Impact Factor
ISSN: 1449-1907
Int J Med Sci 2021; 18(16):3851-3860. doi:10.7150/ijms.60928 This issue Cite
Review
The Emerging Roles of π Subunit-Containing GABAA Receptors in Different Cancers
1. Department of Neurosciences, School of Medical Sciences, Universiti Sains Malaysia, Health Campus, 16150 Kubang Kerian, Kelantan, Malaysia.
2. Centre for Drug Research, Universiti Sains Malaysia, 11800 Minden, Penang, Malaysia.
Received 2021-3-27; Accepted 2021-10-10; Published 2021-10-31
Abstract
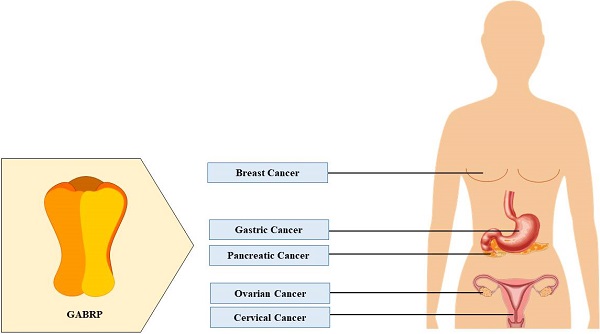
Cancer is one of the leading causes of death in both developed and developing countries. Due to its heterogenous nature, it occurs in various regions of the body and often goes undetected until later stages of disease progression. Feasible treatment options are limited because of the invasive nature of cancer and often result in detrimental side-effects and poor survival rates. Therefore, recent studies have attempted to identify aberrant expression levels of previously undiscovered proteins in cancer, with the hope of developing better diagnostic tools and pharmaceutical options. One class of such targets is the π-subunit-containing γ-aminobutyric acid type A receptors. Although these receptors were discovered more than 20 years ago, there is limited information available. They possess atypical functional properties and are expressed in several non-neuronal tissues. Prior studies have highlighted the role of these receptors in the female reproductive system. New research focusing on the higher expression levels of these receptors in ovarian, breast, gastric, cervical, and pancreatic cancers, their physiological function in healthy individuals, and their pro-tumorigenic effects in these cancer types is reviewed here.
Keywords: Cancer, GABAA receptors, π subunit, female reproductive system
Introduction
Cancer is a broad term used to describe more than 277 different types of diseases (leukemia, melanoma, lymphoma, etc.) that occur in various regions of the body caused by uncontrolled mitotic cell division [1]. This uncontrolled growth and division of cells due to genetic mutations results in neoplasms (tumors); therefore, cancer is a (malignant) neoplastic condition. Mutations leading to cancer may arise due to genetic predispositions [2], environmental carcinogens [3], lifestyle choices (e.g., diet, excessive drinking, tobacco smoking, etc.) [4], radiation exposure [5], viral infections [6, 7], and epigenetic changes (e.g., histone modifications, DNA methylation, and microRNA dysregulation) [8]. Globally, there were an estimated 19.3 million diagnosed cases and approximately 10.0 million deaths in 2020 related to cancer, excluding nonmelanoma skin cancer [9]. Prostate and breast cancer are the most commonly diagnosed cancers in men and women, respectively [10]. Notably, a recent 25-year study revealed a significant increase in the mortality rate of patients with breast cancer in both developed and developing countries [11]. To curb the cancer mortality rate, current antineoplastic options include chemotherapy [12], radiotherapy [13], immunotherapy [14], hormone therapy [15], surgery [16], precision medicine [17], molecular targeted therapy [18], and stem cell transplantation [19]. However, due to the heterogenous and invasive nature of some types of cancer, therapy is not always feasible, leading to poor prognosis and survival rates [20, 21, 22, 23, 24, 25, 26, 27]. Therefore, current research is focused on previously undiscovered pathways and other factors (such as receptors) that are involved in cancer development in order to design newer and more effective antineoplastic agents. Recent studies have implicated γ-aminobutyric acid (GABA) type A receptors (GABAARs), more specifically GABAA receptor containing the π subunit (GABRP), in cancer [28, 29]. Therefore, this review highlights the existing knowledge of GABRP expression and function in healthy individuals and its potential role in cancer.
The GABAA receptors
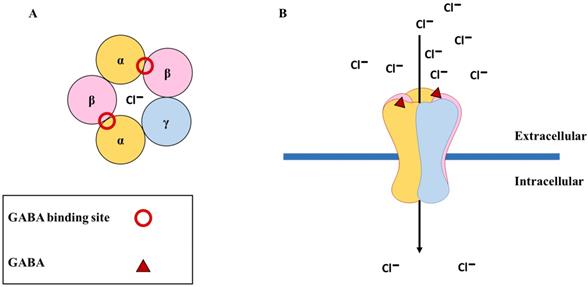
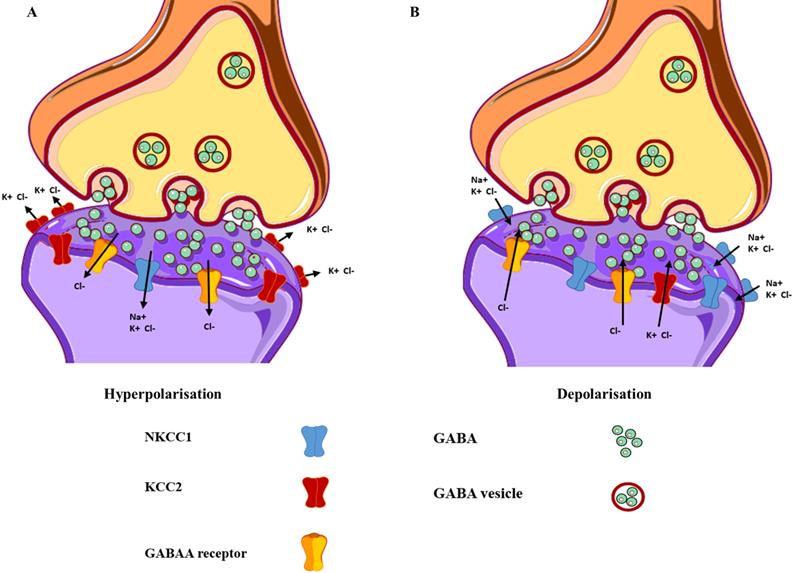
GABA is the main inhibitory neurotransmitter of the central nervous system and it generates fast signaling neurotransmissions through the GABAARs that are cys-loop ligand-gated ion channels comprising five subunits (the main receptor subtype contains two α, two β, and one γ subunit [α-β-α-β-γ]) enclosing a central chloride ion (Cl-) pore in the brain [30]. To date, 19 subunits have been discovered in the human (α1-α6, β1-β3, γ1-γ3, δ, ε, θ, π, and ρ1-ρ3). When two molecules of GABA bind to the β+/α- interface, they cause a conformational change in the receptor structure, resulting in the opening of channels permeable to Cl-. This causes an influx of Cl- into the cell, resulting in hyperpolarization of the membrane and inhibition of neuronal signaling [31] (Figure 1). When GABAARs are activated in healthy neurons, hyperpolarization may occur, only if the extracellular concentration of Cl- is greater than the intracellular concentration, resulting in an influx of Cl- into the cell. This Cl- homeostasis is maintained by two essential cation-chloride cotransporters, sodium-potassium-chloride cotransporter isoform 1 (NKCC1) and potassium-chloride cotransporter isoform 2 (KCC2). NKCC1 assimilates Cl- into the cell, whereas KCC2 expels Cl- from the cell [32]. In healthy neurons, KCC2 is expressed more than NKCC1, and upon binding of GABA to GABAARs, there is an influx of Cl- into the cell, which results in hyperpolarization [33] (Figure 2A). When the intracellular Cl- concentration exceeds the extracellular concentration, the binding of GABA to the GABAARs results in an efflux of Cl- from the cell, causing the membrane potential to become more positive, resulting in depolarization [34] (Figure 2B). This unique ability of GABAARs can also be observed in immature neurons during early postnatal development [35]. While both the α and β subunits can co-assemble with all other GABAAR subunits, the γ and δ subunits cannot coexist within the same receptor subtype [36]. Furthermore, only the ρ1, β3, α, and γ subunits can form homo-oligomeric receptors [37]. GABAARs vary in their affinity for GABA, expression sites (synaptic or extrasynaptic), and biophysical and pharmacological properties based on their subunit composition. For example, α subtypes have functional variation; α1 results in sedation, whereas α2 and α3 are instrumental in anxiolysis [38]. However, the expression of these subunits is dependent on the other subunits that they co-assemble within the receptor. For instance, the α(1/2/3/5)+/γ2- interface is essential for benzodiazepine-mediated sedation, anxiolysis, seizure suppression, and muscle relaxation [38], and only the placement of the α subunit next to the γ subunit (α+/γ2-) can mediate its function. If both subunits exist within the same receptor but not in the correct order, the resulting GABAAR will not be receptive to benzodiazepine. Importantly, not all potential subunit combinations can result in functional GABAARs. Alterations in receptor composition, such as the switch from a γ2 to a δ subunit could desensitize GABAARs to drugs such as benzodiazepines [39]. Several conditions, such as epilepsy [40, 41], traumatic brain injury [42, 43], mental illnesses (such as schizophrenia and mood disorders) [44, 45], addiction [46, 47], Alzheimer's disease [48, 49], and Parkinson's disease [50, 51], can result in alterations in the subunit composition of GABAARs, thus highlighting the critical function of subunit configuration in healthy individuals. For more in-depth understanding of the GABAAR structure and their ligand-binding site interactions, readers can refer to [30, 52, 53].
The physiological function of GABAA receptors. (A) The most common subunit configuration of GABAA receptors along with the binding sites for GABA (shown with red circle); (B) The binding of GABA (shown with red triangle) results in the opening of the channel, causing an influx of chloride ions (Cl-) into the cell.
Differences in the functional and expressional levels of NKCC1 and KCC2. (A) Mature neurons exhibit lower expression levels of NKCC1, as compared to KCC2, resulting in hyperpolarisation. (B) Immature neurons express higher levels of NKCC1 in contrast to KCC2, resulting in depolarisation. The yellow structure represents the GABAA receptors, with the green circles representing the neurotransmitter GABA.
GABRP
Overview
Although GABRP was first discovered more than 20 years ago, there is not much information currently known about this receptor which possesses atypical functional properties. In comparison to other GABAAR subunits, the π subunit is closely related to the β (37%), δ (35%), and ρ (33%) subunits. Previous studies have shown that the π subunit is incapable of forming homo-oligomeric receptors [54]. However, it can be co-assembled with α, β, and γ subunits to create αβπ and αβγπ isoforms [55]. GABRP exhibits differential pharmacological properties as compared to other similar GABAAR isoforms (αβγ, αβδ, and αβε), increased sensitivity to inhibition by zinc ions, no sensitivity to diazepam action, and distinctive neurosteroidal regulation [55]. Located on chromosome 5q34, this receptor is expressed in several non-neuronal regions such as the uterus [56], placenta [57], pancreas [29], gastrointestinal tract [58], lungs [59], kidney [60], immune cells [61], and mammary glands [62].
Female reproductive system
In the uterus, GABRP can alter uterine motility by modulating tissue contractility [54]. Its expression levels change throughout pregnancy, with consistent levels throughout gestation followed by a reduction during the onset of labor [63]. This change in expression can modify the sensitivity of recombinant receptors to pregnanolone and allopregnanolone [54,63]. During gestation, allopregnanolone increases the binding of the GABAAR agonist, muscimol, to uterine GABAARs; in contrast, labor is marked by a limitation in this interaction, which can be attributed to the lower expression levels of GABRP [63]. Endometrial levels of GABRP also change during the secretory phase of the uterus, and elevated levels play a crucial role in acquiring endometrial receptivity for embryo implantation [56]. Similarly, a recent study found constant placental expression levels of GABRP during gestation, followed by a reduction during labor onset and a complete absence at term [64]. This suggests that GABRP has invasive potential and is involved in the development of villous trophoblasts and syncytiotrophoblasts during the first trimester, thus ensuring a secure uterine wall implantation. GABRP can also modulate both anti-apoptotic (B-cell lymphoma 2 [Bcl-2]) and pro-apoptotic (Bcl-2-associated agonist of cell death [Bad] and Bcl-2-like protein 4 [Bax]) protein levels, and elevated placental GABRP levels are implicated in preeclampsia [57], thereby highlighting the pivotal role it plays in the female reproductive system.
Gastrointestinal tract
In the gastrointestinal tract, GABRP regulates electrolyte transport, with GABA administration resulting in increased intestinal secretion in a dose-dependent manner [58]. Increased expression of these receptors has been reported in cases of allergic diarrhea and ulcerative colitis, and treatment with a suitable GABAAR antagonist results in alleviation of symptoms [58, 65].
Lungs
GABRP also plays a role in fetal lung development by governing cell proliferation and/or fluid secretion. During gestation, elevated expression levels of α1, β2, and π subunits have been observed in fetal lung tissue from the initial stage to the later adult stages [59]. In one study, fetuses exposed to GABA exhibited a significant increase in body and lung weight with a 30% increase in the total number of saccules, a common marker for lung maturity, as compared to that in a control group. Exposure to GABA also amplified the number of alveolar epithelial type II cells while reducing the amount of α-smooth muscle actin-positive myofibroblasts [59], which indicate conditions such as asthma when present in large numbers [66]. Therefore, a reduction in this cell type suggests healthy lung development via GABRP. In another study, epithelial cells exposed to GABA also demonstrated higher Ki-67 levels [59]; Ki-67 is another marker for cellular proliferation and the development of healthy lungs, which is absent in resting cells [67]. Additionally, GABA regulates Cl- efflux and resolves pulmonary oedema [68]. Notably, GABRP-knockout studies have reported inhibition of this efflux function [69], further supporting the critical role of GABRP in fetal lung development.
Kidneys
GABRP has also been detected at both the mRNA and protein levels in human and rat kidneys and may have an autocrine/paracrine mechanism for local GABAergic transmissions [60]. Although the receptor composition for GABRP is still debatable due to lack of consistent results from transfection studies, it has been suggested that GABRP in the kidneys is composed of a combination of α1β3π [60].
Breast
Although several studies have detected the presence of glutamic acid decarboxylase (GAD; the enzyme that synthesizes GABA), GABA [70], and significant expression levels of GABRP in healthy breast tissue, their function remain largely unknown.
Summary diagram of how GABRP's regulation of ERK in breast/ovarian, gastric and pancreatic cancers.
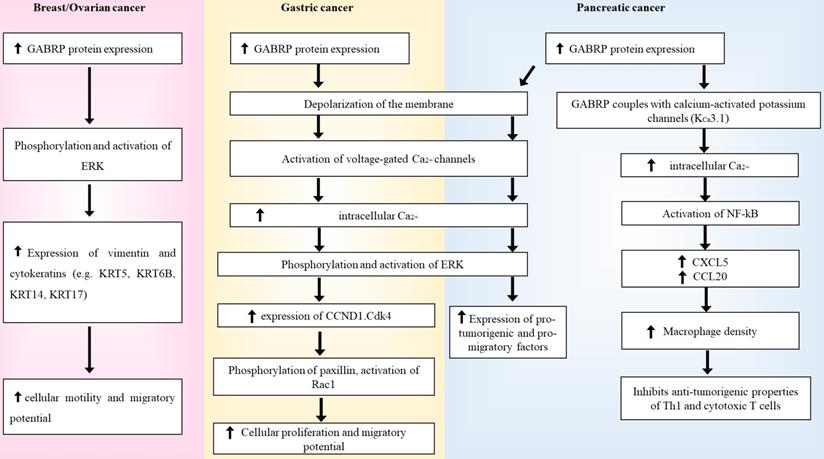
Aberrant GABRP regulation in different types of cancer
Breast cancer
GABRP expression levels are an important indicator of the risk of recurrence of breast cancer and mortality [62]. Almost 50% of all breast cancer types exhibit high GABRP expression levels [71]. Elevated levels of GABRP have been previously reported in circulating breast cancer cells [72,73,74] and isolated lymph nodes from patients with breast cancer [75]. A multigene real-time reverse transcription polymerase chain reaction (RT-PCR) study observed patients with metastatic breast cancer expressing eight times as much GABRP as compared to stages II-IV patients with no evidence of metastasis [76]. This expression level was 30 times higher than that in stage I patients with no evidence of metastasis, suggesting that GABRP expression levels increase with disease progression and metastasis. This elevated expression level of GABRP was also effective in detecting circulating tumor cells in patients with stage I (65%), stages II-IV with no evidence of metastasis (72%), and metastatic (88.5%) breast cancer. Circulating tumor cells serve as an important indicator of the overall survival rate of patients with breast cancer [77]. Detection of GABRP expression levels can therefore prove beneficial in tracking disease progression, for instance, when traditional serum markers fail. In one study, most of the healthy controls (51 out of 53) showed low expression levels of GABRP compared to patients with breast cancer [76], and of the 2 remaining controls that exhibited high GABRP levels, 1 participant was pregnant (first trimester). The elevated expression levels of GABRP can be explained by its known physiological role in the female reproductive system. Similarly, in vitro studies in basal-like breast cancer (BLBC) cell lines (HCC1187 and HCC70) have also reported elevated expression levels of GABRP [78]. Other studies in healthy individuals showed that luminal progenitor breast cells also express high levels of GABRP [79]. These cells have been suggested to generate BLBC cells during carcinogenesis, further suggesting a strong connection between GABRP and the BLBC subtype [80]. Patients with BLBC often develop secondary cancer in visceral organs such as the lung, liver, and brain when the cancer metastasizes [81,82,83].
Sizemore and colleagues found a strong correlation between GABRP and the formation, migration, and aggressiveness of secondary cancer cells, thus implicating GABRP in brain metastases and poor prognosis. Lentiviral knockdown of GABRP in these BLBC cell lines resulted in cytoskeletal alterations, lower expression levels of basal-like cytokeratins (KRT5, KRT6B, KRT14, and KRT17), and reduced phosphorylation of the extracellular signal-regulated kinase (ERK) 1/2 signaling pathway [78]. Cytokeratins are structural proteins that form a major component of the intermediate filaments. Since their expression levels vary depending on cell types and their degree of differentiation, cytokeratins serve as suitable markers for differentiating carcinomas from other subtypes of cancer [84]. Previous studies have linked GABRP with KRT5, KRT6B, KRT14, and KRT17 in breast cancer pathogenesis [85], as several cytokeratins have been implicated in cancer cell migration [86,87,88]. Additionally, cell lines generated with functional GABRP but inhibited ERK 1/2 activity resulted in a lack of this migratory disease phenotype, suggesting that GABRP utilizes the ERK 1/2 signaling pathway to mediate its pro-migratory effects [78]. ERK 1/2 is a member of the mitogen-activated protein kinase (MAPK) family and has a highly regulated pathway that plays a crucial role in cell proliferation, differentiation, and stress response. The entire signaling pathway utilizes various kinases, such as Ras/Raf/MAPK-ERK (MEK), ribosomal s6 kinases, MAP kinase-interacting serine/threonine-protein kinases, mitogen- and stress-activated protein kinases, and cytosolic phospholipase A2 [89]. This pathway is a known modulator of bispecific phosphatases [90,91], subcellular localization of cascade components [92,93], cellular motility [94,95], cytokeratins [78,96,97,98], and other scaffolding proteins [99,100]. The ERK 1/2 signaling pathway has been implicated in several cancer subtypes [89,101,102]; therefore, abnormal manipulation of this pathway by GABRP can result in carcinogenesis. Triple-negative breast cancer (TNBC) cells have also been reported to primarily express GABRP mRNA and proteins [71]. Unlike other types of breast cancer, TNBC cells lack conventional biomarkers such as the oestrogen, progesterone, and human epidermal growth factor receptors and have also been linked to higher rates of relapse and mortality due to its aggressive nature. Therefore, the detection of GABRP mRNA and proteins in these cells could act as potential biomarkers while also providing a site for targeted therapy [103]. In vitro studies have shown that GABRP knockdown inhibits the proliferation of TNBC, whereas GABRP silencing suppresses the development of MDA-MB-468 xenografts in nude mice. Moreover, application of anti-GABRP antibodies or de novo generated Fabs in TNBC cell lines arrests further cancerous growth. When used in combination with mertansine, similar antineoplastic properties were also observed at nanomolar concentrations [71], further underlining the prospective role of GABRP as a therapeutic target in breast cancer.
Ovarian cancer
An in vivo ovarian cancer study detected a >2-fold increase in the transcriptional expression levels of GABRP in metastatic implants of human ovarian carcinoma xenografts in mice compared to SK-OV-3 ovarian carcinoma cells [104]. Another study conducted by the same group found a >4-fold increase in GABRP expression levels in the metastatic tissue of the mice model [28]. Utilizing the SK-OV-3 ovarian carcinoma cell lines, several gain-of-function and loss-of-function studies were performed to analyze the role of GABRP in cellular migration and invasion. It was revealed that GABRP silencing reduced the invasive and migratory potential of SK-OV-3 cells while downregulating the ERK pathway. Similarly, increased expression of GABRP enhanced cellular invasion and migration and upregulated the ERK pathway. The involvement of GABRP in ERK regulation was further highlighted when the administration of U0126, a MAPK/MEK inhibitor, eliminated the invasive and pro-migratory abilities of SK-OV-3 cells, suggesting that GABRP modulates the MAPK/ERK pathway to enhance the metastatic potential of ovarian cancer [28]. Furthermore, a genome-wide DNA methylation profiling study in mouse models detected hypomethylation at the GABRP-963 CpG site. Similar results were also observed in patients who were in the advanced stages of ovarian cancer, implying that the transcriptional regulation of GABRP is governed by a DNA methylation-dependent epigenetic mechanism which further ameliorates the aggressive phenotype of ovarian cancer [28].
Cervical cancer
Cervical cancer studies have also reported higher expression levels of GABRP in metastatic tissue in patients with cancer as compared to that in controls [105]. MicroRNAs are short non-coding RNAs that affect gene silencing by targeting mRNAs at their 3-untranslated region, thus regulating protein expression levels. They are crucial for almost all cellular processes, such as differentiation, development, and homeostasis [106]. The microRNA, miR-320c, has been shown to possess anti-tumorigenic properties in cancer development as it downregulates the migratory potential of cancer cells [105]. It mediates this function by negatively regulating GABRP protein expression levels in these tissues [105]. Rescue studies have shown that patients with reduced expression levels of miR-320c had higher protein expression levels of GABRP, thus developing lymphatic and distant metastases at a higher rate than patients with increased expression levels of miR-320c [105]. These high expression levels of GABRP were shown to reverse the effects of miR-320c and increase the migratory potential of cervical cancer cells. Additionally, the upregulation of miR-320c significantly suppressed the migratory potential of HeLa and C33-A cells due to lower expression levels of the GABRP protein. Western blotting studies have indicated that cervical cancer cells that exhibited higher expression levels of miR-320c had significantly lower protein expression levels of GABRP and lower migratory potential [105], implying a possible role for GABRP in metastatic cervical cancer.
Gastric cancer
In vitro studies in KATO III cell lines revealed GABRP-induced proliferative effects in gastric cancer [107]. RT-PCR and immunohistochemical studies confirmed that these effects are mediated through a GABA-dependent mechanism in an autocrine or paracrine manner. The integral component of this entire process is the upregulation of the ERK 1/2 pathway via GABRP, which in turn strengthens cyclin D1 expression [107]. As previously mentioned, GABAARs have an inhibitory function (hyperpolarization) based on extracellular Cl- levels. In cancer, there often tends to be a Cl- imbalance that results in depolarization of the membrane, which indirectly activates voltage-gated calcium channels [108]. This raises the intracellular calcium ion (Ca2+) concentration, which further activates several downstream kinases and signaling pathways. The ERK 1/2 pathway is one such cascade, which upon activation results in the transcriptional upregulation of several genes such as CCND1 (cyclin D1), which is critical for the progression of the cell cycle from the G1 phase to the S phase [109]. Abnormal expression levels of cyclin D1 increase cancer cell proliferation, migration, and metastasis via the Ccnd1·Cdk4-paxillin-Rac1 axis [110]. Therefore, irregularities in ERK 1/2 activation due to GABRP can result in cancer. Elevated expression levels of GABRP mRNA and proteins have also been detected in oral squamous cell carcinoma cell lines [111]. Additionally, the application of muscimol or GABA further stimulates cellular proliferation, while suppressing apoptosis and arresting the cell cycle in the G2/M phase. Furthermore, when these cells were treated with the GABAAR antagonist, S106, and then later re-treated with GABA, they lacked the anti-apoptotic properties that they previously exhibited, strongly supporting the pro-oncogenic nature of GABRP. The modulation of the cell cycle was achieved via GABRP-mediated activation of the p38 pathway and downregulation of the c-Jun N-terminal kinase (JNK) signaling pathway, both of which belong to the MAPK family [111]. In healthy cells, activation of the JNK pathway results in the phosphorylation and activation of pro-apoptotic proteins such as Bcl-2-interacting mediator of cell death (BIM; homologous to BAX) and Bcl-2-modifying factor (BMF), which further activates downstream caspases. Simultaneously, JNK can also phosphorylate and inactivate anti-apoptotic proteins, such as death protein 5/harakiri, Bcl-2, and B-cell lymphoma extra-large [112]. Therefore, the downregulation of this pathway can result in uncontrolled cell proliferation and can have detrimental effects. In contrast, upregulation of the p38 pathway enhances metastasis and has been correlated with a poor prognosis in cancer [113,114,115].
Pancreatic cancer
Higher expression levels of GABRP have been observed in all grades of pancreatic ductal adenocarcinoma (PDAC) than in healthy control pancreatic tissues, implying that GABRP plays a critical role in the early stages of pancreatic carcinogenesis [29,116,117]. Small interfering RNA-mediated GABRP knockdown in PDAC cells was shown to significantly reduce cancer cell proliferation [117]. Additionally, the introduction of GABA to these cell lines further increased the growth of GABRP-expressing PDAC cells. However, this was not observed for GABRP-negative cells, implying that GABRP and not any other subtype of GABAARs are responsible for the tumorigenic phenotype of the PDAC cells. Treatment of GABRP-positive cells with the GABAAR inhibitor, picrotoxin, and the calcium channel blocker, nifedipine, restricted cellular proliferation. Moreover, treatment with GABA also increased the intracellular Ca2+ levels, which resulted in the activation of the ERK signaling pathway, and picrotoxin or nifedipine could inhibit this activation. Although GABAA receptors cause hyperpolarization in mature neurons, they have been shown to mediate depolarization in immature neurons and glial tumour cells. This activates the voltage gated Ca2+ channels causing an increase in intracellular Ca2+ levels which results in the phosphorylation and activation of the MAPK/ERK pathway [117]. Tissues derived from patients with PDAC have higher levels of GABA due to increased expression levels of GAD1, indicating an autocrine or paracrine-mediated modulation of GABRP in PDAC [117]. As previously discussed, aberrant activation of the ERK pathway results in the phosphorylation and activation of several downstream kinases and transcription factors that are essential for cell proliferation, migration, and survival, thus supporting cancerous growth. Another recent study also suggested GABRP-mediated carcinogenesis in PDAC, but in a GABA-independent manner [29]. Macrophages are important immune cells that protect the body against harmful microorganisms via phagocytosis, while also serving other essential regulatory and repair functions [119]. Moreover, these cells possess the ability to inhibit Th1 cells and the anti-tumor abilities of cytotoxic T lymphocytes, contribute to matrix remodeling, and promote tumor cell invasion and migration [120,121,122]. GABRP can govern macrophage infiltration in PDAC cells by coupling with a calcium-activated potassium channel (KCa3.1), which causes an influx of Ca2+ and activates the nuclear factor κB. Consequently, this accelerates the expression levels of CXCL5 and CCL20 [29], which are known macrophage-recruiting chemokines [123]. This results in increased macrophage density which has often been correlated with a poor prognosis in cancer [118]. Pharmacological deletion of macrophages by liposomal clodronate greatly reduced the cancer proliferation of GABRP in PDAC cells [29]. GABRP knockdown reduced the expression levels of chemokines [29], thereby suggesting a unique immunomodulatory role for GABRP in PDAC.
Conclusion
Although this review summarizes our current knowledge of the GABRP and its enigmatic role in cancer, there are still several areas that require thorough research. A critical component requiring further investigation is its subunit composition, since there is an almost negligible amount of data currently available. Unlike its famous counterparts, the GABRP subunit composition remains shrouded in mystery. GABRP is expressed in several organs and has a few known physiological roles. These recently discovered roles in various cancer subtypes will hopefully direct more attention to these receptors. Although several studies have already highlighted the role of ERK in cancer, there are several factors that could potentially modulate this pathway; GABRP being one of them. GABRP has the potential to serve as a diagnostic marker as well as a possible therapeutic target in cancer. However, further research is needed to better understand these receptors and utilize them as potential targets in cancer therapy.
Abbreviations
Bcl-2: B-cell lymphoma 2; Bad: Bcl-2-associated agonist of cell death; Bax: Bcl-2-like protein 4; BIM: BCL-2-interacting mediator of cell death; BMF: Bcl-2-modifying factor; BLBC: basal-like breast cancer; ERK: extracellular signal-regulated kinase; GABA: γ-aminobutyric acid; GABAARs: γ-aminobutyric acid type A receptors; GABRP: GABA receptor π subunit; JNK: c-Jun N-terminal kinase; KCC2: potassium-chloride cotransporter isoform 2; MAPK: mitogen-activated protein kinase; NKCC1: sodium-potassium-chloride cotransporter isoform 1; PDAC: pancreatic ductal adenocarcinoma; RT-PCR: real-time reverse transcription polymerase chain reaction; TNBC: Triple-negative breast cancer.
Acknowledgements
Funding
This review work was funded by the Fundamental Research Grant Scheme (FRGS) 203/PPSP/6171233, sponsored by Ministry of Higher Education, Malaysia.
Availability of data and material
Data sharing is not applicable to this article as no new data were created or analysed in this review work.
Authors' contributions
- Iman Imtiyaz Ahmed Juvale: Designing and drafting the manuscript;
- Zurina Hassan: Revising the manuscript;
- Ahmad Tarmizi Che Has: Revising the manuscript critically and approving the manuscript to be published.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Hassanpour S, Dehghani M. Review of cancer from perspective of molecular. J Cancer Res Pract. 2017;4(4):127-129
2. Riahi A, Chabouni-Bouhamed H, Kharrat M. Prevalence of BRCA1 and BRCA2 large genomic rearrangements in Tunisian high risk breast/ovarian cancer families: Implications for genetic testing. Cancer Genet. 2017;210:22-27
3. Ceccaroli C, Pulliero A, Geretto M. et al. Molecular Fingerprints of Environmental Carcinogens in Human Cancer. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2015;33(2):188-228
4. Jacob L, Freyn M, Kalder M. et al. Impact of tobacco smoking on the risk of developing 25 different cancers in the UK: a retrospective study of 422,010 patients followed for up to 30 years. Oncotarget. 2018;9(25):17420-17429
5. Shah D, Sachs R, Wilson D. Radiation-induced cancer: a modern view. Br J Radiol. 2012;85(1020):e1166-e1173
6. Yi Z, Yuan Z. Hepatitis C Virus-Associated Cancers. Adv Exp Med Biol. 2017;1018:129-146
7. Farahmand M, Monavari SH, Shoja Z. et al. Epstein-Barr virus and risk of breast cancer: a systematic review and meta-analysis. Future Oncol. 2019;15(24):2873-2885
8. Kinnaird A, Zhao S, Wellen K. et al. Metabolic control of epigenetics in cancer. Nat Rev Cancer. 2016;16(11):694-707
9. Sung H, Ferlay J, Siegel R. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin. 2021;71(3):209-249
10. Siegel R, Miller K, Jemal A. Cancer statistics, 2016. CA: Cancer J Clin. 2016;66(1):7-30
11. Azamjah N, Soltan-Zadeh Y, Zayeri F. Global Trend of Breast Cancer Mortality Rate: A 25-Year Study. Asian Pac J Cancer Prev. 2019;20(7):2015-2020
12. Gupta S. Adjuvant chemotherapy in locally advanced cervical cancer: the ceiling remains unbroken. J Gynecol Oncol. 2019;30(4):1-3
13. Demaria S, Golden E, Formenti S. Role of Local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol. 2015;1(9):1325
14. Liu M, Guo F. Recent updates on cancer immunotherapy. Precis Clin Med. 2018;1(2):65-74
15. Shelley M, Kumar S, Wilt T. et al. A systematic review and meta-analysis of randomised trials of neo-adjuvant hormone therapy for localised and locally advanced prostate carcinoma. Cancer Treat Rev. 2009;35(1):9-17
16. Zheng Y, Yang H, Wang H. et al. Fluorescence-guided surgery in cancer treatment: current status and future perspectives. Ann Transl Med. 2019;7(S1):S6-S6
17. Lheureux S, Braunstein M, Oza A. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA: Cancer J Clin. 2019;69(4):280-304
18. Lee Y, Tan Y, Oon C. Molecular targeted therapy: Treating cancer with specificity. Eur J Pharmacol. 2018;834:188-196
19. Singh A and McGuirk. Allogeneic Stem Cell Transplantation: A Historical and Scientific Overview. Cancer Res. 2016;76(22):6445-6451
20. Aslam M, Naveed S, Ahmed A. et al. Side Effects of Chemotherapy in Cancer Patients and Evaluation of Patients Opinion about Starvation Based Differential Chemotherapy. J Cancer Ther. 2014;05(08):817-822
21. Zimmermann S, Dziadziuszko R, Peters S. Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat Rev. 2014;40(6):716-722
22. Inamoto Y, Shah NN, Savani BN. et al. Secondary solid cancer screening following hematopoietic cell transplantation. Bone Marrow Transplant. 2015;50(8):1013-23
23. Tohme S, Simmons R, Tsung A. Surgery for Cancer: A Trigger for Metastases. Cancer Res. 2017;77(7):1548-1552
24. Inamoto Y, Lee S. Late effects of blood and marrow transplantation. Haematologica. 2017;102(4):614-625
25. Keklikoglou I, Cianciaruso C, Güç E. et al. Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat Cell Biol. 2018;21(2):190-202
26. Axelrad JE, Bazarbashi A, Zhou J. et al. Hormone Therapy for Cancer Is a Risk Factor for Relapse of Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2020;18(4):872-880
27. Santarone S, Natale A, Angelini S. et al. Secondary oral cancer following hematopoietic cell transplantation. Bone Marrow Transplant. 2021;56:1038-1046
28. Sung HY, Yang SD, Ju W. et al. Aberrant epigenetic regulation of GABRP associates with aggressive phenotype of ovarian cancer. Exp Mol Med. 2017;49(5):e335-e335
29. Jiang S, Zhu L, Zhang M. et al. GABRP regulates chemokine signalling, macrophage recruitment and tumour progression in pancreatic cancer through tuning KCNN4-mediated Ca2+ signalling in a GABA-independent manner. Gut. 2019;68(11):1994-2006
30. Sieghart W. Allosteric modulation of GABAA receptors via multiple drug-binding sites. Adv Pharmacol. 2015;72:53-96
31. Sigel E, Steinmann ME. Structure, function, and modulation of GABA(A) receptors. J Biol Chem. 2012;287(48):40224-40231
32. Liu R, Wang J, Liang S. et al. Role of NKCC1 and KCC2 in Epilepsy: From Expression to Function. Front Neurol. 2020;10(1407):1-12
33. Tillman L, Zhang J. Crossing the Chloride Channel: The Current and Potential Therapeutic Value of the Neuronal K+-Cl-Cotransporter KCC2. Biomed Res Int. 2019 doi:10.1155/2019/8941046
34. Herbison A, Moenter S. Depolarising and Hyperpolarising Actions of GABAA Receptor Activation on Gonadotrophin-Releasing Hormone Neurones: Towards an Emerging Consensus. J Neuroendocrinol. 2011;23(7):557-569
35. Kirmse K, Kummer M, Kovalchuk Y. et al. GABA depolarizes immature neurons and inhibits network activity in the neonatal neocortex in vivo. Nat Commun. 2015;6(7750):1-13
36. Sarto-Jackson I, Sieghart W. Assembly of GABA(A) receptors (Review). Mol Membr Biol. 2008;25(4):302-10
37. Hannan S, Smart T. Cell surface expression of homomeric GABAAreceptors depends on single residues in subunit transmembrane domains. J Biol Chem. 2018;293(35):13427-13439
38. Juvale IIA, Che Has A. Possible interplay between the theories of pharmacoresistant epilepsy. Eur J Neurosci. 2021;53(6):1998-2026
39. Goetz T, Arslan A, Wisden W. et al. GABAA receptors: Structure and function in the basal ganglia. Prog Brain Res. 2007;160:21-41
40. Grabenstatter H, Cogswell M, Cruz Del Angel Y. et al. Effect of spontaneous seizures on GABAA receptor α4 subunit expression in an animal model of temporal lobe epilepsy. Epilepsia. 2014;55(11):1826-1833
41. Rocha L, Alonso-Vanegas M, Martínez-Juárez I. et al. GABAergic Alterations in Neocortex of Patients with Pharmacoresistant Temporal Lobe Epilepsy Can Explain the Comorbidity of Anxiety and Depression: The Potential Impact of Clinical Factors. Front Cell. Neurosci. 2015;8(442):1-10
42. Gibson C, Meyer R, Hamm R. Traumatic brain injury and the effects of diazepam, diltiazem, and MK-801 on GABA-A receptor subunit expression in rat hippocampus. J Biomed Sci. 2010;17(1):38
43. Drexel M, Puhakka N, Kirchmair E. et al. Expression of GABA receptor subunits in the hippocampus and thalamus after experimental traumatic brain injury. Neuropharmacology. 2015;88:122-133
44. Fatemi S, Folsom T, Rooney R. et al. Expression of GABAA α2, β1 and ɛ receptors are altered significantly in the lateral cerebellum of subjects with schizophrenia, major depression and bipolar disorder. Transl Psychiatry. 2013;3(9):e303-e303
45. Liao J, Wang S, Yang H. et al. The mRNA expression levels of GABAA receptor α1 and α2 subunits in patients with major depressive disorder during onset and remission. Int J Neurosci. 2021 doi:10.1080/00207454.2020.1829618
46. Stephens D, King S, Lambert J. et al. GABAA receptor subtype involvement in addictive behaviour. Genes Brain Behav. 2016;16(1):149-184
47. Barker J, Hines R. Regulation of GABAA Receptor Subunit Expression in Substance Use Disorders. Int J Mol Sci. 2020;21(12):4445
48. Limon A, Reyes-Ruiz J, Miledi R. Loss of functional GABAA receptors in the Alzheimer diseased brain. Proc Natl Acad Sci USA. 2012;109(25):10071-10076
49. Kwakowsky A, Calvo-Flores Guzmán B, Pandya M. et al. GABAA receptor subunit expression changes in the human Alzheimer's disease hippocampus, subiculum, entorhinal cortex and superior temporal gyrus. J Neurochem. 2018;145(5):374-392
50. Luchetti S, Huitinga I, Swaab D. Neurosteroid and GABA-A receptor alterations in Alzheimer's disease, Parkinson's disease and multiple sclerosis. Neuroscience. 2011;191:6-21
51. Wang T, Zhang L, Zhang Q. et al. Involvement of lateral habenula α1 subunit-containing GABAA receptor-mediated inhibitory transmission in the regulation of depression-related behaviors in experimental Parkinson's disease. Neuropharmacology. 2017;116:399-411
52. Zhu S, Noviello C, Teng J. et al. Structure of a human synaptic GABAA receptor. Nature. 2018;559(7712):67-72
53. Masiulis S, Desai R, Uchański T. et al. Author Correction: GABAA receptor signalling mechanisms revealed by structural pharmacology. Nature. 2019;566(7744):E8-E8
54. Hedblom E, Kirkness E. A Novel Class of GABAAReceptor Subunit in Tissues of the Reproductive System. J Biol Chem. 1997;272(24):15346-15350
55. Neelands T, Macdonald R. Incorporation of the π Subunit into Functional γ-Aminobutyric Acid A Receptors. Mol Pharmacol. 1999;56(3):598-610
56. Quezada M, Henríquez S, Vargas M. et al. Proenkephalin A and the γ-aminobutyric acid A receptor π subunit: expression, localization, and dynamic changes in human secretory endometrium. Fertil Steril. 2006;86(6):1750-1757
57. Lu J, Zhang Q, Tan D. et al. GABA A receptor π subunit promotes apoptosis of HTR-8/SVneo trophoblastic cells: Implications in preeclampsia. Int J Mol Med. 2016;38(1):105-112
58. Li Y, Xiang Y, Lu W. et al. A novel role of intestine epithelial GABAergic signaling in regulating intestinal fluid secretion. Am J Physiol Gastrointest Liver Physiol. 2012;303(4):G453-G460
59. Chintagari N, Jin N, Gao L. et al. Role of GABA Receptors in Fetal Lung Development in Rats. PLoS ONE. 2010;5(11):e14171
60. Takano K, Yatabe M, Abe A. et al. Characteristic Expressions of GABA Receptors and GABA Producing/Transporting Molecules in Rat Kidney. PLoS ONE. 2014;9(9):e105835
61. Auteri M, Zizzo M, Serio R. GABA and GABA receptors in the gastrointestinal tract: from motility to inflammation. Pharmacol Res. 2015;93:11-21
62. Andres S, Brock G, Wittliff J. Interrogating differences in expression of targeted gene sets to predict breast cancer outcome. BMC Cancer. 2013;13(326):1-18
63. Fujii E, Mellon S. Regulation of Uterine γ-Aminobutyric Acid A Receptor Subunit Expression throughout Pregnancy. Endocrinology. 2001;142(5):1770-1777
64. Karvas R, McInturf S, Zhou J. et al. Use of a human embryonic stem cell model to discover GABRP, WFDC2, VTCN1 and ACTC1 as markers of early first trimester human trophoblast. Mol Hum Reprod. 2020;26(6):425-440
65. Ma X, Sun Q, Sun X. et al. Activation of GABAA Receptors in Colon Epithelium Exacerbates Acute Colitis. Front immunol. 2018;9(987):1-18
66. Boser S, Mauad T, Araújo-Paulino B. et al. Myofibroblasts are increased in the lung parenchyma in asthma. PLoS ONE. 2017;12(8):e0182378
67. Cuylen S, Blaukopf C, Politi A. et al. Ki-67 acts as a biological surfactant to disperse mitotic chromosomes. Nature. 2016;535(7611):308-312
68. Chintagari N, Liu L. GABA receptor ameliorates ventilator-induced lung injury in rats by improving alveolar fluid clearance. Crit Care. 2012;16(2):R55
69. Jin N, Kolliputi N, Gou D. et al. A Novel Function of Ionotropic γ-Aminobutyric Acid Receptors Involving Alveolar Fluid Homeostasis. J Biol Chem. 2006;281(47):36012-36020
70. Mazurkiewicz M, Opolski A, Wietrzyk J. et al. GABA level and GAD activity in human and mouse normal and neoplastic mammary gland. J Exp Clin Cancer Res. 1999;18(2):247-53
71. Wali V, Patwardhan G, Pelekanou V. et al. Identification and Validation of a Novel Biologics Target in Triple Negative Breast Cancer. Sci Rep. 2019;9(14934):1-10
72. Backus J, Laughlin T, Wang Y. et al. Identification and Characterization of Optimal Gene Expression Markers for Detection of Breast Cancer Metastasis. J Mol Diagn. 2005;7(3):327-336
73. Reinholz MM, Nibbe A, Jonart LM. et al. Evaluation of a panel of tumor markers for molecular detection of circulating cancer cells in women with suspected breast cancer. Clin Cancer Res. 2005;11:3722-3732
74. Lacroix M. Significance, detection and markers of disseminated breast cancer cells. Endocr Relat Cancer. 2006;13:1033-1067
75. Zehentner BK, Dillon DC, Jiang Y. et al. Application of a multigene reverse transcription-PCR assay for detection of mammaglobin and complementary transcribed genes in breast cancer lymph nodes. Clin Chem. 2002;48:1225-1231
76. Zehentner B, Secrist H, Hayes D. et al. Detection of Circulating Tumor Cells in Peripheral Blood of Breast Cancer Patients During or After Therapy Using a Multigene Real-Time RT-PCR Assay. Mol Diagn Ther. 2006;10(1):41-47
77. Ivanova E, Ward A, Wiegmans A. et al. Circulating Tumor Cells in Metastatic Breast Cancer: From Genome Instability to Metastasis. Front Mol Biosci. 2020;7(134):1-11
78. Sizemore G, Sizemore S, Seachrist D. et al. GABA(A) Receptor Pi (GABRP) Stimulates Basal-like Breast Cancer Cell Migration through Activation of Extracellular-regulated Kinase 1/2 (ERK1/2). J Biol Chem. 2014;289(35):24102-24113
79. Lim E, Vaillant F, Wu D. et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15:907-913
80. Christin J, Wang C, Chung C. et al. Stem Cell Determinant SOX9 Promotes Lineage Plasticity and Progression in Basal-like Breast Cancer. Cell Rep. 2020;31(10):107742
81. Paolillo M, Schinelli S. Brain infiltration by cancer cells: different roads to the same target? J cancer metastatis treat. 2015;2:90-100
82. Jin L, Han B, Siegel E. et al. Breast cancer lung metastasis: Molecular biology and therapeutic implications. Cancer Biol Ther. 2018;19(10):858-868
83. Wang S, Li G, Tan C. et al. FOXF2 reprograms breast cancer cells into bone metastasis seeds. Nat Commun. 2019;10(2707):1-16
84. Weng Y, Cui Y, Fang J. Biological Functions of Cytokeratin 18 in Cancer. Mol Cancer Res. 2012;10(4):485-493
85. Symmans WF, Fiterman DJ, Anderson SK. et al. A single-gene biomarker identifies breast cancers associated with immature cell type and short duration of prior breastfeeding. Endocr Relat Cancer. 2005;12:1059-1069
86. Kuo W, Chang Y, Lai L. et al. Molecular Characteristics and Metastasis Predictor Genes of Triple-Negative Breast Cancer: A Clinical Study of Triple-Negative Breast Carcinomas. PLoS ONE. 2012;7(9):e45831
87. Tanikawa C, Zhang Y, Yamamoto R. et al. The Transcriptional Landscape of p53 Signalling Pathway. EBioMedicine. 2017;20:109-119
88. Blommel K, Knudsen C, Wegner K. et al. Meta-analysis of gene expression profiling reveals novel basal gene signatures in MCF-10A cells transformed with cadmium. Oncotarget. 2020;11(39):3601-3617
89. Guo Y, Pan W, Liu S. et al. ERK/MAPK signalling pathway and tumorigenesis (Review). Exp Ther Med. 2020;9(3):1997-2007
90. Patterson K, Brummer T, O'brien P. et al. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J. 2009;418(3):475-489
91. Seternes O, Kidger A, Keyse S. Dual-specificity MAP kinase phosphatases in health and disease. Biochim Biophys Acta Mol Cell Res. 2019;1866(1):124-143
92. Andreadi C, Noble C, Patel B. et al. Regulation of MEK/ERK pathway output by subcellular localization of B-Raf. Biochem Soc Trans. 2012;40(1):67-72
93. Wainstein E, Seger R. The dynamic subcellular localization of ERK: mechanisms of translocation and role in various organelles. Curr Opin Cell Biol. 2016;39:15-20
94. Tanimura S, Hashizume J, Arichika N. et al. ERK signaling promotes cell motility by inducing the localization of myosin 1E to lamellipodial tips. J Cell Biol. 2016;214(4):475-489
95. Hirata E, Kiyokawa E. ERK Activity Imaging During Migration of Living Cells in vitro and In vivo. Int J Mol Sci. 2019;20(3):679
96. Busch T, Armacki M, Eiseler T. et al. Keratin 8 phosphorylation regulates keratin reorganization and migration of epithelial tumor cells. J Cell Sci. 2012;125:2148-2159
97. Zhang W, Dang E, Shi X. et al. The pro-inflammatory cytokine IL-22 up-regulates keratin 17 expression in keratinocytes via STAT3 and ERK1/2. PLoS One. 2012;7:e40797
98. Ohtsuka T, Sakaguchi M, Yamamoto H. et al. Interaction of cytokeratin 19 head domain and HER2 in the cytoplasm leads to activation of HER2-Erk pathway. Sci Rep. 2016;6(39557):1-13
99. Casar B, Crespo P. ERK Signals: Scaffolding Scaffolds? Front Cell Dev Biol. 2016;4(49):1-11
100. Zou J, Lei T, Guo P. et al. Mechanisms shaping the role of ERK1/2 in cellular sene scence (Review). Mol Med Rep. 2018;19(2):759-770
101. Khotskaya Y, Holla V, Farago A. et al. Targeting TRK family proteins in cancer. Pharmacol Ther. 2017;173:58-66
102. García-Gómez R, Bustelo X, Crespo P. Protein-Protein Interactions: Emerging Oncotargets in the RAS-ERK Pathway. Trends Cancer. 2018;4(9):616-633
103. Kwa M, Adams S. Checkpoint inhibitors in triple-negative breast cancer (TNBC): Where to go from here. Cancer. 2018;124(10):2086-2103
104. Kim NH, Sung HY, Choi EN. et al. Aberrant DNA methylation in the IFITM1 promoter enhances the metastatic phenotype in an intraperitoneal xenograft model of human ovarian cancer. Oncol Rep. 2014;31(5):2139-46
105. Li Y, Huang Y, Zhou C. et al. MiR-320c prevents the malignant development of cervical cancer by regulating GABRP level. Eur Rev Med Pharmacol Sci. 2020;24(17):8731-8739
106. Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20(1):21-37
107. Maemura K, Shiraishi N, Sakagami K. et al. Proliferative effects of γ-aminobutyric acid on the gastric cancer cell line are associated with extracellular signal-regulated kinase 1/2 activation. J Gastroenterol Hepatol. 2009;24(4):688-696
108. Rahmati N, Hoebeek F, Peter S. et al. Chloride Homeostasis in Neurons With Special Emphasis on the Olivocerebellar System: Differential Roles for Transporters and Channels. Front Cell Neurosci. 2018;12(101):1-23
109. Modi P, Komaravelli N, Singh N. et al. Interplay between MEK-ERK signaling, cyclin D1, and cyclin-dependent kinase 5 regulates cell cycle reentry and apoptosis of neurons. Mol Biol Cell. 2012;23(18):3722-3730
110. Fusté N, Fernández-Hernández R, Cemeli T. et al. Cytoplasmic cyclin D1 regulates cell invasion and metastasis through the phosphorylation of paxillin. Nat Commun. 2016;7(1):1-14
111. Ma J, Zhang Y, Wang J. et al. Proliferative effects of gamma-amino butyric acid on oral squamous cell carcinoma cells are associated with mitogen-activated protein kinase signaling pathways. Int J Mol Med. 2016;38(1):305-311
112. Yarza R, Vela S, Solas M. et al. c-Jun N-terminal Kinase (JNK) Signaling as a Therapeutic Target for Alzheimer's Disease. Front Pharmacol. 2016;6(321):1-12
113. Koul HK, Pal M, Koul S. Role of p38 MAP Kinase Signal Transduction in Solid Tumors. Genes Cancer. 2013;4(9-10):342-359
114. Huth H, Santos D, Gravina H. et al. Upregulation of p38 pathway accelerates proliferation and migration of MDA-MB-231 breast cancer cells. Oncol Rep. 2017;37(4):2497-2505
115. Goldsmith C, Kim S, Karunarathna N. et al. Inhibition of p38 MAPK activity leads to cell type-specific effects on the molecular circadian clock and time-dependent reduction of glioma cell invasiveness. BMC Cancer. 2018;18(43):1-12
116. Johnson SK, Haun RS. The gamma-aminobutyric acid A receptor pi subunit is overexpressed in pancreatic adenocarcinomas. JOP. 2005;6(2):136-42
117. Takehara A, Hosokawa M, Eguchi H. et al. Gamma-aminobutyric acid (GABA) stimulates pancreatic cancer growth through overexpressing GABAA receptor pi subunit. Cancer Res. 2007;67(20):9704-12
118. Jung KY, Cho SW, Kim YA. et al. Cancers with Higher Density of Tumor-Associated Macrophages Were Associated with Poor Survival Rates. J Pathol Transl Med. 2015;49(4):318-324
119. Gordon S, Plüddemann A. Tissue macrophages: heterogeneity and functions. BMC Biol. 2017;15(53):1-18
120. Qian B, Pollard J. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell. 2010;141(1):39-51
121. Rivera L, Bergers G. Location, Location, Location: Macrophage Positioning within Tumors Determines Pro- or Antitumor Activity. Cancer Cell. 2013;24(6):687-689
122. Dehne N, Mora J, Namgaladze D. et al. Cancer cell and macrophage cross-talk in the tumor microenvironment. Curr Opin Pharmacol. 2017;35:12-19
123. Argyle D, Kitamura T. Targeting Macrophage-Recruiting Chemokines as a Novel Therapeutic Strategy to Prevent the Progression of Solid Tumors. Front immunol. 2018;9(2629):1-15
Author contact
![]() Corresponding author: Ahmad Tarmizi Che Has, Department of Neurosciences, School of Medical Sciences, Universiti Sains Malaysia, Health Campus, 16150 Kubang Kerian, Kelantan, Malaysia. Tel.: +6097676320; Fax: +6097673833; E-mail ahmadtarmizimy. ORCID (Corresponding author): 0000-0002-3949-8243.
Corresponding author: Ahmad Tarmizi Che Has, Department of Neurosciences, School of Medical Sciences, Universiti Sains Malaysia, Health Campus, 16150 Kubang Kerian, Kelantan, Malaysia. Tel.: +6097676320; Fax: +6097673833; E-mail ahmadtarmizimy. ORCID (Corresponding author): 0000-0002-3949-8243.

 Global reach, higher impact
Global reach, higher impact