelial cell dysfunction and angiogenesis induced by hyperglycemia [81]. eNOs, a member of NOS families, is expressed in vascular smooth muscle [82]. eNOS plays a crucial role in the pathogenesis of pre-eclampsia, since it makes a great contribution to fight against oxidative stress by producing NO and inhibiting the generation of ROS [83]. SIRT1 can directly deacetylate or phosphorylate eNOs, or indirectly stimulate eNOs activity by FOXOs and AMPK pathway [84], which might participate in the pathogenesis of pre-eclampsia. This evidence demonstrated that SIRT1 might protect endothelial cells from oxidative stress and inflammation by interacting with various substrates, which might be associated with pre-eclampsia.
3.2. SIRT1 can also protect endothelial cells by autophagy
In endothelial cells, autophagy is mainly regulated by SIRT1/FOXO1 pathway, which might play a crucial role in the pathogenesis of pre-eclampsia [85]. Studies showed that SIRT1 actives FOXO1 to protect vascular endothelial cells by regulating autophagy [86]. More specifically, SIRT1 deacetylates and activates FOXO1, while FOXO1 can also positively regulate the expression of SIRT1 after activation [87]. FOXO1 is closely related to autophagy, since FOXO1 modulates the expression of many autophagy related proteins such as LC3, ATG5 and Beclin-1[88]. These results suggested that SIRT1 protects vascular endothelial cells by regulating autophagy via many pathways.
3.3. SIRT1 can also protect endothelial cells from senescence
Vascular endothelial senescence is a major risk factor for cardiovascular disease and a leading cause of death in patients [89, 90]. Interestingly, patients with pre-eclampsia exhibit senescence and dysfunction of endothelial progenitor cells [91, 92]. And SIRT1 protects endothelial cells from senescence by various pathways, such as p53, eNOs, Nrf2, FOXO3, and p21/p16, which can be regulated by several miRNA, including miR-217, miR-34a, miR-155, and miR-22 [93-99]. However, more evidence is needed to further verify the functions of SIRT1 in endothelial aging.
This evidence demonstrated that SIRT1 can protect endothelial cells from oxidative stress, inflammatory response, senescence and autophagy by deacetylating various substrates, which might be involved in the pathogenesis of pre-eclampsia.
4. SIRT1 attenuates the performances of pre-eclampsia in animal models
4.1. SIRT1 knockdown drives the development of pre-eclampsia
Studies reported that SIRT1 is decreased in placentas and serum samples of pre-eclampsia patients, as well as in placentas of pre-eclampsia mice model [100]. Importantly, in our previous research, we found that SIRT1 knockdown mice (SIRT1+/- mice) exhibits significant pre-eclampsia-like performances, such as hypertension, proteinuria, fetal growth restriction, kidney injury, and narrow labyrinthine layer, while the manifestations could be reversed after intraperitoneally injecting SRT2104, which is a highly selective agonist of SIRT1[17]. Similarly, Arul Nambi Rajan et al. [22] also found that embryos and placentas were smaller in SIRT1 absence mice, with placentas showing abnormalities in both the labyrinthine layer and junctional zone. Additionally, SIRT1 deficiency mice show multiple developmental defects, ranging from embryonic lethality to postnatal lethality during embryogenesis, with embryo growth restriction [1, 101-103]. Furthermore, placentas of SIRT1-KO mice exhibit senescence markers and morphological disruption [50], which is closely associated with the development of pre-eclampsia.
4.2. Supplement of SIRT1 attenuates the performances of pre-eclampsia
Recently, Huang et al. found that supplement with SIRT1 recombinant protein improved the blood pressure, angiogenic imbalance, inflammation, and pregnancy outcome in RUPP pre-eclampsia rat model [8]. Interestingly, in our previous research, the pre-eclampsia-like performances were reversed after intraperitoneally injecting SRT2104 that can elevate SIRT1 protein expression [17]. However, more animal experiments and clinical trials are needed to further verify the role of SIRT1 in pre-eclampsia.
5. The effect of SIRT1 agonist resveratrol in pre-eclampsia
Resveratrol (3,5,4´-trihydoxy-trans-stilbene, RESV) is a plant polyphenol found in grape skins and red wine, and mainly functions as SIRT1 agonist. Studies have shown that resveratrol involves in various biological processes, such as anti-oxidation, anti-inflammation, anti-aging and anti-cancer [104]. And resveratrol is considered in the treatment of pre-eclampsia according to various pre-clinical experiments and clinical trial.
5.1. The effect of resveratrol on trophoblasts or endothelial cells—in vitro experiments
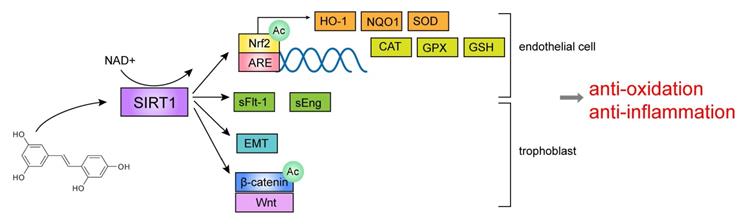
Some studies suggested that resveratrol has an anti-hypertensive effect, which is mainly related to inhibiting the release of sFlt-1 (soluble fms-like tyrosine kinase-1) and sEng (soluble endoglin), reducing the expression of pro-inflammatory molecules, and increasing the expression of anti-oxidant molecules. Resveratrol reduced sFlt-1 and sEng secretion from primary trophoblasts and HUVECs [105, 106], and the elevation of sFlt-1 and sEng is an important feature of pre-eclampsia. Additionally, it is reported that resveratrol could reduce oxidative stress by improving some anti-oxidant markers in endothelial cells of pre-eclampsia, including HO-1, NQO1, Nrf2, GSH (glutathione), SOD (superoxide dismutase) and ARE (antioxidant responsive element) [39, 107-109], which are all crucial molecules regulated by SIRT1. Nrf2, a redox-sensitive transcription factor, can be deacetylated and activated by SIRT1 and promotes the genes transcription of downstream detoxification enzymes and antioxidant enzymes [110, 111], such as SOD and HO-1[112-114]. In addition, Nrf2 can combine with specific DNA sequence ARE to stimulate the transcription of downstream target genes and antioxidant genes including CAT, SOD, and GPX (glutathione peroxidase) [115]. Therefore, resveratrol may play an antioxidant role by upregulating the expression level of SIRT1, thereby activating downstream antioxidant molecules. In addition, some research also reported that resveratrol might promote trophoblast invasion, migration and tube formation by activating epithelial-mesenchymal transition (EMT) and Wnt/β-catenin pathway in pre-eclampsia [116]. The above-mentioned pathways are shown in Figure 3. These reports demonstrated that resveratrol, as an agonist of SIRT1, can regulate the functions of trophoblasts and endothelial cells in vitro.
5.2. The effect of resveratrol on blood pressure in animal model of pre-eclampsia—in vivo experiments
Interestingly, it is reported that resveratrol can alleviate the symptoms of pre-eclampsia in animal models. Poudel et al. [117] showed that resveratrol improves artery blood flow and increases fetal weight in COMT-/- mice but not in eNOS-/- mice, which are both animal models of pre-eclampsia. Furthermore, resveratrol reverses the blood pressure and the concentration of urine protein, and inhibits the oxidative stress in L-NAME-induced pre-eclampsia rat model [109, 116]. However, Ozlem's research is different from the results of the above-mentioned studies, possibly due to the differentially experimental methodology [5]. Therefore, resveratrol reduces blood pressure in pre-eclampsia animal models, indicating that SIRT1 might modulate the progression of pre-eclampsia.
The effect of SIRT1 agonist resveratrol in pre-eclampsia.
5.3. The effect of resveratrol on blood pressure in pre-eclampsia—clinical trials
Furthermore, several clinical trials also found that resveratrol can decrease blood pressure in hypertensive patients. A randomized clinical trial showed that taking resveratrol can significantly reduce hypertensive symptoms in pre-eclampsia patients, compared with the control group [118]. Several meta-analyses and reviews also verify the efficacy of resveratrol in pre-eclampsia [119-122]. Moreover, resveratrol also improves flow-mediated dilatation in obese patients and has a controversial anti-hypertensive effect on hypertensive patients [123-127]. This evidence suggested that resveratrol might reduce the blood pressure in hypertensive patients, and might play a crucial role in improving the symptoms of pre-eclampsia in a SIRT1 dependent manner. However, resveratrol might also play an anti-hypertensive effect through other pathways, which needs more experiments to verify.
Discussion
In this review, we systematically concluded the role of SIRT1 in pre-eclampsia. SIRT1 can affect the development, differentiation, autophagy and senescence of trophoblasts, thereby regulating their invasion and migration, and participating in the remodeling process of spiral arteries [22, 39, 50]. In addition, SIRT1 can also participate in vascular endothelial dysfunction by mediating inflammatory response, oxidative stress, autophagy and aging, and reverse the progression of pre-eclampsia [69, 70, 86]. Interestingly, SIRT1 knockout mice exhibited significant pre-eclampsia-like performances, which can be attenuated by SIRT1 supplementation [8, 17]. Moreover, the SIRT1 agonist resveratrol also shows a strong anti-hypertensive effect, and might function by increasing the expression level of SIRT1 protein [109, 116-118]. However, since resveratrol can also act in other ways, further validation is needed. This evidence suggests that SIRT1 might be an important marker in the pathogenesis of pre-eclampsia.
However, there are still many problems needed further experimental validation. For example, some studies have found that SIRT1 can regulate trophoblast autophagy, but the regulatory mechanisms are not yet completely definite. In addition, SIRT1 is also an important anti-aging molecule involved in a variety of aging-related diseases [8]. However, the specific mechanisms of SIRT1 in placental aging need to be further elucidated. Furthermore, it is not clear whether SIRT1 is involved in the progression of pre-eclampsia through other ways, such as abnormal placental metabolism. A typical example is that lipid abnormalities develop in placentas of pre-eclampsia patients. Recent research showed a possible role for LXRβ (liver X receptors beta) as a transcriptional regulator in pre-eclampsia [128]. LXRβ is a key regulator of lipid homeostasis, and can be deacetylated by SIRT1[129]. However, the functions and mechanisms of SIRT1 and LXRβ in pre-eclampsia remain unclear. Moreover, the upstream molecular mechanisms of SIRT1 in pre-eclampsia also needs to be further elucidated. Our previous study found that progesterone can significantly improve the pre-eclampsia-like symptoms in SIRT1 knockdown mice [17], indicating that progesterone might act as an upstream regulator of SIRT1. These issues still need more experiments and clinical trials to further verify, which is also the direction of our future research.
Abbreviations
PE: pre-eclampsia; ECs : endothelial cells; SIRT1: sirtuin1; Ac: acetylation; P: phosphorylation; NF-κB: nuclear factor-kappaB; PPARγ: peroxisome proliferator-activated receptor gamma; PGC-1α: peroxisome proliferator-activated receptor-gamma co-activator-1alpha; TFEB: transcription factor EB; LC3: microtubule associated protein 1 light chain 3; LAMP1: lysosomal associated membrane protein 1; LAMP2: lysosomal associated membrane protein 2; CTSD: cathepsin D; ATG3: autophagy-related gene 3; ATG5: autophagy-related gene 5; ATG7: autophagy-related gene 7; ATG8: autophagy-related gene 8; SQSTM1: sequestosome 1; HUVECs : human umbilical vein endothelial cells; ROS : reactive oxygen species; AMPK: AMP-activated protein kinase; LKB1: liver kinase B1; NAM: nicotinamide; Nampt: nicotinamide phosphoribosyltransferase; NOX: NADPH oxidases; Nrf2: nuclear factor-erythroid 2 (NF-E2)-related factor2; eNOs: neuronal nitricoxide synthase; FOXO: forkhead box O; MnSOD: manganese superoxide dismutase; sFlt-1: soluble fms-like tyrosine kinase-1; sEng: soluble endoglin; HO-1: heme oxygenase-1; GSH: glutathione; SOD: superoxide dismutase; ARE: antioxidant responsive element; CAT: catalase; GPX: glutathione peroxidase; NQO1: NADPH quinone oxidoreductase 1; EMT: epithelial-mesenchymal transition; NCoR1: nuclear receptor corepressor 1; SMRT: silencing mediator of retinoic acid and thyroid hormone; Prdm16: PR domain-containing protein 16; NO: nitric oxide; STAT3: activating signal transducers and activators of transcription 3; RUPP: reduced uterine perfusion pressure; L-NAME: NG-Nitro-L-arginine methyl ester; LXRβ: liver X receptors beta; HELLP: hemolysis, elevated liver enzymes, and low platelet count; FGR: fetal growth restriction; LSD1: lysine-specific demethylase 1; WB: western blotting; qPCR: quantitative real-time PCR; IHC: immunohistochemistry.
Acknowledgements
This work was supported by the National Natural Sciences Foundation of China (No. 81971408, 81801469, and 81801468); the National Key R&D Program of China (No. 2016YFC1000403); and the 2020 "Diligence· Excellence" Clinical Innovative Team Project “Study on the comprehensive management of preeclampsia and its pathogenesis” conducted by Obstetrics and Gynecology Hospital of Fudan University (No. 2021fckbc06).
Author Contributions
Zhenzhen Liu, Chengjie Wang, and Jiangnan Pei prepared the manuscript. Mingqing Li and Weirong Gu was responsible for overall supervision. All authors reviewed the article critically for intellectual content and agreed to the published version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet Gynecol. 2019;133:1
2. Goel A, Maski MR, Bajracharya S, Wenger JB, Zhang D, Salahuddin S. et al. Epidemiology and Mechanisms of De Novo and Persistent Hypertension in the Postpartum Period. Circulation. 2015;132:1726-33
3. Ives CW, Sinkey R, Rajapreyar I, Tita ATN, Oparil S. Preeclampsia-Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;76:1690-702
4. Eddy AC, Chapman H, George EM. Heparanase regulation of sFLT-1 release in trophoblasts in vitro. Placenta. 2019;85:63-8
5. Moraloglu O, Engin-Ustun Y, Tonguc E, Var T, Tapisiz OL, Ergun H. et al. The effect of resveratrol on blood pressure in a rat model of preeclampsia. J Matern Fetal Neonatal Med. 2012;25:845-8
6. Pankiewicz K, Fijalkowska A, Issat T, Maciejewski TM. Insight into the Key Points of Preeclampsia Pathophysiology: Uterine Artery Remodeling and the Role of MicroRNAs. International journal of molecular sciences. 2021;22:3132
7. Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y. et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011-5
8. Chen C, Zhou M, Ge Y, Wang X. SIRT1 and aging related signaling pathways. Mech Ageing Dev. 2020;187:111215
9. Kong P, Yu Y, Wang L, Dou YQ, Zhang XH, Cui Y. et al. circ-Sirt1 controls NF-kappaB activation via sequence-specific interaction and enhancement of SIRT1 expression by binding to miR-132/212 in vascular smooth muscle cells. Nucleic Acids Res. 2019;47:3580-93
10. Rada P, Pardo V, Mobasher MA, Garcia-Martinez I, Ruiz L, Gonzalez-Rodriguez A. et al. SIRT1 Controls Acetaminophen Hepatotoxicity by Modulating Inflammation and Oxidative Stress. Antioxidants & redox signaling. 2018;28:1187-208
11. Xu C, Wang L, Fozouni P, Evjen G, Chandra V, Jiang J. et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nat Cell Biol. 2020;22:1170-9
12. Cole P. [Destructive respiratory infection]. Kansenshogaku Zasshi. 1996;70:1133-9
13. Huang K, Chen C, Hao J, Huang J, Wang S, Liu P. et al. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating Sirt1 to resist AGEs-induced upregulation of fibronetin and transforming growth factor-beta1 in rat glomerular messangial cells. Mol Cell Endocrinol. 2015;399:178-89
14. Huang K, Huang J, Xie X, Wang S, Chen C, Shen X. et al. Sirt1 resists advanced glycation end products-induced expressions of fibronectin and TGF-beta1 by activating the Nrf2/ARE pathway in glomerular mesangial cells. Free radical biology & medicine. 2013;65:528-40
15. Huang K, Li R, Wei W. Sirt1 activation prevents anti-Thy 1.1 mesangial proliferative glomerulonephritis in the rat through the Nrf2/ARE pathway. Eur J Pharmacol. 2018;832:138-44
16. Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB. et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14855-60
17. Pei J, Liu Z, Wang C, Chu N, Liu L, Tang Y. et al. Progesterone Attenuates SIRT1-Deficiency-Mediated Pre-Eclampsia. Biomolecules. 2022;12:422
18. Fisher SJ. Why is placentation abnormal in preeclampsia? Am J Obstet Gynecol. 2015;213:S115-22
19. Huynh J, Dawson D, Roberts D, Bentley-Lewis R. A systematic review of placental pathology in maternal diabetes mellitus. Placenta. 2015;36:101-14
20. Kwak-Kim J, Bao S, Lee SK, Kim JW, Gilman-Sachs A. Immunological modes of pregnancy loss: inflammation, immune effectors, and stress. Am J Reprod Immunol. 2014;72:129-40
21. Pham J, Arul Nambi Rajan K, Li P, Parast MM. The role of Sirtuin1-PPARgamma axis in placental development and function. Journal of molecular endocrinology. 2018;60:R201-R12
22. Arul Nambi Rajan K, Khater M, Soncin F, Pizzo D, Moretto-Zita M, Pham J. et al. Sirtuin1 is required for proper trophoblast differentiation and placental development in mice. Placenta. 2018;62:1-8
23. Lappas M, Mitton A, Lim R, Barker G, Riley C, Permezel M. SIRT1 is a novel regulator of key pathways of human labor. Biol Reprod. 2011;84:167-78
24. Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR. et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585-95
25. Borg AJ, Yong HE, Lappas M, Degrelle SA, Keogh RJ, Da Silva-Costa F. et al. Decreased STAT3 in human idiopathic fetal growth restriction contributes to trophoblast dysfunction. Reproduction. 2015;149:523-32
26. Erlebacher A, Price KA, Glimcher LH. Maintenance of mouse trophoblast stem cell proliferation by TGF-beta/activin. Dev Biol. 2004;275:158-69
27. Tang S, Huang G, Fan W, Chen Y, Ward JM, Xu X. et al. SIRT1-mediated deacetylation of CRABPII regulates cellular retinoic acid signaling and modulates embryonic stem cell differentiation. Mol Cell. 2014;55:843-55
28. Takahashi Y, Takahashi M, Carpino N, Jou ST, Chao JR, Tanaka S. et al. Leukemia inhibitory factor regulates trophoblast giant cell differentiation via Janus kinase 1-signal transducer and activator of transcription 3-suppressor of cytokine signaling 3 pathway. Mol Endocrinol. 2008;22:1673-81
29. Wang W, Li F, Xu Y, Wei J, Zhang Y, Yang H. et al. JAK1-mediated Sirt1 phosphorylation functions as a negative feedback of the JAK1-STAT3 pathway. J Biol Chem. 2018;293:11067-75
30. Tache V, Ciric A, Moretto-Zita M, Li Y, Peng J, Maltepe E. et al. Hypoxia and trophoblast differentiation: a key role for PPARgamma. Stem Cells Dev. 2013;22:2815-24
31. Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R. et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771-6
32. Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y. et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell. 2012;150:620-32
33. Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining "clean" cells. Autophagy. 2005;1:131-40
34. Doherty J, Baehrecke EH. Life, death and autophagy. Nat Cell Biol. 2018;20:1110-7
35. Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069-75
36. Shimada Y, Klionsky DJ. Autophagy contributes to lysosomal storage disorders. Autophagy. 2012;8:715-6
37. Yoshimori T. Autophagy: paying Charon's toll. Cell. 2007;128:833-6
38. Nakashima A, Cheng SB, Ikawa M, Yoshimori T, Huber WJ, Menon R. et al. Evidence for lysosomal biogenesis proteome defect and impaired autophagy in preeclampsia. Autophagy. 2020;16:1771-85
39. Wang P, Huang CX, Gao JJ, Shi Y, Li H, Yan H. et al. Resveratrol induces SIRT1-Dependent autophagy to prevent H2O2-Induced oxidative stress and apoptosis in HTR8/SVneo cells. Placenta. 2020;91:11-8
40. Chen C, Xia B, Tang L, Wu W, Tang J, Liang Y. et al. Echinacoside protects against MPTP/MPP(+)-induced neurotoxicity via regulating autophagy pathway mediated by Sirt1. Metab Brain Dis. 2019;34:203-12
41. Jiang Q, Hao R, Wang W, Gao H, Wang C. SIRT1/Atg5/autophagy are involved in the antiatherosclerosis effects of ursolic acid. Molecular and cellular biochemistry. 2016;420:171-84
42. Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R. et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol. 2015;17:288-99
43. Napolitano G, Esposito A, Choi H, Matarese M, Benedetti V, Di Malta C. et al. mTOR-dependent phosphorylation controls TFEB nuclear export. Nat Commun. 2018;9:1-10
44. Akaishi R, Yamada T, Nakabayashi K, Nishihara H, Furuta I, Kojima T. et al. Autophagy in the placenta of women with hypertensive disorders in pregnancy. Placenta. 2014;35:974-80
45. Gao L, Qi HB, Kamana KC, Zhang XM, Zhang H, Baker PN. Excessive autophagy induces the failure of trophoblast invasion and vasculature: possible relevance to the pathogenesis of preeclampsia. J Hypertens. 2015;33:106-17
46. Oh SY, Choi SJ, Kim KH, Cho EY, Kim JH, Roh CR. Autophagy-related proteins, LC3 and Beclin-1, in placentas from pregnancies complicated by preeclampsia. Reprod Sci. 2008;15:912-20
47. Feng L, Chen M, Li Y, Li M, Hu S, Zhou B. et al. Sirt1 deacetylates and stabilizes p62 to promote hepato-carcinogenesis. Cell death & disease. 2021;12:1-13
48. Huang R, Xu Y, Wan W, Shou X, Qian J, You Z. et al. Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol Cell. 2015;57:456-66
49. Pi QZ, Wang XW, Jian ZL, Chen D, Zhang C, Wu QC. Melatonin Alleviates Cardiac Dysfunction Via Increasing Sirt1-Mediated Beclin-1 Deacetylation and Autophagy During Sepsis. Inflammation. 2021;44:1184-93
50. Xiong L, Ye X, Chen Z, Fu H, Li S, Xu P. et al. Advanced Maternal Age-associated SIRT1 Deficiency Compromises Trophoblast Epithelial-Mesenchymal Transition through an Increase in Vimentin Acetylation. Aging cell. 2021;20:e13491
51. Sultana Z, Maiti K, Dedman L, Smith R. Is there a role for placental senescence in the genesis of obstetric complications and fetal growth restriction? Am J Obstet Gynecol. 2018;218:S762-S73
52. Biron-Shental T, Sukenik-Halevy R, Sharon Y, Goldberg-Bittman L, Kidron D, Fejgin MD. et al. Short telomeres may play a role in placental dysfunction in preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol. 2010;202:381 e1-7
53. Tasta O, Swiader A, Grazide MH, Rouahi M, Parant O, Vayssiere C. et al. A role for 4-hydroxy-2-nonenal in premature placental senescence in preeclampsia and intrauterine growth restriction. Free radical biology & medicine. 2021;164:303-14
54. Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. 2000;273:793-8
55. Shin-Ichiro Imal, Christopher M.Armstrong, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:796-800
56. Hannan NJ, Beard S, Binder NK, Onda K, Kaitu'u-Lino TJ, Chen Q. et al. Key players of the necroptosis pathway RIPK1 and SIRT2 are altered in placenta from preeclampsia and fetal growth restriction. Placenta. 2017;51:1-9
57. Yu Y, An X, Fan D. Histone Deacetylase Sirtuin 2 Enhances Viability of Trophoblasts Through p65-Mediated MicroRNA-146a/ACKR2 Axis. Reprod Sci. 2021;28:1370-81
58. Yu H, Zhang Y, Liu M, Liao L, Wei X, Zhou R. SIRT3 deficiency affects the migration, invasion, tube formation and necroptosis of trophoblast and is implicated in the pathogenesis of preeclampsia. Placenta. 2022;120:1-9
59. Castex J, Willmann D, Kanouni T, Arrigoni L, Li Y, Friedrich M. et al. Inactivation of Lsd1 triggers senescence in trophoblast stem cells by induction of Sirt4. Cell death & disease. 2017;8:e2631-e2631
60. Sandvoss M, Potthast AB, von Versen-Hoynck F, Das AM. HELLP Syndrome. Reprod Sci. 2017;24:568-74
61. Bartho LA, O'Callaghan JL, Fisher JJ, Cuffe JSM, Kaitu'u-Lino TJ, Hannan NJ. et al. Analysis of mitochondrial regulatory transcripts in publicly available datasets with validation in placentae from pre-term, post-term and fetal growth restriction pregnancies. Placenta. 2021;112:162-71
62. Lim R, Barker G, Lappas M. SIRT6 is decreased with preterm labor and regulates key terminal effector pathways of human labor in fetal membranes. Biol Reprod. 2013;88:1-10
63. Viana-Mattioli S, Nunes P, Cavalli R, Sandrim V. Analysis of SIRT1 Expression in Plasma and in an In Vitro Model of Preeclampsia. Oxid Med Cell Longev. 2020;2020:4561083
64. Yin Y, Feng Y, Zhao H, Zhao Z, Yua H, Xu J. et al. SIRT1 inhibits releases of HMGB1 and HSP70 from human umbilical vein endothelial cells caused by IL-6 and the serum from a preeclampsia patient and protects the cells from death. Biomed Pharmacother. 2017;88:449-58
65. Rochette L, Zeller M, Cottin Y, Vergely C. Diabetes, oxidative stress and therapeutic strategies. Biochim Biophys Acta. 2014;1840:2709-29
66. Kaludercic N, Di Lisa F. Mitochondrial ROS Formation in the Pathogenesis of Diabetic Cardiomyopathy. Front Cardiovasc Med. 2020;7:12
67. Keane KN, Cruzat VF, Carlessi R, de Bittencourt PI Jr, Newsholme P. Molecular Events Linking Oxidative Stress and Inflammation to Insulin Resistance and beta-Cell Dysfunction. Oxid Med Cell Longev. 2015;2015:181643
68. Volpe CMO, Villar-Delfino PH, Dos Anjos PMF, Nogueira-Machado JA. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell death & disease. 2018;9:1-9
69. Caldeira-Dias M, Viana-Mattioli S, de Souza Rangel Machado J, Carlstrom M, de Carvalho Cavalli R, Sandrim VC. Resveratrol and grape juice: Effects on redox status and nitric oxide production of endothelial cells in in vitro preeclampsia model. Pregnancy Hypertens. 2021;23:205-10
70. Viana-Mattioli S, Cinegaglia N, Bertozzi-Matheus M, Bueno-Pereira TO, Caldeira-Dias M, Cavalli RC. et al. SIRT1-dependent effects of resveratrol and grape juice in an in vitro model of preeclampsia. Biomed Pharmacother. 2020;131:110659
71. Mishra M, Duraisamy AJ, Kowluru RA. Sirt1: A Guardian of the Development of Diabetic Retinopathy. Diabetes. 2018;67:745-54
72. Karbasforooshan H, Karimi G. The role of SIRT1 in diabetic retinopathy. Biomed Pharmacother. 2018;97:190-4
73. Kume S, Uzu T, Kashiwagi A, Koya D. SIRT1, a calorie restriction mimetic, in a new therapeutic approach for type 2 diabetes mellitus and diabetic vascular complications. Endocr Metab Immune Disord Drug Targets. 2010;10:16-24
74. Orimo M, Minamino T, Miyauchi H, Tateno K, Okada S, Moriya J. et al. Protective role of SIRT1 in diabetic vascular dysfunction. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:889-94
75. Ota H, Eto M, Kano MR, Kahyo T, Setou M, Ogawa S. et al. Induction of endothelial nitric oxide synthase, SIRT1, and catalase by statins inhibits endothelial senescence through the Akt pathway. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:2205-11
76. Pal PB, Sonowal H, Shukla K, Srivastava SK, Ramana KV. Aldose reductase regulates hyperglycemia-induced HUVEC death via SIRT1/AMPK-alpha1/mTOR pathway. Journal of molecular endocrinology. 2019;63:11-25
77. Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628-35
78. Zhang W, Huang Q, Zeng Z, Wu J, Zhang Y, Chen Z. Sirt1 Inhibits Oxidative Stress in Vascular Endothelial Cells. Oxid Med Cell Longev. 2017;2017:7543973
79. Giannakou ME, Partridge L. The interaction between FOXO and SIRT1: tipping the balance towards survival. Trends Cell Biol. 2004;14:408-12
80. Kobayashi Y, Furukawa-Hibi Y, Chen C, Horio Y, Isobe K, Ikeda K. et al. SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. International journal of molecular medicine. 2005;16:237-43
81. Huang X, Sun J, Chen G, Niu C, Wang Y, Zhao C. et al. Resveratrol Promotes Diabetic Wound Healing via SIRT1-FOXO1-c-Myc Signaling Pathway-Mediated Angiogenesis. Frontiers in pharmacology. 2019;10:421
82. Qian J, Fulton D. Post-translational regulation of endothelial nitric oxide synthase in vascular endothelium. Frontiers in physiology. 2013;4:347
83. Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829-37 37a-37d
84. Chen Z, Peng IC, Sun W, Su MI, Hsu PH, Fu Y. et al. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circulation research. 2009;104:496-505
85. Cornelius DC, Wallace K. Autophagy in preeclampsia: A new target? EBioMedicine. 2020;57:102864
86. Wu Q, Hu Y, Jiang M, Wang F, Gong G. Effect of Autophagy Regulated by Sirt1/FoxO1 Pathway on the Release of Factors Promoting Thrombosis from Vascular Endothelial Cells. International journal of molecular sciences. 2019;20:4132
87. Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M. et al. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10042-7
88. Liu J, Bi X, Chen T, Zhang Q, Wang SX, Chiu JJ. et al. Shear stress regulates endothelial cell autophagy via redox regulation and Sirt1 expression. Cell death & disease. 2015;6:e1827-e1827
89. Heo JI, Kim KI, Woo SK, Kim JS, Choi KJ, Lee HJ. et al. Stromal Cell-Derived Factor 1 Protects Brain Vascular Endothelial Cells from Radiation-Induced Brain Damage. Cells. 2019;8:1230
90. Tian XL, Li Y. Endothelial cell senescence and age-related vascular diseases. J Genet Genomics. 2014;41:485-95
91. Hwang HS, Maeng YS, Park YW, Koos BJ, Kwon YG, Kim YH. Increased senescence and reduced functional ability of fetal endothelial progenitor cells in pregnancies complicated by preeclampsia without intrauterine growth restriction. Am J Obstet Gynecol. 2008;199:259 e1-7
92. Sugawara J, Mitsui-Saito M, Hayashi C, Hoshiai T, Senoo M, Chisaka H. et al. Decrease and senescence of endothelial progenitor cells in patients with preeclampsia. J Clin Endocrinol Metab. 2005;90:5329-32
93. Guo Q, Zhang H, Zhang B, Zhang E, Wu Y. Tumor Necrosis Factor-alpha (TNF-alpha) Enhances miR-155-Mediated Endothelial Senescence by Targeting Sirtuin1 (SIRT1). Med Sci Monit. 2019;25:8820-35
94. Guo Y, Chao L, Chao J. Kallistatin attenuates endothelial senescence by modulating Let-7g-mediated miR-34a-SIRT1-eNOS pathway. Journal of cellular and molecular medicine. 2018;22:4387-98
95. Ming GF, Wu K, Hu K, Chen Y, Xiao J. NAMPT regulates senescence, proliferation, and migration of endothelial progenitor cells through the SIRT1 AS lncRNA/miR-22/SIRT1 pathway. Biochem Biophys Res Commun. 2016;478:1382-8
96. Wang Z, Shi D, Zhang N, Yuan T, Tao H. MiR-217 promotes endothelial cell senescence through the SIRT1/p53 signaling pathway. J Mol Histol. 2021;52:257-67
97. Chen L, Holder R, Porter C, Shah Z. Vitamin D3 attenuates doxorubicin-induced senescence of human aortic endothelial cells by upregulation of IL-10 via the pAMPKalpha/Sirt1/Foxo3a signaling pathway. PLoS One. 2021;16:e0252816
98. Chen Z, Yu J, Fu M, Dong R, Yang Y, Luo J. et al. Dipeptidyl peptidase-4 inhibition improves endothelial senescence by activating AMPK/SIRT1/Nrf2 signaling pathway. Biochem Pharmacol. 2020;177:113951
99. Duan JL, Ruan B, Song P, Fang ZQ, Yue ZS, Liu JJ. et al. Shear stress-induced cellular senescence blunts liver regeneration through Notch-sirtuin 1-P21/P16 axis. Hepatology. 2022;75:584-99
100. Huang Y, Zheng XD, Li H. Protective role of SIRT1-mediated Sonic Hedgehog signaling pathway in the preeclampsia rat models. J Assist Reprod Genet. 2021;38:1843-51
101. Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P. et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10794-9
102. McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR. et al. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol. 2003;23:38-54
103. Wang RH, Sengupta K, Li C, Kim HS, Cao L, Xiao C. et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008;14:312-23
104. Breuss JM, Atanasov AG, Uhrin P. Resveratrol and Its Effects on the Vascular System. International journal of molecular sciences. 2019;20:1523
105. Al-Ani B. Resveratrol inhibits proteinase-activated receptor-2-induced release of soluble vascular endothelial growth factor receptor-1 from human endothelial cells. EXCLI J. 2013;12:598-604
106. Cudmore MJ, Ramma W, Cai M, Fujisawa T, Ahmad S, Al-Ani B. et al. Resveratrol inhibits the release of soluble fms-like tyrosine kinase (sFlt-1) from human placenta. Am J Obstet Gynecol. 2012;206:253 e10-5
107. Caldeira-Dias M, Montenegro MF, Bettiol H, Barbieri MA, Cardoso VC, Cavalli RC. et al. Resveratrol improves endothelial cell markers impaired by plasma incubation from women who subsequently develop preeclampsia. Hypertens Res. 2019;42:1166-74
108. Gurusinghe S, Cox AG, Rahman R, Chan ST, Muljadi R, Singh H. et al. Resveratrol mitigates trophoblast and endothelial dysfunction partly via activation of nuclear factor erythroid 2-related factor-2. Placenta. 2017;60:74-85
109. Zou Y, Zuo Q, Huang S, Yu X, Jiang Z, Zou S. et al. Resveratrol inhibits trophoblast apoptosis through oxidative stress in preeclampsia-model rats. Molecules. 2014;19:20570-9
110. Yamamoto M, Kensler TW, Motohashi H. The KEAP1-NRF2 System: a Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol Rev. 2018;98:1169-203
111. Xu JJ, Cui J, Lin Q, Chen XY, Zhang J, Gao EH. et al. Protection of the enhanced Nrf2 deacetylation and its downstream transcriptional activity by SIRT1 in myocardial ischemia/reperfusion injury. International journal of cardiology. 2021;342:82-93
112. Dinkova-Kostova AT. The Role of Sulfhydryl Reactivity of Small Molecules for the Activation of the KEAP1/NRF2 Pathway and the Heat Shock Response. Scientifica (Cairo). 2012;2012:606104
113. Giudice A, Arra C, Turco MC. Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Methods Mol Biol. 2010;647:37-74
114. Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549-57
115. Nagappan A, Kim JH, Jung DY, Jung MH. Cryptotanshinone from the Salvia miltiorrhiza Bunge Attenuates Ethanol-Induced Liver Injury by Activation of AMPK/SIRT1 and Nrf2 Signaling Pathways. International journal of molecular sciences. 2019;21:265
116. Zou Y, Li S, Wu D, Xu Y, Wang S, Jiang Y. et al. Resveratrol promotes trophoblast invasion in pre-eclampsia by inducing epithelial-mesenchymal transition. Journal of cellular and molecular medicine. 2019;23:2702-10
117. Poudel R, Stanley JL, Rueda-Clausen CF, Andersson IJ, Sibley CP, Davidge ST. et al. Effects of resveratrol in pregnancy using murine models with reduced blood supply to the uterus. PLoS One. 2013;8:e64401
118. Ding J, Kang Y, Fan Y, Chen Q. Efficacy of resveratrol to supplement oral nifedipine treatment in pregnancy-induced preeclampsia. Endocr Connect. 2017;6:595-600
119. Cicero AFG, Fogacci F, Colletti A. Food and plant bioactives for reducing cardiometabolic disease risk: an evidence based approach. Food & function. 2017;8:2076-88
120. de Alwis N, Binder NK, Beard S, Kaitu'u-Lino TJ, Tong S, Brownfoot F. et al. Novel approaches to combat preeclampsia: from new drugs to innovative delivery. Placenta. 2020;102:10-6
121. Ozarowski M, Karpinski TM, Szulc M, Wielgus K, Kujawski R, Wolski H. et al. Plant Phenolics and Extracts in Animal Models of Preeclampsia and Clinical Trials-Review of Perspectives for Novel Therapies. Pharmaceuticals (Basel). 2021;14:269
122. Tenorio MB, Ferreira RC, Moura FA, Bueno NB, Goulart MOF, Oliveira ACM. Oral antioxidant therapy for prevention and treatment of preeclampsia: Meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2018;28:865-76
123. Biesinger S, Michaels HA, Quadros AS, Qian Y, Rabovsky AB, Badger RS. et al. A combination of isolated phytochemicals and botanical extracts lowers diastolic blood pressure in a randomized controlled trial of hypertensive subjects. Eur J Clin Nutr. 2016;70:10-6
124. Marques B, Trindade M, Aquino JCF, Cunha AR, Gismondi RO, Neves MF. et al. Beneficial effects of acute trans-resveratrol supplementation in treated hypertensive patients with endothelial dysfunction. Clin Exp Hypertens. 2018;40:218-23
125. Movahed A, Ostovar A, Iranpour D, Thandapilly SJ, Raj P, Louis XL. et al. The efficacy of resveratrol in controlling hypertension: study protocol for a randomized, crossover, double-blinded, placebo-controlled trial. Trials. 2016;17:296
126. Wong RH, Berry NM, Coates AM, Buckley JD, Bryan J, Kunz I. et al. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J Hypertens. 2013;31:1819-27
127. Wong RH, Howe PR, Buckley JD, Coates AM, Kunz I, Berry NM. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr Metab Cardiovasc Dis. 2011;21:851-6
128. Weedon-Fekjaer MS, Johnsen GM, Anthonisen EH, Sugulle M, Nebb HI, Duttaroy AK. et al. Expression of liver X receptors in pregnancies complicated by preeclampsia. Placenta. 2010;31:818-24
129. Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28:91-106
Author contact
![]() Corresponding authors: Dr. Weirong Gu, The Department of Obstetrics, Obstetrics and Gynecology Hospital of Fudan University, No. 419 Fangxie Road, Shanghai 200011, China. E-mail: guweirongedu.cn. Prof. Mingqing Li, Shanghai Key Laboratory of Female Reproductive Endocrine Related Diseases, No. 413 Zhaozhou Road, Shanghai 200011, China. E-mail: mqliedu.cn.
Corresponding authors: Dr. Weirong Gu, The Department of Obstetrics, Obstetrics and Gynecology Hospital of Fudan University, No. 419 Fangxie Road, Shanghai 200011, China. E-mail: guweirongedu.cn. Prof. Mingqing Li, Shanghai Key Laboratory of Female Reproductive Endocrine Related Diseases, No. 413 Zhaozhou Road, Shanghai 200011, China. E-mail: mqliedu.cn.

 Global reach, higher impact
Global reach, higher impact