3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(3):588-595. doi:10.7150/ijms.61712 This issue Cite
Research Paper
Prognostic Factors of AL-PCMM and AL-MM: A Single-Center Retrospective Study
1. Department of Hematology, Peking University First Hospital, Beijing, China
2. Department of Cardiology, Cardiovascular Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, China
†This is the first author of this study.
Received 2021-4-16; Accepted 2022-2-27; Published 2022-3-14
Abstract
Background: Patients with amyloid light-chain (AL) amyloidosis with a bone marrow plasma cell ratio > 10% (AL-PCMM) have a poorer prognosis than patients with AL amyloidosis with a bone marrow plasma cell ratio of <10% (AL-only), similar to that of patients with AL amyloidosis and multiple myeloma (AL-MM). However, the prognostic factors for AL-PCMM and AL-MM have not been studied.
Methods: A total of 49 patients with AL-PCMM or AL-MM in the Peking University First Hospital registry in 2010-2018 were enrolled. Clinical and follow-up data were collected. The relationship between clinical parameters and survival time was also assessed.
Results: Compared with patients with AL-PCMM, patients with AL-MM only had a higher incidence of bone marrow plasma cell ratio ≥ 20%. In AL-PCMM and AL-MM, the survival time was significantly shorter in patients with alkaline phosphatase (ALP) ≥ 187.5 IU/L, γ-glutamyl transpeptidase (GGT) ≥ 85 IU/L, total bilirubin (TBIL) ≥ 20 µmol/L, cardiac troponin I (CTNI) ≥ 0.1 ng/mL, ejection fraction (EF) < 50%, initial therapeutic effect (ITE) < very good partial response (VGPR), and Boston University (BU) staging system stage ≥ III. ALP at diagnosis was correlated with brain natriuretic peptide (BNP) level, CTNI level, and EF rather than TBIL level. Cox regression analyses revealed that BU staging system stage ≥ III (P=0.001, hazard ratio [HR]=5.579), ALP ≥ 187.5 IU/L (P=0.011, HR=3.563), and ITE < VGPR (P=0.002, HR=7.462) were independent significant risk factors for a poor prognosis of AL-PCMM and AL-MM.
Conclusion: ALP level, which is related to cardiac amyloidosis rather than liver involvement, can be a prognostic factor for this group of patients. A BU staging system stage ≥ III, ALP ≥ 187.5 IU/L, and ITE < VGPR were independent significant risk factors for a poor prognosis of AL-PCMM and AL-MM.
Keywords: AL amyloidosis, AL-PCMM, AL-MM, prognostic factors, BU staging system
Introduction
Primary amyloid light-chain (AL) amyloidosis is a systemic plasma cell disease with a poor prognosis that is related to the number and degree of involvement of affected organs. In 2004, Dispenzieri et al. collected and analyzed the clinical data of 242 AL amyloidosis patients from the Mayo Clinic and found that an N-terminal pro B-type natriuretic peptide (NT-proBNP) > 332 ng/L, cardiac troponin T (CTNT) > 0.035 μg/L, or cardiac troponin Ⅰ (CTNI) > 0.1 μg/L level was a poor prognostic factor [1]. In 2012, Kumar et al. found that the difference between involved and uninvolved free light chains (dFLC) ≥ 180 mg/L was also an important prognostic factor [2]. Therefore, a staging system combining CTNI, NT-proBNP, and dFLC was proposed. The median overall survival (OS) times for patients with stages I-IV disease were 94.1, 40.3, 14.0, and 5.8 months (P<0.001), respectively. In clinical practice, we often use the 2004 and 2012 Mayo Clinical AL Amyloidosis Staging System to stratify patients with AL amyloidosis. However, many centers are not capable of detecting NT-proBNP levels.
In 2019, the Boston University School of Medicine and Boston Medical Center proposed the Boston University (BU) staging system, which had the same effectiveness as the 2004 Mayo Clinical AL amyloidosis staging system for predicting the survival time of AL amyloidosis [3]. In addition, Mollee et al. provided external validation of the BU staging system for AL amyloidosis, and only one patient was miscategorized according to the 2004 Mayo Clinical AL amyloidosis staging system [4]. In addition to these prognostic staging systems, some studies also found that a bone marrow plasma cell (BMPC) ratio > 10%, M protein > 1 g/24 h, total bilirubin (TBIL), alkaline phosphatase (ALP), red blood cell distribution width, reactive vasodilation, D-dimer, preserved left ventricular ejection fraction, immunoparesis, and cytogenetic abnormalities such as T (11;14) are poor prognostic factors for AL amyloidosis [5-15].
There is consensus that patients with AL amyloidosis and concomitant hypercalcemia, renal failure, anemia, and lytic bone lesions attributable to clonal expansion of plasma cells (CRAB criteria) also have multiple myeloma. Patients with AL amyloidosis and a bone marrow plasma cell ratio > 10% (AL-PCMM) and AL amyloidosis with multiple myeloma (AL-MM) have a poorer prognosis than patients with AL amyloidosis with a BMPC ratio < 10% (AL-only) [16]. However, the prognostic factors for AL-PCMM and AL-MM have not yet been studied. Reliable prognostic indicators for this group of patients are currently lacking. This study aimed to bridge this knowledge gap by collecting and analyzing clinical information and follow-up data to determine the prognostic factors of AL-PCMM and AL-MM.
Methods
Patients and institutional review board approval
Between January 1, 2010 and December 31, 2018, 52 patients with AL-PCMM or AL-MM were registered at Peking University First Hospital. This tertiary first-class hospital treats approximately 30 patients with AL amyloidosis each year. All patient data were anonymized. We searched the hospital medical records system using the disease codes to include all patients who met the enrollment criteria. Finally, only 49 patients were included in this study. One patient with incomplete data and two patients with a diagnosis interval of AL amyloidosis and MM of more than one month were excluded. Light and polarized microscopy for the presence of Congo red deposits and green birefringence in the biopsies of the affected parts were used to confirm the AL amyloidosis.
Organ involvement in AL amyloidosis was assessed according to the consensus criteria reported in the 10th International Symposium on Amyloid and Amyloidosis [17]. AL-MM was defined as AL amyloidosis with CRAB (serum Ca > 2.75 mmol/L, serum creatinine > 177 µmol/L, hemoglobin value > 20 g/L below the lower limit of normal, and one or more osteolytic lesions on skeletal radiography, computed tomography (CT), or positron emission tomography/CT (PET/CT). AL-PCMM was defined as AL amyloidosis with a BMPC ratio > 10% but without CRAB. Written informed consent was obtained from all participants. The Ethics Committee of Peking University First Hospital approved this study (no. 2017[1304]). This study was conducted in accordance with the principles of the Declaration of Helsinki.
Data collection
Patient clinical information was obtained by hospital record review. The following clinical information at the time of diagnosis was extracted retrospectively: Ca, hemoglobin (Hb), 24-h urinary protein quantity (24-h UTP), albumin (Alb), serum creatinine (Scr), total bilirubin (TBIL), γ-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), cardiac troponin I (CTNI), brain natriuretic peptide (BNP), ejection fraction (EF), lactate dehydrogenase (LDH), and β-2 microglobulin (β2-MG). Bone destruction was determined using radiography, CT, magnetic resonance imaging (MRI), or PET/CT. A bone puncture was performed to determine the bone marrow plasma cell ratio. Based on the above-mentioned information, the best BU staging system for AL amyloidosis was determined.
Treatment, initial therapeutic effect, and follow-up
At the time of diagnosis, the treatment regimens were divided into two drug combination regimens, melphalan and prednisone (MP), bortezomib and dexamethasone (BD), and lenalidomide and dexamethasone (RD), and a three-drug combination regimen, including melphalan plus prednisone and thalidomide (MPT), bortezomib plus cyclophosphamide and dexamethasone (BCD), bortezomib plus thalidomide and dexamethasone (BTD), bortezomib plus doxorubicin and dexamethasone (PAD), and bortezomib plus lenalidomide and dexamethasone (RVD). The 2016 International Myeloma Working Group consensus criteria were used to evaluate the response of multiple myeloma after the fourth course of chemotherapy [18], including progressive disease, stable disease, minimal response, partial response (PR), very good partial response (VGPR), complete response (CR), and stringent complete response (sCR).
A better initial therapeutic effect was defined as better than PR in terms of sCR, CR, and VGPR. Telephone interviews and retrospective review of hospital files were used to obtain patient survival data. The inability to communicate with the patient on the phone, which resulted in loss of survival data, was considered lost to follow-up. OS was defined as the time from diagnosis to the time of death (regardless of cause) or the last documented contact with the patient. AL amyloidosis-related death included renal failure caused by renal amyloidosis, cardiac amyloidosis (heart failure and arrhythmia), and digestive tract hemorrhage caused by digestive tract involvement.
Statistical analyses
All analyses were performed using SPSS software (version 20.0; SPSS Institute). Categorical variables are reported as number and percentage. Chi-square and Fisher's exact tests were used to compare categorical variables among the different groups of patients. OS curves were analyzed using the Kaplan-Meier method and compared using the log-rank test for univariate analysis. Factors with values of P<0.10 on univariate analyses or acknowledged as clinically meaningful were included in the multivariate analysis. Multivariate analyses were performed using a Cox proportional hazards model. The Kolmogorov-Smirnov test was used to detect whether the values were normally distributed. Correlation analyses were performed using Spearman's correlation test. All quoted P values were obtained from two-sided tests. Statistical significance was set at P<0.05.
Results
Clinical characteristics and laboratory examination of AL-PCMM and AL-MM
A total of 49 patients with AL-PCMM or AL-MM were registered between January 1, 2010 and December 31, 2018. It is worth noting that 28/49 (57.1%) patients had PCMM and 21/49 (42.9%) had MM. Clinical information and laboratory examination results of all patients at the time of diagnosis are shown in Table 1. Patients with AL-MM had the same incidence of Alb < 25 g/L, 24-h UTP > 3.5 g, ALP ≥ 187.5IU/L, GGT ≥ 85 IU/L, TBIL ≥ 20 µmol/L, BNP ≥ 1500 pg/mL, EF < 50%, and BU staging system stage ≥ III, which represented AL amyloidosis severity. For tumor burden indicators, the incidence of LDH ≥ 240 IU/L and β2-MG > 3.5 mmol/L were the same for AL-PCMM and AL-MM, while AL-MM had a higher incidence of a BMPC ratio ≥ 20%. In addition, 7/28 (25.0%) patients with AL-MM had Hb levels of < 85 g/L. A total of 19/28 (67.8%) patients with AL-MM had bone destruction > 3; 3/28 (10.7%) patients with AL-MM had hypercalcemia, and 11/28 (39.2%) patients with AL-MM had renal dysfunction.
Clinical characteristics of AL-PCMM and AL-MM
| Amyloidosis n=49, cases (%) | AL-MM n=28, cases (%) | AL-PCMM n=21, cases (%) | P | |
|---|---|---|---|---|
| Age ≥ 65 years | 14 (28.5) | 6 (21.4) | 8 (30.1) | 0.222 |
| Male sex | 34 (69.3) | 18 (64.2) | 16 (76.1) | 0.533 |
| Alb < 25 g/L | 20 (40.8) | 11 (39.2) | 9 (42.8) | 1.000 |
| 24-h UTP > 3.5 g | 23 (46.9) | 10 (35.7) | 13 (61.9) | 0.088 |
| ALP ≥ 187.5 IU/L | 7 (14.2) | 4 (14.2) | 3 (14.2) | 1.000 |
| GGT ≥ 85 IU/L | 11 (22.4) | 6 (21.4) | 5 (23.8) | 1.000 |
| TBIL ≥ 20 µmol/L | 5 (10.2) | 3 (10.7) | 2 (9.5) | 1.000 |
| CTNI ≥ 0.1 ng/mL | 11 (22.4) | 7 (25.0) | 4 (19.0) | 0.737 |
| BNP ≥ 1500 pg/mL | 7 (14.2) | 4 (14.2) | 3 (14.2) | 1.000 |
| EF < 50% | 9 (16.3) | 6 (21.4) | 3 (14.2) | 0.714 |
| BU staging system stage ≥ III | 10 (20.4) | 7 (25.0) | 3 (14.2) | 0.482 |
| LDH ≥ 240 IU/L | 13 (26.5) | 9 (32.1) | 4 (19.0) | 0.348 |
| β2-MG > 3.5 mmol/L | 38 (77.5) | 23 (82.1) | 15 (71.4) | 0.494 |
| BMPC ratio ≥ 20% | 21 (34.6) | 16 (57.1) | 5 (23.8) | 0.024 |
| ITE < VGPR | 30 (61.2) | 16 (57.1) | 14 (66.6) | 0.564 |
24-h UTP, 24-h urine total protein; Alb, albumin; AL-MM, amyloid light-chain with multiple myeloma; ALP, alkaline phosphatase; AL-PCMM, amyloid light-chain and a bone marrow plasma cell ratio > 10%; β2-MG, β-2 microglobulin; BMPC, bone marrow plasma cell; BNP, brain natriuretic peptide; BU, Boston University; CTNI, cardiac troponin I; EF, ejection fraction; GGT, γ-glutamyl transpeptidase; ITE, initial therapeutic effect; LDH, lactate dehydrogenase; TBIL, total bilirubin
Treatment and initial therapeutic effect of AL-PCMM and AL-MM
The treatment strategies are summarized in Table 2. At the time of diagnosis, 37 of the 49 patients were treated with a two-drug combination regimen, including 33 with BD, 3 with MP, and 1 with RD. Furthermore, 7 of 49 patients were treated with a three-drug combination regimen, including 2 with PAD, 2 with RVD, 1 with MTP, 1 with BCD, and 1 with BTD. In AL-PCMM, 16 patients were treated with a two-drug combination regimen, including 13 with BD, 2 with MP, and 1 with RD. In addition, 3 patients were treated with a three-drug combination regimen, including 1 with PAD, 1 with RVD, and 1 with BCD. In the AL-MM group, 21 patients were treated with a two-drug combination regimen, including 20 with BD and 1 with MP. In addition, 4 patients were treated with a three-drug combination regimen: 1 with PAD, 1 with RVD, 1 with MPT, and 1 with BCD. Finally, 5 patients received no treatment, including 2 with AL-PCMM and 3 with AL-MM. Among the two-drug and three-drug combination regimens, bortezomib-based regimens accounted for 89.1% and 85.7%, respectively. Only 4 (10.8%) patients receiving two-drug combination regimens and 1 (14.2%) patient receiving three-drug combination regimens underwent autologous stem cell transplantation during the course of the disease. There was no statistical difference in the proportion of patients with AL-MM with an ITE < VGPR (57.1% vs. 66.6%, P=0.564; Table 1).
Treatment of patients with AL-PCMM versus AL-MM
| Treatment | AL amyloidosis n=49 | AL-PCMM n=21 | AL-MM n=28 | |
|---|---|---|---|---|
| Two-drug | BD | 33 | 13 | 20 |
| MP | 3 | 2 | 1 | |
| RD | 1 | 1 | 0 | |
| Three-drug | PAD | 2 | 1 | 1 |
| RVD | 2 | 1 | 1 | |
| MPT | 1 | 0 | 1 | |
| BCD | 1 | 1 | 0 | |
| BTD | 1 | 0 | 1 | |
| No treatment | 5 | 2 | 3 | |
AL, amyloid light-chain; AL-MM, AL amyloidosis with multiple myeloma; AL-PCMM, AL amyloidosis and a bone marrow plasma cell ratio > 10%; BCD, bortezomib plus cyclophosphamide and dexamethasone; BD, bortezomib and dexamethasone; BTD, bortezomib plus thalidomide and dexamethasone; MP, melphalan and prednisone; MPT, melphalan plus prednisone and thalidomide; PAD, bortezomib plus doxorubicin and dexamethasone; RD, lenalidomide and dexamethasone; RVD, bortezomib plus lenalidomide and dexamethasone.
Organ involvement, death rate, and causes of death in patients with AL-PCMM and AL-MM
Organ involvement among the 49 patients with AL-PCMM or AL-MM was as follows: kidney (36 [73.4%]), liver (13 [26.5%]), heart (27 [55.1%]), skin (8 [16.3%]), gastrointestinal tract (6 [12.2%]), nerve (4 [8.2%]), soft tissue (4 [8.2%]), and pulmonary (1 [2%]). Fifteen (30.6%) patients had ≥ 3 involved organs, while 34 patients (69.4%) had 1 or 2 involved organs. There was no statistically significant difference in the incidence of the involvement of various organs and the number of organs involved between patients with AL-MM and AL-PCMM, especially the most commonly affected organs (kidneys, 71.4% vs. 76.1%; heart, 60.7% vs. 47.6%; liver, 25.0% vs. 28.5%) (Table S1).
A total of 31 of the 49 (63.3%) patients with AL amyloidosis died during the study period, including 19 of 34 patients (55.8%) with 1 or 2 involved organs and 12 of 15 patients (80%) with ≥3 involved organs. Specifically, 19 of 26 (73.0%) patients with heart involvement died. All 13 patients with liver involvement died, 6 of whom died of cardiac amyloidosis. A total of 24 of 36 patients (66.7%) with kidney involvement died, 12 of whom died of cardiac amyloidosis. Five of 6 patients (83.3%) with gastrointestinal tract involvement died, 3 of whom died of cardiac amyloidosis. Causes of death are listed in Table S2. Twelve (38.6%) patients did not have clear causes of death; among the remaining 19 patients, 13 (41.9%) died of cardiac amyloidosis, making it the leading cause of death. Other causes included renal failure caused by renal amyloidosis (2 [6.5%]), digestive tract hemorrhage caused by digestive tract involvement (2 [6.5%]), and other causes such as infection and hypercalcemia (2 [6.5%]).
Survival analysis of AL-PCMM and AL-MM
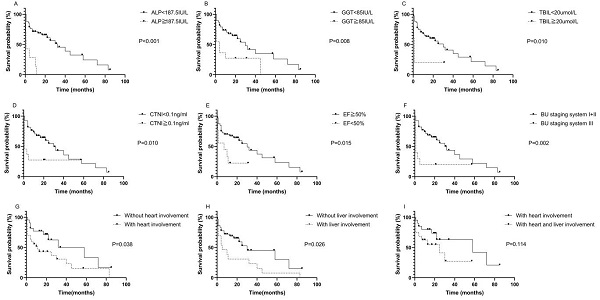
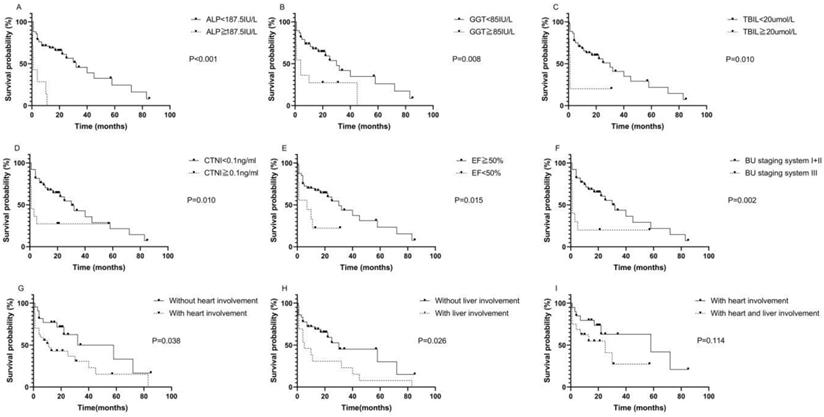
The median survival time of the 49 patients was 32.26 months. The median follow-up time was 41.96 months. Kaplan-Meier survival curves were used to describe OS. There was no statistically significant difference in OS between patients with AL-PCMM and those with AL-MM (Fig. S1; P=0.354). In AL-MM and AL-PCMM, the survival time was significantly shorter in patients with an ALP ≥ 187.5 IU/L (Fig. 1A; median survival time of 1.0 vs. 32.0 months; P<0.001), GGT ≥ 85 IU/L (Fig. 1B; median survival time of 4.0 vs. 30.0 months; P=0.008), TBIL ≥20 µmol/L (Fig. 1C; median survival time of 1.0 vs. 30.0 months; P=0.010), CTNI ≥ 0.1 ng/mL (Fig. 1D; median survival time of 1.0 vs. 30.0 months; P=0.010), EF < 50% (Fig. 1E; median survival time of 7.0 vs. 30.0 months; P=0.015), and BU staging system stage ≥ III (Fig. 1F; median survival time of 1.0 vs. 30.0 months; P=0.002). Subsequently, we analyzed the survival times of patients with AL-MM and AL-PCMM by affected organ. The results showed that the median survival time was significantly shorter in patients with heart involvement (Fig. 1G; median survival time of 11.0 vs 58.0 months; P=0.038) and liver involvement (Fig. 1H; 5.0 vs 30.0 months; P=0.026). Interestingly, the median survival of patients with both liver and heart involvement was the same as that of patients with heart involvement only (Fig. 1I; 25.0 vs 58.0 months; P=0.114).
Univariate and multivariate COX regression analyses of patients with AL-PCMM and AL-MM
Univariate Cox regression analyses found that ALP ≥ 187.5 IU/L (P<0.001; HR=6.694), GGT ≥ 85 IU/L (P=0.013; HR=2.767), EF < 50% (P=0.023; HR=2.809), TBIL ≥ 20 µmol/L (P=0.019; HR=3.698), CTNI ≥ 0.1 ng/mL (P=0.016; HR=2.792), ITE < VGPR (P=0.002; HR=6.711), and BU staging system stage ≥ III (P=0.004; HR=3.431) were related to the prognosis of AL-PCMM and AL-MM. Sex, age, Hb, bone destruction, Ca, creatinine, 24-h UTP, albumin, BNP, LDH, β2-MG, treatment regimen, and bone marrow plasma cell ratio did not affect the prognoses of AL-PCMM and AL-MM. However, age, Alb, ALP, GGT, TBIL, BNP, CTNI, BU staging system, and initial therapeutic effect were included in multivariate Cox regression analysis. Multivariate Cox regression analysis showed that a BU staging system stage ≥ III (P=0.001, HR=5.579), ALP ≥ 187.5 IU/L (P=0.011, HR=3.563), and ITE < VGPR (P=0.002, HR=7.462) were independent significant risk factors for poor prognosis (Table 3).
Cox regression analyses of 49 patients with AL-PCMM and AL-MM
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| OS HR (95% CI) | P value | OS HR (95% CI) | P value | |
| ITE < VGPR | 6.711 (2.020-22.22) | 0.002 | 7.462 (2.083-27.027) | 0.002 |
| ALP ≥ 187.5 IU/L | 6.694 (2.609-17.174) | <0.001 | 3.563 (1.343-9.453) | 0.011 |
| BU staging system stage ≥ III | 3.431 (1.477-7.969) | 0.004 | 5.579 (2.018-15.420) | 0.001 |
| CTNI ≥ 0.1 ng/mL | 2.792 (1.208-6.452) | 0.016 | ||
| EF < 50% | 2.809 (1.153-6.842) | 0.023 | ||
| GGT ≥ 85 IU/L | 2.767 (1.237-6.187) | 0.013 | ||
| TBIL ≥ 20 µmol/L | 3.698 (1.242-11.015) | 0.019 | ||
| BNP ≥ 700 pg/mL | 1.725 (0.836-3.560) | 0.140 | ||
| Male sex | 1.388 (0.901-2.138) | 0.137 | ||
| Age ≥ 65 years | 1.985 (0.917-4.298) | 0.082 | ||
| Alb < 25 g/L | 1.405 (0.679-2.906) | 0.359 | ||
| Hb > 85 g/L | 0.275 (0.065-1.172) | 0.081 | ||
| Bone destruction > 3 | 0.828 (0.397-1.726) | 0.615 | ||
| Ca > 2.65 mmol/L | 0.733 (0.098-5.496) | 0.763 | ||
| With MM | 0.686 (0.323-1.457) | 0.327 | ||
| 24-h UTP > 3.5 g | 1.120 (0.537-2.337) | 0.762 | ||
| β2-MG > 3.5 mmol/L | 1.130 (0.490-2.604) | 0.774 | ||
| Scr ≥ 177 µmol/L | 0.467 (0.162-1.349) | 0.159 | ||
| LDH ≥ 240 IU/L | 1.033 (0.456-2.340) | 0.937 | ||
| BMPC ratio ≥ 20% | 1.070 (0.513-2.231) | 0.858 | ||
| Treatment regimen | 0.285 (0.039-2.108) | 0.219 | ||
24-h UTP, 24-h urinary protein quantity; β2-MG, β-2 microglobulin; Alb, albumin; ALP, alkaline phosphatase; BMPC, bone marrow plasma cell; BNP, brain natriuretic peptide; BU, Boston University; CI, confidence interval; CTNI, cardiac troponin I; EF, ejection fraction; GGT, γ-glutamyl transpeptidase; Hb, hemoglobin; HR, hazard ratio; ITE, initial therapeutic effect; LDH, lactate dehydrogenase; MM, multiple myeloma; OS, overall survival; Scr, serum creatinine; TBIL, total bilirubin; VGPR, very good partial response
Correlation of ALP with BNP, CTNI, and TBIL
The Kolmogorov-Smirnov test showed that ALP, CTNI, BNP, EF, and TBIL values were not normally distributed. To investigate the relationship between ALP and BNP, CTNI, EF, and TBIL levels, we performed Spearman's correlation tests. ALP at diagnosis was correlated with BNP (r=0.388; P=0.006; Fig. S2A), CTNI (r=0.313; P=0.028; Fig. S2B), and EF (r=-0.379; P=0.007; Fig. S2C). However, there was no correlation between ALP and TBIL levels (r=0.141; P=0.335; Fig. S2D).
Kaplan-Meier survival curves of AL-PCMM and AL-MM. A. Survival difference between patients with an ALP < 187.5 IU/L and those with an ALP ≥ 187.5 IU/L (P<0.001). B. Survival difference between patients with a GGT < 85 IU/L and those with a GGT ≥ 85 IU/L (P=0.008). C. Survival difference between patients with a TBIL< 20 µmol/L and those with a TBIL ≥ 20 µmol/L (P=0.010). D. Survival difference between patients with a CTNI < 0.1 ng/mL and those with a CTNI ≥ 0.1 ng/mL (P=0.010). E. Survival difference between patients with an ejection fraction < 50% and those with an ejection fraction ≥ 50% (P=0.015). F. Survival difference between patients with BU staging system stages I + II and those with BU staging system stage III (P=0.002). G. Survival difference between patients with heart and those without heart involvement (P=0.038). H. Survival difference between patients with liver involvement and those without liver involvement (P=0.025). I. Survival difference between patients with both heart and liver involvement and those with only heart involvement (P=0.114). AL-MM, amyloid light-chain amyloidosis and multiple myeloma; AL-PCMM, amyloid light-chain amyloidosis with a bone marrow plasma cell ratio > 10%; ALP, alkaline phosphatase; BU, Boston University; CTNI, cardiac troponin I; GGT, γ-glutamyl transpeptidase; TBIL, total bilirubin
Discussion
Patients with AL-PCMM have a poorer prognosis than those with AL-only, similar to patients with AL-MM [16]. We also found no statistically significant difference in OS between patients with AL-PCMM and those with AL-MM. However, why was there no difference in survival between these two groups of patients? We found that the indicators of tumor burden, such as Hb, bone destruction, Ca, Scr, β2-MG, LDH, and BMPC ratio, were not associated with the prognosis of AL-PCMM or AL-MM. However, amyloid organ involvement significantly impacts the prognosis of this group of patients, particularly those with cardiac amyloidosis. Compared with AL-PCMM, AL-MM displayed a difference in only the incidence of CRAB and BMPC ratio, which represented the tumor burden; however, there was no difference in the number or severity of involved organs, which resulted in death and a shorter survival time. Moreover, in our study, most patients were treated with the BD regimen, which resulted in no difference in treatment effect between these two groups of patients, resulting in this phenomenon. It should be noted that if these two groups of patients were treated with the recommended BCD or VRD regimen, the doses of cyclophosphamide and lenalidomide for those with AL-MM required adjustment according to the level of renal function, which might have led to a difference in the treatment effect and survival time of the two groups. However, this requires further investigation.
The Boston staging system, equivalent to the 2004 Mayo Clinical AL amyloidosis staging system, was used to predict the surival time in patients with AL amyloidosis, with a median survival time not reached for stage I, 30 months for stage II, and 5 months for stage III [3,4]. Our study also demonstrated that the survival time was significantly shorter in patients with BU staging system stages III and IIIB than in those with BU staging system stages I and II (1 month vs. 30 months). In a previous study, the 2004 Mayo Clinical AL amyloidosis staging system and the 2012 Mayo Clinical AL amyloidosis staging system were independent poor prognostic factors for AL-PCMM and AL-MM [16]. In our study, multivariate Cox regression analysis also found that a BU staging system stage ≥ III was an independent significant risk factor for a poor prognosis in AL-PCMM and AL-MM. The clinical stage of cardiac amyloidosis is the main factor affecting the prognosis of such patients. Therefore, more attention should be paid to evaluating cardiac amyloidosis severity in clinical settings. In addition, the BU staging system will be particularly widely used in this group of patients because not all centers have the capability to test NT-proBNP levels. However, whether the predictive value of the BU and Mayo staging systems is equivalent in this group of patients requires external validation.
Studies have reported that total bilirubin, an indicator of liver involvement severity in AL amyloidosis, is an independent adverse risk factor for progression-free survival (PFS) and OS in AL amyloidosis [19,20]. However, in our study, multivariate Cox regression analysis showed that TBIL level was not an independent prognostic factor for AL-PCMM and AL-MM. Therefore, liver involvement appears to have little effect on the survival time of these patients. Interestingly, we found that an ALP ≥ 187.5 IU/L, which was the diagnostic criterion for liver involvement, was an independent prognostic factor for AL-PCMM and AL-MM. In a retrospective study, Raymond et al. found that an increased ALP level was associated with increased 1-year mortality [20]. However, whether ALP is related to liver involvement severity and, thus, affects the survival time of these patients remains unclear. We found that ALP was related to BNP, CTNI, and EF rather than TBIL, which suggests liver involvement severity.
There may be two reasons for the elevated ALP levels in patients with primary systemic AL amyloidosis. First, chronic heart failure caused by cardiac amyloidosis leads to liver congestion, which manifests as elevated levels of biliary-related enzymes. Some studies also found that ALP was related to central venous pressure (CVP) and right ventricular free wall strain in patients with chronic heart failure, which supports this finding [21]. Second, amyloid deposition in the liver obstructs the small bile ducts, leading to increased ALP levels. In our study, the former may have been even stronger. Therefore, ALP, which is related to cardiac amyloidosis rather than liver involvement, can be used as a prognostic factor for this group of patients. Moreover, as ALP is closely related to right heart failure caused by cardiac amyloidosis, ALP ≥ 187.5 IU/L may have a high misdiagnosis rate as a diagnostic criterion for liver involvement. Liver puncture can be actively improved to assess the probability of misdiagnosis when the patient has cardiac amyloidosis with an ALP ≥ 187.5 IU/L under safe conditions. In addition, it may be necessary to identify more suitable specific indicators to diagnose liver involvement.
Our study had some limitations. We lacked data from the 2004 and 2012 Mayo Clinic AL amyloidosis staging systems; thus, we could not determine whether the Mayo staging system was a predictor of a poor prognosis for this type of patient. In addition, we had insufficient data to evaluate the degree of hematological remission and organ response to AL amyloidosis in each patient. Therefore, we could not determine whether the 2016 International Myeloma Working Group consensus criteria for the response to multiple myeloma are correlated with the hematologic response criteria for AL amyloidosis; in principle, we should not include patients whose treatment effects cannot be evaluated. However, because the number of cases was too small, we classified the treatment effect of these patients as disease progression to ensure that a sufficient number of cases were included in the group. This may have resulted in a certain degree of bias.
Conclusions
In summary, our study found that ALP, which is related to cardiac amyloidosis rather than liver involvement, can be a prognostic factor for this group of patients. BU staging system stage ≥ III, ALP ≥ 187.5 IU/L, and ITE < VGPR representing the degree of AL amyloidosis rather than tumor burden of multiple myeloma are independent significant risk factors for a poor prognosis of AL-PCMM and AL-MM.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
Funding
This study was supported by the Beijing Municipal Science Technology Commission (no. Z191100006619026).
Author Contributions
JHX designed this project, collected the details, performed the statistical analysis, and wrote the paper; XNC and MJW designed and supervised this project; and ZXQ, MY, ZYS, BJW, HHL, and BCL provided the clinical data. All authors have read and approved the final manuscript.
Availability of data and materials
The data supporting the study have been presented in the manuscript, and the original data can be obtained from the corresponding author upon suitable request.
Ethics approval and consent to participate
The Ethics Committee of Peking University First Hospital approved this study (no. 2017[1304]). Signed written consent was obtained from all patients.
Consent for publication
All patients approved the publication of this manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Dispenzieri A, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM. et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22:3751-7
2. Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C. et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30:989-95
3. Lilleness B, Ruberg FL. Development and validation of a survival staging system incorporating BNP in patients with light chain amyloidosis. 2019; 133: 215-23.
4. Tomlinson R, Matigian N, Mollee P. Validation of the Boston University staging system in AL amyloidosis. Amyloid: the international journal of experimental and clinical investigation: the official journal of the International Society of Amyloidosis. 2019;26:125-7
5. Kobayashi H, Abe Y, Miura D, Narita K, Kitadate A, Takeuchi M. et al. Prevalence and clinical implications of t(11;14) in patients with amyloid light-chain amyloidosis with or without concurrent multiple myeloma. Japanese journal of clinical oncology. 2019;49:195-8
6. Zhang CL, Qiu Y, Shen KN, Miao HL, Feng J, Cao XX. et al. Clinical presentation and prognosis of immunoglobulin light-chain amyloidosis with high percentage of bone marrow plasma cells. Leukemia research. 2019;81:19-24
7. Tovar N, Rodríguez-Lobato LG. Bone marrow plasma cell infiltration in light chain amyloidosis: impact on organ involvement and outcome. 2018; 25: 79-85.
8. Muchtar E, Gertz MA, Kourelis TV, Sidana S. Bone marrow plasma cells 20% or greater discriminate presentation, response, and survival in AL amyloidosis. 2020; 34: 1135-43.
9. Barros-Gomes S, Williams B, Nhola LF, Grogan M, Maalouf JF, Dispenzieri A. et al. Prognosis of Light Chain Amyloidosis With Preserved LVEF: Added Value of 2D Speckle-Tracking Echocardiography to the Current Prognostic Staging System. JACC Cardiovascular imaging. 2017;10:398-407
10. Yogo T, Okazuka K, Nashimoto J, Uto Y, Sato K, Miyazaki K. et al. Red blood cell distribution width is a simple and novel biomarker for survival in light-chain amyloidosis. International journal of hematology. 2019;110:431-7
11. Stamatelopoulos K, Georgiopoulos G, Athanasouli F, Nikolaou PE, Lykka M, Roussou M. et al. Reactive Vasodilation Predicts Mortality in Primary Systemic Light-Chain Amyloidosis. Circulation research. 2019;125:744-58
12. Pudusseri A, Sanchorawala V. Prevalence and prognostic value of D-dimer elevation in patients with AL amyloidosis. 2019; 94: 1098-103.
13. Dispenzieri A, Kyle RA, Gertz MA, Therneau TM, Miller WL, Chandrasekaran K. et al. Survival in patients with primary systemic amyloidosis and raised serum cardiac troponins. Lancet (London, England). 2003;361:1787-9
14. Muchtar E, Dispenzieri A, Kumar SK, Buadi FK, Lacy MQ, Zeldenrust S. et al. Immunoparesis in newly diagnosed AL amyloidosis is a marker for response and survival. Leukemia. 2017;31:92-9
15. Sachchithanantham S, Berlanga O. Immunoparesis defined by heavy+light chain suppression is a novel marker of long-term outcomes in cardiac AL amyloidosis. 2017; 179: 575-85.
16. Kourelis TV, Kumar SK, Gertz MA, Lacy MQ, Buadi FK, Hayman SR. et al. Coexistent multiple myeloma or increased bone marrow plasma cells define equally high-risk populations in patients with immunoglobulin light chain amyloidosis. J Clin Oncol. 2013;31:4319-24
17. Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN. et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18-22 April 2004. Am J Hematol. 2005;79:319-28
18. Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P. et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. The Lancet Oncology. 2016;17:e328-e46
19. Cappelli F, Baldasseroni S, Bergesio F, Spini V, Fabbri A, Angelotti P. et al. Liver dysfunction as predictor of prognosis in patients with amyloidosis: utility of the Model for End-stage Liver disease (MELD) scoring system. Internal and emergency medicine. 2017;12:23-30
20. Zhang LL, Shen KN, Zhang CL, Qiu Y, Miao HL, Feng J. et al. Clinical presentation and prognostic analysis of Chinese patients with systemic light chain amyloidosis with liver involvement. Leukemia research. 2019;86:106226
21. Borovac JA, Glavas D, Susilovic Grabovac Z, Supe Domic D, Stanisic L, D'Amario D. et al. Right Ventricular Free Wall Strain and Congestive Hepatopathy in Patients with Acute Worsening of Chronic Heart Failure: A CATSTAT-HF Echo Substudy. 2020; 9.
Author contact
![]() Corresponding authors: Xinan Cen, Department of Hematology, Peking University First Hospital, No.8 Xi Shi Ku Street, Xi Cheng District, Beijing 100034, China. TEL: 86-10-83575746 E-mail: cenxnedu.cn and/or Mangju Wang, Department of Hematology, Peking University First Hospital, No.8 Xi Shi Ku Street, Xi Cheng District, Beijing 100034, China. TEL: 86-10-83575746 E-mail: wang_m_jcom
Corresponding authors: Xinan Cen, Department of Hematology, Peking University First Hospital, No.8 Xi Shi Ku Street, Xi Cheng District, Beijing 100034, China. TEL: 86-10-83575746 E-mail: cenxnedu.cn and/or Mangju Wang, Department of Hematology, Peking University First Hospital, No.8 Xi Shi Ku Street, Xi Cheng District, Beijing 100034, China. TEL: 86-10-83575746 E-mail: wang_m_jcom

 Global reach, higher impact
Global reach, higher impact