3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(5):813-820. doi:10.7150/ijms.68118 This issue Cite
Research Paper
Clinical and Cellular Evidence of Glycyrrhiza glabra and Platycodon grandiflorus for Vocal Fold Nodules Complementary Treatment
1. Department of Chinese Medicine, Chiayi Chang Gung Memorial Hospital, Chiayi 61363, Taiwan.
2. School of Chinese Medicine, College of Medicine, Chang Gung University, Taoyuan 33302, Taiwan.
3. Department of Medical Research and Development, Chiayi Chang Gung Memorial Hospital, Chiayi 61363, Taiwan.
4. Department of Pharmacy, Chiayi Chang Gung Memorial Hospital, Chiayi, Taiwan.
5. Department of Neurology, National Cheng-Kung University Hospital, Tainan, Taiwan.
6. Department of Otolaryngology-Head and Neck Surgery, Chiayi Chang Gung Memorial Hospital, Chiayi 61363, Taiwan.
7. Graduate Institute of Clinical Medical Sciences, College of Medicine, Chang Gung University, Taoyuan 33302, Taiwan.
8. Department of Otolaryngology, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung 83301, Taiwan.
9. School of Medicine, College of Medicine, Chang Gung University, Taoyuan 33302, Taiwan.
Received 2021-10-16; Accepted 2022-4-18; Published 2022-5-1
Abstract
Vocal fold nodules (VFNs) are the most frequent cause of hoarseness. The management comprised medical, surgical and physical therapy but the effectiveness is not always satisfactory. In this study, we try to figure out an alternative treatment from our clinical experience summary. We retrospectively reviewed VFNs patients who received traditional Chinese medicine (TCM) treatments from July 2018 to August 2020 and traced their Chinese Voice Handicap Index-10 (VHI-C10) and multidimensional voice program (MDVP) analysis results. For further evaluation, we conducted an inflammatory response of porcine vocal fold epithelial (PVFE) cells with 50 ng/mL TNF-alpha. The inflamed PVFE cells were separately cultured in the aqueous extract of Glycyrrhiza glabra (G. glabra) and Platycodon grandifloras (P. grandifloras). In these VFNs patients (n = 22), the average VHI-C10 score decreased from 17.6 to 6.6 (p < 0.001). MDVP analysis revealed improvements in jitter, shimmer, noise-harmonic ratio, and GRBAS scoring system. Of the TCM prescription patterns, G. glabra and P. grandiflorus were used most frequently. In the MTT assay of PVFE cells, no adverse effects of our extracts were observed at doses of 1-200 µg/mL. Western blot analysis revealed downregulation of p65 and mitogen activated protein kinase pathway proteins. The results from both the clinical and in vitro aspects of this study revealed that the herbs G. glabra and P. grandiflorus may offer beneficial outcomes as alternative treatments for VFNs after precise diagnosis.
Keywords: vocal fold nodules, traditional Chinese medicine, herbs, Glycyrrhiza glabra, Platycodon grandifloras
Introduction
Vocal fold nodules (VFNs) as benign vocal fold lesions are quite a challenge for otolaryngologists. Hoarse and raspy voices are distinguished symptoms for VFNs. It is popular among professional voice users like teachers or sales. The definition of VFNs are mucosal lesions occurring on both sides of the vocal fold on the border of the anterior and middle third of the vocal fold [1, 2]. Such lesions are often found on the superficial layer of the lamina propia [3]. Long term exposure to irritants may cause chronic laryngeal inflammation, which may further lead to the formation of VFNs.
Medical treatments, voice therapy and excision through microlaryngeal surgery are considered as VFNs treatment. Physiological, medical, and psychological factors should be considered during diagnosis and treatment [4, 5].
While voice therapy can alter voice production patterns to minimize phonotrauma, thus considered as the first-line treatment for VFNs because it typically resolves voice problems and prevents recurrence in most patients, vocal hygiene education were also proved to be a cost-effective conservative treatment especially in the countries with few speech pathologists [4,6]. Both of above treatments involve education on vocal fold mechanics and etiological factors, as well as specific modifications to behaviors that exacerbate inappropriate voice production.
The compliance of the voice therapy and vocal hygiene education may be affected by various factors. For example, for the patients who live in the rural areas, the compliance and self-awareness of vocal hygiene may be hard to achieve. When the above conservative treatments failed, surgery is still the standard VFNs treatment. However, serious complications associated with surgery may occur, including damage to the vocal folds [7]. Intracordal steroid injection can be beneficial for managing VFNs. However, the technique of injection on vocal folds requires a well-trained laryngologist. Nevertheless, VFNs may persist after injection.
Indeed, there are increasing patients inclined to choose complementary medicine other than original management due to concerning about the risks of surgery or side effects of steroid [8, 9]. As for physiotherapy, such as massage, spinal manipulative therapy and acupuncture, there are several trials revealing that these treatments might be effective for voice disorders [10]. In an small-scaled acupuncture study, anti-inflammatory significantly increased was found in the experiment group. This may be the mechanism why complementary medicine is effective to the voice disorders [11].
When it comes to traditional Chinese medicine (TCM), there are limited evidence of effects on vocal nodules and vocal polyp management. Moreover, the methodological quality remained unsatisfactory and the exact contents and the dosage were uncertain in these researches [12, 13]. Therefore, it is a challenge to verify the exact effect of TCM, especially cellular mechanism.
For in vitro part, some herbal aqueous and ethanol extracts such as clove and ginger exhibit anti-inflammatory ability in human tonsil epithelial cells [12]. There are several cell types in the upper airway. The vocal fold comprised stratified squamous epithelial cells [13]. Since it is difficult to obtain healthy human vocal fold epithelial cells without risking vocal function, the fresh porcine vocal fold tissue is readily and easily available [14]. Moreover, compared to other animals, the morphology of porcine vocal fold epithelium is the most similar to that of humans [15].
So far, few researches reported the effects of herbs on both vocal fold nodule and inflammatory vocal fold cells. In our study, we retrospectively evaluated the clinical effects by the clinical results of our TCM herbal treatment. Also, we further investigated the effects of these herbs in an in vitro study.
Materials and Methods
Subjects
We reviewed the medical records of 22 patients with diagnosis of VFNs which are based on videostroboscopy or pathological result from July 2018 to August 2020. Other benign vocal fold lesions were excluded from the present study, such as vocal polyps, vocal cysts, and Reinke's edema. The patients were examined with laryngeal videostroboscopy using a Kay Elemetrics Stroboscopy Unit (Model, Lincoln Park, NJ, USA) at their initial visit and were followed up 3 months after TCM treatment. The TCM treatment was given with finished herbal products (FHPs) after patient evaluation respectively. The TCM treatment was under national healthcare insurance.
Speech pathologist skilled in voice training and an otolaryngologist evaluated the recorded data. Both of them were blinded to the TCM treatment protocols. The present study was approved by the Institutional Review Board of Chiayi Chang Gung Memorial Hospital (No. 201901034B0). All participants gave written informed consent prior to the beginning of the study.
Objective and Subjective Voice Analysis
The acoustic parameters recorded automatically were as follows: average fundamental frequency (F0, in Hertz), jitter, shimmer, and noise-to-harmonic ratio (NHR). Jitter is defined as the cycle-to-cycle variation in fundamental frequency [16, 17], and shimmer is expressed in decibels, representing the variability in the peak-to-peak amplitude. NHR is used for evaluation of dysphonic voice by measuring the amount of additive noise in the voice signal [18]. The acoustic parameters were measured by the Computerized Speech Laboratory (core model CSL 4500, KayPentax, Lincoln Park, NJ, USA). The duration parameter measured in this study was the maximum phonation time (MPT). A speaker's MPT is measured by a stopwatch, as their best attempt to sustain the vowel /a/ sound at a comfortable intensity [19]. Twenty-two patients completed a pre- and post-test Chinese Voice Handicap Index-10 questionnaire (VHI-C10) [20, 21]. Pre- and post-TCM perceptual evaluation was performed by using the GRBAS scoring system (G = grade, R = roughness, B = breathiness, A = asthenia, and S = strain; 0 = normal, 1 = mild, 2 = moderate, and 3 = severe). It the difference between the two GRBAS scores exceeded two points, a reassessment is required. The voice parameters mentioned above were measured by a speech pathologist and an otolaryngologist in a double-blinded manner.
Aqueous Extracts of TCM Herb Glycyrrhiza glabra and Platycodon grandifloras
The raw herbs used for the aqueous extracts (AE) were obtained from Chiayi Chang Gung Memorial Hospital (Taiwan), 20 g Glycyrrhiza. glabra from Batch No. 42050309 and 20 g Platycodon. grandiflorus from Batch No. N15529. The raw herb materials were separately macerated in 1000 mL reverse osmosis water and then boiled for 10 minutes. These filtrates were combined and concentrated under reduced pressure at 40°C by using a vacuum rotary evaporator to obtain crude AEs. Approximately 4 g of the resulting G. glabra AE (20% yield) and 11 g of the resulting P. grandifloras AE (55% yield) were stored at -20 °C before use.
Primary Culture PVFE Cells
The porcine vocal fold epithelial (PVFE) cells for the primary culture were purchased from GeneDirex, Inc. The PVFE cells were isolated and cultured as reported [14]. Epithelial cells were derived from porcine vocal folds, and then further expanded in culture. Epithelial cells identification and characterization were done by immunostaining with pan-Cytokeratin antibodies.
MTT Assay
PVFE cells were treated with various concentrations of G. glabra and P. grandiflorus; subsequently. The percentages of metabolically active cells were determined based on the mitochondrial conversion of 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) into formazine. In brief, after cells were treated with G. glabra and P. grandiflorus for various incubation times, culture media were replaced with DMEM/F-12 (1:3 ratio) containing 0.02% MTT (Sigma-Aldrich) and incubated for 4 hours; subsequently, the medium was replaced with 200 μL of dimethyl sulfoxide per well. The results were assessed in a 96-well format plate reader by measuring the absorbance at a wavelength of 595 nm on a Victor Nivo Multimode microplate reader (PerkinElmer, Akron, OH, USA).
Inflammatory Response Induction and Culture in TCM Aqueous Extract
The PVFE cells were seeded at a density of 35×103 cells/well in PLL-coated 24-well plates and incubated overnight at 35 °C for 20 hours. PVFE cells were then exposed to the inflammatory cytokine tumor necrosis factor alpha (TNF-α) 50 ng/mL for 24 hours incubation at 35 °C to trigger inflammatory reaction. In addition, the corticosteroid dexamethasone was included as a positive control anti-inflammatory drug.
Western Blot Analysis
Cells were collected in lysis buffer containing protease and phosphatase inhibitors for protein isolation. We prepared cellular extracts by sonication. And total protein concentrations were determined for the Western blot analyses. Proteins were separated on 4%-20% Tris-Glycine gels (Invitrogen, Carlsbad, CA, USA) and transferred to nitrocellulose membranes. After the membranes were blocked in TBST (10 mM Tris-HCl buffer, pH 8.0; 150 mM NaCl; and 0.1% Tween 20) and 5% (w/v) BSA at room temperature for 60 mins, they were incubated overnight at 4 °C with antigen-specific primary antibodies. The primary antibodies used were against phospho-NF-κB p65 (Ser536) (Cell Signaling Technology, Danvers, MA, USA). The blots were under incubation with species-specific HRP-conjugated secondary antibodies for 2 hours at room temperature. And then, the proteins were visualized through incubation with a chemiluminescent substrate kit (Thermo Fisher Scientific, Waltham, MA, USA).
Statistics
Experiments were performed at least three times. The clinical and in vitro data were analyzed using SPSS 13.0 (Chicago, IL, USA). The results are expressed as means ± standard deviations. P value less than 0.05 was considered statistically significant.
Results
Laryngeal videostroboscopy image outcomes
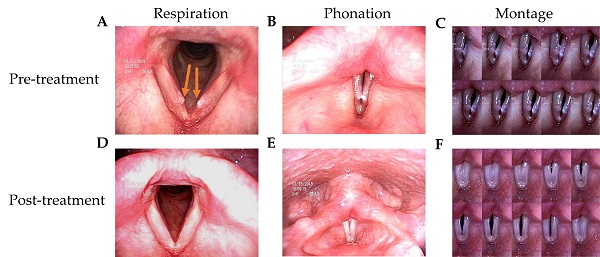
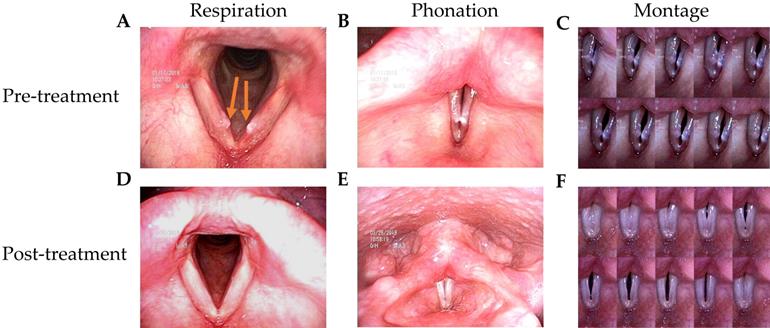
The patients in this study had husky voices for more than 6 months. VFNs with mild to moderate phonatory gaps were seen under videostroboscopy. Figure 1 presents the bilateral vocal nodules at rest (Figure 1A) and the glottal gap during phonation (Figure 1B).
Patients were followed up 3 months after TCM treatment. Videostroboscopy, acoustic analysis, and perceptual assessment were conducted. Figure 1D and 1E presents the post-TCM glottal gap at rest and during phonation, respectively. We found that after TCM treatment, most patients achieved marked improvements in glottal closure, mucosal wave, and the amplitude of mucosal wave under videostroboscopy. No additional morbidities or complications were observed during treatment.
Videostroboscopy record of a 42-year-old female patient before and after 2-week TCM treatment. (A) Bilateral vocal nodule before TCM (respiration). (B) Glottal gap (phonation). (C) Persistent glottal gap in montage photograph during phonation. (D) No vocal nodule after TCM (respiration). (E) No glottal gap (phonation). (F) Montage photograph of normal glottal gap in during phonation.
Objective and Subjective Voice Parameters
The acoustic analyses before and after TCM herb treatments are presented in Table 1. No significant differences were noted between MPT and NHR among the two groups (P > 0.05). Subjective VHI-C10 questionnaire scores, assessed by a blinded voice therapist, decreased significantly after TCM treatment. Jitter, shimmer, and GRBAS scores also improved significantly. A significant decrease was also observed in pre- and post-TCM perceptual assessments, which were conducted according to total GRBAS scores (Table 2).
Demographic data and pre- and post-TCM acoustic analysis results of study patients
| Characteristics | Total patients (n = 22) | ||
|---|---|---|---|
| Pre | Post | p value | |
| Sex (Male/Female) | 4/18 | ||
| Mean age (Min-Max) | 56 (32-75) | ||
| Mean follow up time (days) | 77 (12-150) | ||
| MPT (sec) | 9.02 ± 4.27 | 8.75 ± 4.52 | 0.770 |
| Jitter (%) | 2.86 ± 1.77 | 1.80 ± 1.28 | 0.001* |
| Shimmer (dB) | 0.67 ± 0.48 | 0.53 ± 0.50 | 0.003* |
| NHR | 0.19 ± 0.10 | 0.16 ± 0.10 | 0.017* |
| VHI-C10 | 17.6 ± 8.06 | 6.6 ± 6.33 | <0.001* |
| GRBAS | 5.91 ± 3.73 | 2.93 ± 3.34 | <0.001* |
| G | 1.39 ± 0.74 | 0.84 ± 0.82 | 0.005 |
| R | 1.39 ± 0.74 | 0.75 ± 0.74 | 0.002 |
| B | 1.14 ± 0.77 | 0.57 ± 0.73 | 0.002 |
| A | 0.95 ± 0.84 | 0.36 ± 0.66 | 0.001 |
| S | 1.05 ± 0.84 | 0.41 ± 0.67 | <0.001 |
TCM Medication Review
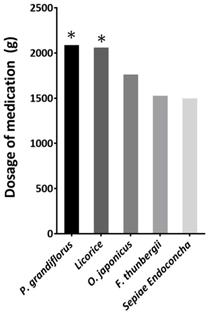
We pooled the prescribed medications of the study participants and calculated the total doses. The following herbs were prescribed with total dosages of >1500 g (Figure 2): Platycodon. grandiflorus, Glycyrrhiza glabra(licorice), Ophiopogon japonicus, Fritillaria thunbergii, and Sepiae Endoconcha (cuttlebone). The total prescribed amount of P. grandifloras and licorice are more than 2000 grams. In our prescription pattern, a 1:1 ratio of G. glabra to P. grandiflorus may be more beneficial for VFNs patients. The average duration and doses of P. grandifloras and licorice for the patients are summarized in Table 2.
Total dosage ranking of the medication prescribed to 22 patients. The total prescribed amount of P. grandifloras and licorice are more than 2000 grams.
P65 and mitogen-activated protein kinase Pathway Inhibition by Glycyrrhiza glabra and Platycodon grandiflorus in vitro
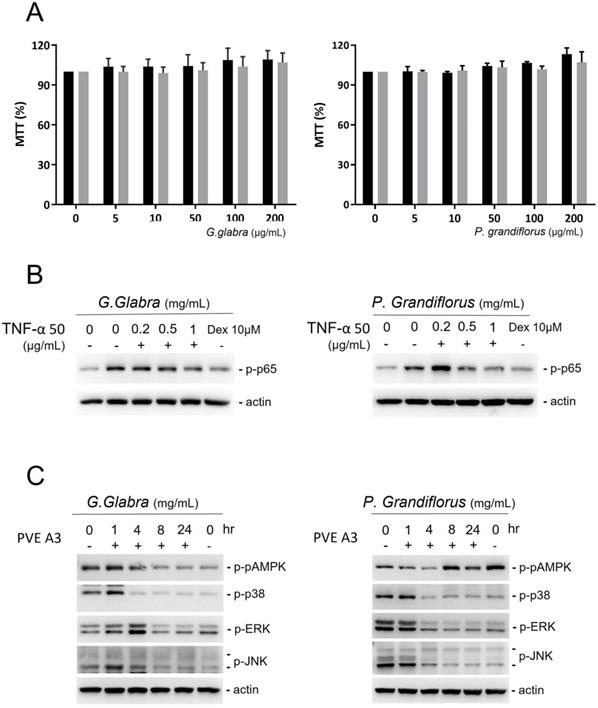
G. glabra and P. grandiflorus did not hinder the growth of PVFE cells even at high concentrations (Figure 3A). In PVFE cells treated with 50 ng/mL TNFα, NF-kβ p65, a downstream target of the mitogen-activated protein kinase (MAPK) pathways, was stimulated by phosphorylation. G. glabra, P. grandiflorus and the corticosteroid dexamethasone could reduce the expression of phosphorylated p65 in TNFα-treated cells (Figure 3B). To further study the mechanism of the anti-inflammatory effect of TCM, the change in p-AMPK, p38, extracellular signal-regulated kinases (ERK), and Jun N-terminal kinases (JNK) in response to TCM treatment in VFE cells was investigated (Figure 3C). G. glabra and P. grandiflorus significantly inhibited the p38, ERK, and JNK pathways, but their effect on the MAPK pathway was unclear.
Discussion
In this study, we retrospectively collected medical records of 22 vocal fold nodule patients treating with TCM from July 2018 to August 2020. We found the higher prescription frequency and greater consumed weight of P. grandiflorus and licorice.
For the clinical part, in the TCM outpatient clinics, the patients with vocal nodules were referred from the otolaryngologists for TCM medication prescription. The composition varied for every single patient since TCM medication was generally thought to be more individualized.
The duration and dosage for each patient of Licorice and P. grandiflorus obtained from a medication chart review of 22 patients
| Licorice | P. grandiflorus | |
|---|---|---|
| Duration (days) | 63.95 ± 32.44 | 62.73 ± 32.89 |
| Median (range) | 69.5 (14-143) | 63 (14-143) |
| Dosage (grams) | 97.09 ± 78.45 | 93.98 ± 79.73 |
| Median (range) | 68.5 (5.61-296) | 60 (7.14-296) |
PVFE cells were treated with G. Radix and P. grandiflorus. (A) G. Radix and P. grandiflorus did not result in cell toxicity in PVFE cells. (B) G. Radix and P. grandiflorus reduced the expression of phosphorylated p65 in TNFα-treated cells. (C) P38, ERK, and JNK pathways were inhibited by G. Radix and P. grandiflorus, but their effect on the AMPK pathway was unclear.
The 22 patients regularly returned to TCM clinic every 2 to 4 weeks. In the meanwhile, they were required to come back for voice analysis. The medication we prescribed was in the form of granulated compounds called finished herbal products (FHP).
The finished herbal products (FHP) we prescribed are granulated compounds concentrated from Chinese herbal remedies, including single herbs and herbal formulae [22]. They are officially approved in the national healthcare systems not only in Taiwan, but also in Japan, Korea and Mainland China. They are widely used in the East Asia [23]. Compared to traditional herbal decoction, the form of FHP is more portable for the patients and easier for us to calculate the weight consumed by our patients.
Since the medication should be different from patient to patient, we found that the prescription for these patients had similarity in their components. Therefore, after chart review for the TCM medication, we can see from Figure 2, the most used herbs were G. glabra and P. grandifloras. The total amount was more than 2000 grams.
G. glabra, the licorice specie we used here, was prepared by Chiayi Chang Gung Memorial Hospital (Taiwan). G. glabra is grown in Eurasia, northern Africa and western Asia [24]. Its rhizomes and roots are the most important medicinal parts, which have been reported to be used alone or in combination with other herbs for treating multiple diseases such as digestive disorders, respiratory tract disorders, epilepsy, fever, sexual disability, and etc [25]. P. grandiflorus contains numerous amino acids, vitamins, and trace elements. Also, it has the ability to treat the following disorders: cough and asthma relief, antitumor activity, anti-inflammatory and antibacterial effects, antioxidation, liver protection, and immunity enhancement [26]. Both G. glabra and P. grandiflorus can be used as food and flavoring agents.
The combined use of these two herbs can be traced to ancient China. In the Chinese classic Shang Han Lun (English title: Treatise on Cold Damage Diseases, written by Zhang Zhong-Jing, 150-219 A.D.), the two herbs were combined in a 2:1 ratio and were used mainly to treat sore throat and Fei Yon (pulmonary abscess) [27, 28]. Pharmacologically, they can affect the metabolic process of each other, to improve the bioavailability of their compounds [29, 30].
In second part of the study, we used these two herbs for porcine PVFE culture. And the two herbs significantly inhibited the P38, ERK, and JNK pathways in TNF-α inflammatory process induced by TNF-α. For the design of our experiments, since inflammation is commonly observed in patients with chronic voice disorders, such as vocal fold nodules. It was demonstrated that an immediate increase of inflammatory cytokines, including TNF-α, is observed in response to vocal fold injury. The effect of TNF-α on the vocal fold epithelium upregulates mucin expression [14]. TNF-α may activate the NF-κB p65 pathway in PVFE cells, but this inflammatory pathway is inhibited by the corticosteroid dexamethasone (Dex) and high doses of G. glabra and P. grandiflorus. This means that the anti-inflammatory effect of G. glabra and P. grandiflorus. on the vocal folds depends on their effect in vocal epithelium cells.
MAPK families are critical in complex cellular programs like proliferation, differentiation, development, transformation, and apoptosis. The MAPK pathways involve a series of protein kinase cascades, which play a crucial role in the regulation of cell proliferation. The three major MAPK families, namely mammalian ERKs, c-JNK, and p38 kinases, are activated by many stimuli. JNK and p38 are activated by cytokines and stressors, and ERK is mainly activated by growth factors [31]. In our study, it revealed that G. glabra and P. grandiflorus downregulated the activation of p38, p-ERK, and p-JNK. This result explained the effectiveness of G. glabra and P. grandiflorus on vocal nodules by downregulation of the MAPK pathway [32].
However, there were some limitations in this study. Our sample size is relatively small. Also, the dosage and duration varied, which may be related to individual difference. For in vitro study, though the above inflammatory pathway down-regulation was seen, whether there exists synergistic effect or other interaction between the herbs are still unknown in clinical practice. Further prospective and functional studies are needed to confirm the results of this study and clarify the mechanism of action.
In conclusion, by reviewing clinical patient data and conducting an in vitro study, we confirmed that TCM herbal treatment is an alternative treatment option for VFNs. Patients' voices exhibited improvement after daily TCM herb use, and VFNs were reduced. From the above results, we may imply that our most prescribed herbs G. glabra and P. grandiflorus as potential VFNs alternative treatment.
Abbreviations
VFNs: Vocal fold nodules; VHI-C10: Chinese Voice Handicap Index-10; MDVP: multidimensional voice program; PVFE: porcine vocal fold epithelial; G. glabra (licorice): Glycyrrhiza glabra; P. grandifloras: Platycodon grandifloras; TCM: traditional Chinese medicine; F0: fundamental frequency; NHR: noise-to-harmonic ratio NHR; MTT: 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; MAPK: mitogen-activated protein kinase; ERK: extracellular signal-regulated kinases; JNK: Jun N-terminal kinases; FHP: finished herbal products; Dex: dexamethasone.
Acknowledgements
The authors wish to thank the Health Information and Epidemiology Labor-atory (CLRPG6G0041) of Chiayi Chang Gung Memorial Hospital for data analysis, statistical analysis, and discussions. The National Health Insurance Research Database used in this study was provided by Taiwan's National Health Research Institutes.
Funding
This research was funded by Chang Gung Memorial Hospital, grant numbers CMRPG6L0261, CMRPG6L0471, CMRPG6K0121, CMRPG6L0271, CMRPG6H0131, CMRPG6H0132 and CMRPG6K0391. The APC was funded by Chang Gung Memorial Hospital.
Institutional Review Board Statement
The present study was approved by the Institutional Re-view Board of Chiayi Chang Gung Memorial Hospital (No. 201901034B0). All participants gave written informed consent prior to the beginning of the study.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Author Contributions
Conceptualization, C.-M.H. and C.-Y. W.; methodology, M.-Y.Y., H.-C.S. and Y.-S.L.; formal analysis, S.-F.C., M.-S.T. and Y.-H.Y.; writing - original draft preparation, C.-M.H. and I.-Y.L.; writing - review and editing, M.-Y.Y., G.-H.C. P.-R.Y and Y.-T.T.; supervision, C.-M.H.; project administration, C.-M.H. All authors have read and agreed to the published version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sataloff RT, Hawkshaw M, Hoover CA, Spiegel JR. Vocal fold nodule and cyst. Ear Nose Throat J. 1998;77:728
2. Shah RK, Engel SH, Choi SS. Relationship between voice quality and vocal nodule size. Otolaryngol Head Neck Surg. 2008;139:723-6
3. Wallis L, Jackson-Menaldi C, Holland W, Giraldo A. Vocal fold nodule vs. vocal fold polyp: answer from surgical pathologist and voice pathologist point of view. J Voice. 2004;18:125-9
4. Leonard R. Voice therapy and vocal nodules in adults. Curr Opin Otolaryngol Head Neck Surg. 2009;17:453-7
5. McHugh-Munier C, Scherer KR, Lehmann W, Scherer U. Coping strategies, personality, and voice quality in patients with vocal fold nodules and polyps. J Voice. 1997;11:452-61
6. Hosoya M, Kobayashi R, Ishii T, Senarita M, Kuroda H, Misawa H. et al. Vocal Hygiene Education Program Reduces Surgical Interventions for Benign Vocal Fold Lesions: A Randomized Controlled Trial. Laryngoscope. 2018;128:2593-9
7. Salturk Z, Ozdemir E, Sari H, Keten S, Kumral TL, Berkiten G. et al. Assessment of Resonant Voice Therapy in the Treatment of Vocal Fold Nodules. J Voice. 2018
8. Harris PE, Cooper KL, Relton C, Thomas KJ. Prevalence of complementary and alternative medicine (CAM) use by the general population: a systematic review and update. Int J Clin Pract. 2012;66:924-39
9. Huang Y, Wu T, Zeng L, Li S. Chinese medicinal herbs for sore throat. Cochrane Database Syst Rev. 2012: CD004877.
10. Cardoso R, Meneses RF, Lumini-Oliveira J. The Effectiveness of Physiotherapy and Complementary Therapies on Voice Disorders: A Systematic Review of Randomized Controlled Trials. Front Med (Lausanne). 2017;4:45
11. Yiu EM, Chan KM, Li NY, Tsang R, Verdolini Abbott K, Kwong E. et al. Wound-healing effect of acupuncture for treating phonotraumatic vocal pathologies: A cytokine study. Laryngoscope. 2016;126:E18-22
12. Wijesundara NM, Sekhon-Loodu S, Rupasinghe HV. Phytochemical-rich medicinal plant extracts suppress bacterial antigens-induced inflammation in human tonsil epithelial cells. PeerJ. 2017;5:e3469
13. Levendoski EE, Leydon C, Thibeault SL. Vocal fold epithelial barrier in health and injury: a research review. J Speech Lang Hear Res. 2014;57:1679-91
14. Erickson-DiRenzo E, Leydon C, Thibeault SL. Methodology for the establishment of primary porcine vocal fold epithelial cell cultures. Laryngoscope. 2019;129:E355-e64
15. Gill GA, Buda A, Moorghen M, Dettmar PW, Pignatelli M. Characterisation of adherens and tight junctional molecules in normal animal larynx; determining a suitable model for studying molecular abnormalities in human laryngopharyngeal reflux. J Clin Pathol. 2005;58:1265-70
16. Murphy PJ. Spectral characterization of jitter, shimmer, and additive noise in synthetically generated voice signals. The Journal of the Acoustical Society of America. 2000;107:978-88
17. Wertzner HF, Schreiber S, Amaro L. Analysis of fundamental frequency, jitter, shimmer and vocal intensity in children with phonological disorders. Braz J Otorhinolaryngol. 2005;71:582-8
18. Jotz GP, Cervantes O, Abrahão M, Settanni FAP, de Angelis EC. Noise-to-harmonics ratio as an acoustic measure of voice disorders in boys. Journal of voice. 2002;16:28-31
19. Tsai MS, Yang MY, Chang GH, Tsai YT, Lin MH, Hsu CM. Autologous thyroid cartilage graft implantation in medialization laryngoplasty: a modified approach for treating unilateral vocal fold paralysis. Sci Rep. 2017;7:4790
20. Lam PK, Chan KM, Ho WK, Kwong E, Yiu EM, Wei WI. Cross-cultural adaptation and validation of the Chinese Voice Handicap Index-10. Laryngoscope. 2006;116:1192-8
21. Xu W, Han D, Li H, Hu R, Zhang L. Application of the Mandarin Chinese version of the Voice Handicap Index. J Voice. 2010;24:702-7
22. WEN K-C. The turnover rate of marker constituents in chinese herbal medicine. J Food Drug Anal. 2000;8:270-7
23. Hsieh S-C, Lai j-n, Lee C-F, Hu F-C, Tseng W-L, Wang J-D. The prescribing of Chinese herbal products in Taiwan: A cross-sectional analysis of the national health insurance reimbursement database. Pharmacoepidemiology and drug safety. 2008;17:609-19
24. Shah SL, Wahid F, Khan N, Farooq U, Shah AJ, Tareen S. et al. Inhibitory Effects of<i> Glycyrrhiza glabra</i> and Its Major Constituent Glycyrrhizin on Inflammation-Associated Corneal Neovascularization. Evidence-Based Complementary and Alternative Medicine. 2018;2018:8438101
25. El-Saber Batiha G, Magdy Beshbishy A, El-Mleeh A, Abdel-Daim MM, Prasad Devkota H. Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules. 2020 10
26. Ji M-Y, Bo A, Yang M, Xu J-F, Jiang L-L, Zhou B-C. et al. The Pharmacological Effects and Health Benefits of Platycodon grandiflorus—A Medicine Food Homology Species. Foods. 2020;9:142
27. Kuwamura A, Komasawa N, Takahashi R, Tanaka M, Minami T. Preoperative Oral Administration of Kikyo-To, a Kampo Medicine, Alleviates Postoperative Sore Throat: A Prospective, Double-Blind, Randomized Study. J Altern Complement Med. 2016;22:294-7
28. Ishimaru N, Maeno T, Suzuki M, Maeno T. Rapid effects of Kikyo-to on sore throat pain associated with acute upper respiratory tract infection. J Complement Integr Med. 2013;11:51-4
29. Mao YC, Peng LX, Kang A, Xie T, Xu JY, Shen CS. et al. Influence of Jiegeng on Pharmacokinetic Properties of Flavonoids and Saponins in Gancao. Molecules. 2017;22:11
30. Shan J-j, Zou J, Xie T, Kang A, Zhou W, Xu J-y. et al. Effects of Gancao on pharmacokinetic profiles of platycodin D and deapio-platycodin D in Jiegeng. Journal of ethnopharmacology. 2015;170:50-6
31. Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807-69
32. Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9-18
Author contact
![]() Corresponding author: Cheng-Ming Hsu, MD, PhD, Department of Otolaryngology-Head and Neck Surgery, Chiayi Chang Gung Memorial Hospital, No.6, W. Sec., Jiapu Rd., Puzi City, Chiayi County 61363, Taiwan. Tel: 886-5-3621000; E-mail: scm0031org.tw.
Corresponding author: Cheng-Ming Hsu, MD, PhD, Department of Otolaryngology-Head and Neck Surgery, Chiayi Chang Gung Memorial Hospital, No.6, W. Sec., Jiapu Rd., Puzi City, Chiayi County 61363, Taiwan. Tel: 886-5-3621000; E-mail: scm0031org.tw.

 Global reach, higher impact
Global reach, higher impact