Impact Factor
ISSN: 1449-1907
Int J Med Sci 2019; 16(12):1614-1620. doi:10.7150/ijms.37040 This issue Cite
Research Paper
Incidence and hemodynamic feature of risky esophageal varices with lower hepatic venous pressure gradient
1. Department of Gastroenterology, Juntendo University, 2-1-1, Hongo, Bunkyo-ku, Tokyo, 113-8421, Japan;
2. Department of Gastroenterology, Chiba University Graduate School of Medicine, 1-8-1, Inohana, Chuo-ku, Chiba, 260-8670, Japan.
Received 2019-5-27; Accepted 2019-10-14; Published 2019-11-9
Abstract
Background: To examine the incidence of cirrhosis patients with high-risk esophageal varices (EV) who show hepatic venous pressure gradient (HVPG) < 10 mmHg and to identify their hemodynamic features.
Methods: This prospective study consisted of 110 cirrhosis patients with EV, all with the candidate for primary or secondary prophylaxis. Sixty-one patients had red sign, and 49 patients were bleeders. All patients underwent both Doppler ultrasound and HVPG measurement.
Results: There were 18 patients (16.4%) with HVPG < 10 mmHg. The presence of venous-venous communication (VVC) was more frequent in patients with HVPG < 10 mmHg (10/18) than in those with HVPG ≥ 10 mmHg (19/92; p = 0.0021). The flow volume in the left gastric vein (LGV) and the incidence of red sign were higher in the former (251.9 ± 150.6 mL/min; 16/18) than in the latter (181 ± 100.5 mL/min, p = 0.02; 45/92; p = 0.0018). The patients with red sign had lower HVPG (13.3 ± 4.5) but advanced LGV hemodynamics (velocity 13.2 ± 3.8 cm/s; flow volume 217.5 ± 126.6 mL/min), whereas those without red sign had higher HVPG (16.2 ± 4.6, p = 0.001) but poorer LGV hemodynamics (10.9 ± 2.3, p = 0.002; 160.1 ± 83.1, p = 0.02).
Conclusion: Patients with high-risk EV with HVPG < 10 mmHg showed 16.4% incidence. Although low HVPG may be underestimated by the presence of VVC, the increased LGV hemodynamics compensates for the severity of portal hypertension, which may contribute to the development of red sign.
Keywords: esophageal varices, ultrasound, left gastric vein, portal hemodynamics
Introduction
Portal hypertension is a major underlying pathogenesis of cirrhosis. It originates from increased portal inflow and/or increased outflow resistance, and the development of intra-/extrahepatic collateral vessels also affects the hemodynamic condition [1]. Consequently, cirrhosis patients suffer from various complications such as gastroesophageal and ectopic varices, hepatic encephalopathy, and ascites [2].
Esophageal varices (EV) is a major complication of cirrhosis. Its frequency is approximately 30% to 40% in compensated cirrhosis and 60% in patients with ascites [3]. It is also reported that the overall bleeding rate is about 25% over 2 years, and the mortality rate related to EV bleeding is about 20% [4, 5]. Better understanding of the pathophysiology of risky varices may be a pivotal issue for proper management against an unfavorable event [6].
The hepatic venous pressure gradient (HVPG) is a typical surrogate marker for portal pressure; significantly increased risk of complications caused by portal hypertension is associated with HVPG >10 mmHg, defined as clinically significant portal hypertension (CSPH), and severer conditions such as variceal bleeding are linked to HVPG with 12 mmHg or more [7, 8]. Moreover, the HVPG may be one of the most important parameters for patients with cirrhosis for possible prediction of their prognosis [9, 10].
However, as the HVPG value is affected by the presence of intrahepatic venous-venous communications (VVC) with the incidence from 13% to 35% in cirrhosis [11-13], it could be determined as a marker, which is subject to hemodynamic modification. Because there is such a variety of portal hemodynamics and because they are so complicated, the HVPG may not always reflect the substantial severity of portal hypertension.
Against the background, we have designed this prospective study to examine the incidence and characteristics of cirrhosis patients with high-risk EV showing HVPG < 10 mmHg, which represents a sub-CSPH condition. Further, we identified the hemodynamic features in such cases, particularly focused on the evaluation of left gastric vein (LGV), which is the main inflow route to the EV with respect to the development of red sign.
Method
Study
This is a newly designed cross-sectional study performed at our university hospital between December 2011 and September 2018. The study was approved by the ethical committee of our department as having an appropriate design for publication. The inclusion criteria of the study were as follows: (1) those diagnosed with cirrhosis by a laboratory test combined with two different imaging modalities (ultrasound [US] and computed tomography [CT]/magnetic resonance imaging [MRI]); (2) those who were candidates for primary prophylaxis, with medium- or large-grade EV, and/or any-grade EV with red sign diagnosed by endoscopy; (3) those who were candidates for secondary prophylaxis; (4) those with no history of EV treatment; (5) those with no history of beta-blocker medication (it is not approved for portal hypertension in our country); and (6) those who were scheduled for hepatic venous catheterization. If the patient decided to participate in the study, Doppler US for the assessment of portal hemodynamics was performed at the time of admission for hepatic venous catheterization, and upper gastrointestinal endoscopy was conducted following Doppler examination.
However, the study excluded the following patients: (1) those with advanced hepatocellular carcinoma (HCC) showing C/D stage by the Barcelona-Clinic Liver Cancer Staging System [14]; (2) those with portal vein obstruction caused by thrombus or tumor thrombus, cavernoma, or intrahepatic arterioportal shunt detected by US and/or CT/MRI; (3) When hepatic venography showed the findings characteristic to idiopathic portal hypertension typified by weeping willow appearance or no retrograde detection of intrahepatic portal vein [15], the patient was not included in the study because of the suspicion of idiopathic portal hypertension.; (4) those with a history of abdominal surgery, partial splenic embolization, or transjugular intrahepatic portosystemic shunt; and (5) those who were pregnant at the time of the study.
Ultrasound
The study used an SSA-770A or 790A (Toshiba, Tokyo, Japan) with a 3.75-MHz convex probe. The operator (H.M.) had more than 20 years of US experience and was blinded to the endoscopic findings. The patients underwent US examination in the supine position after fasting for 4 hours or more. After the routine observation, the portal system was carefully assessed, and the diameter and the velocity in the portal trunk and in the main part of the LGV, and in the other collaterals were measured. Briefly, the pulsed Doppler technique used the sampling width corresponding to the vessel diameter, and the blood flow was assessed at an angle < 60 degrees between the US beam and the vessel. The mean flow volume (mL/min) was calculated by determining the mean velocity for 1 second for the cross-section of the vessel and multiplying it by 60 seconds [16].
The spleen size (mm2) was determined by multiplying the distance from the splenic hilum to the caudal polar angle, measured with two intersecting lines, according to the literature [16]. The upper limit of normal used in the study was 2000 mm2. The data used for analysis were the average values, which were calculated using measurements taken 2 to 4 times.
The presence or absence or the degree of ascites was assessed based on clinical and US findings. Mild ascites was defined as that detectable only by US examination, moderate ascites was defined as that causing moderate symmetrical distention of the abdomen, and severe ascites was defined as that causing marked abdominal distension.
Endoscopy
Endoscopic examination was performed using a GIF-H260 or GIF-Q240 system (Olympus Corp., Tokyo, Japan) and was performed by either S.K. or K.K, each of whom had more than 7 years of experience and were blinded to the US findings. Gastroesophageal varices were classified as small, medium, or large [17]. In addition, the presence or absence of red sign and portal hypertensive gastropathy (PHG) were assessed [17].
The study defined EV bleeding by the presence of both of the following findings: (i) an apparent bleeding history and (ii) endoscopic evidence of active bleeding or a fibrin clot on the varices. However, even in cases without evidence of active bleeding or a fibrin clot, the varices were considered to be the source of bleeding when no other cause for gastrointestinal bleeding could be identified.
Hepatic Venous Catheterization
The study performed hepatic venous catheterization with the standard method [18]. Free and wedged hepatic venous pressure were measured in the right main hepatic vein using a balloon catheter (5 Fr, 9 mm; Terumo Clinical Supply Co. Ltd, Gifu, Japan), and the HVPG was calculated as the difference between them. The presence or absence of VVC was assessed by two independent reviewers, H.M and S.K. or K.K. When VVC was detected on the venogram, the measurement was repeated in a different position of the hepatic vein and in a different hepatic vein.
Statistical Analysis
Student's t test, analysis of variance, or Pearson product-moment correlation coefficient was used for continuous variables, and Fisher's exact test or chi-square test was used for categorical variables. A p-value <0.05 was considered statistically significant. The statistical values were calculated using SAS software (SAS Institute Inc., Cary, NC, USA).
Results
Patient Characteristics
The study included 110 cirrhosis patients (73 male, 37 female) with an average age of 62.4 years (standard deviation [SD], 12.0; range, 23-86; Table 1). The degree of EV by endoscopy was small in 13 patients, medium in 58 patients, and large in 39 patients. Sixty-one patients (55.5%) had red sign, and 49 patients (44.5%) were bleeders. Seventy-three patients had cardiac varices, and 16 had gastric fundal varices. Liver function reserve presented by Child-Pugh classification was A in 61, B in 41, and C in 8. The HVPG ranged from 2.9 to 30.3 mmHg (14.6 ± 4.8).
The diameter (mm), velocity (cm/s), and flow volume (mL/min) in the portal trunk and in the LGV are summarized in Table 2. The median interval between US examination and hepatic venous catheterization was 1 day (range, 0-3 days), between bleeding episode and US examination was 11 days (range, 0-20 days), and between bleeding episode and hepatic venous catheterization was 15 days (range, 3-29 days).
Patient characteristics
| Number | 110 |
|---|---|
| Age | 62.4 ± 12.0 (23-86) |
| Sex (Male/Female) | 73 / 37 |
| Body mass index (kg/m2) | 23.4 ± 4.0 (14.7-35.5) |
| Etiology, HCV/HBV/HBV+HCV/alcohol/NASH/PBC/ PSC/NBNC | 31 / 8 / 2 / 23 / 18 / 13 / 3 / 12 |
| Esophageal varices, n (%) | |
| Small | 13 (11.8%) |
| Medium | 58 (52.7%) |
| Large | 39 (35.5%) |
| Red sign, -/+ | 49 / 61 |
| Bleeding, -/+ | 61 / 49 |
| Cardiac varices, -/+ | 37 / 73 |
| Gastric fundal varices, -/+ | 94 / 16 |
| Portal hypertensive gastropathy, -/+ | 71 / 39 |
| Ascites, -/mild/moderate to severe | 68 / 30 / 12 |
| Splenomegaly, -/+ | 17 / 93 |
| Hepatocellular carcinoma, -/+ | 82 / 28 |
| Blood test | |
| Platelet count (×109/L) | 84.9 ± 55.8 (19-391) |
| Aspartate aminotransferase (IU/L) | 50.4 ± 42.5 (3-420) |
| Alanine aminotransferase (IU/L) | 35.3 ± 47.1 (2-501) |
| Albumin (g/dL) | 3.4 ± 0.6 (1.8-4.8) |
| Total bilirubin (mg/dL) | 1.6 ± 1.0 (0.4-8.2) |
| Prothrombin time (%) | 80 ± 15.8 (38-128) |
| Child-Pugh score | 6.8 ± 1.6 (5-13) |
| Child-Pugh classification A/B/C | 61 / 41 / 8 |
| Hepatic venous pressure gradient | 14.6 ± 4.8 (2.9-30.3) |
Data are expressed as number or mean ± standard deviation (range).
HCV hepatitis C virus, HBV hepatitis B virus, NASH non-alcoholic steatohepatitis, PBC primary biliary cholangitis, PSC primary sclerosing cholangitis, NBNC, non B non C.
Measurement data in the portal trunk and in the left gastric vein
| Diameter (mm) | Velocity (cm/s) | Flow volume (mL/min) | |
|---|---|---|---|
| Portal vein (Forward flow, n=107*) | 11.4 ± 1.6 (8.1 - 15.8) | 12.1 ± 2.4 (6.3 - 19.4) | 763.1 ± 277.8 (237.5 - 1825) |
| Left gastric vein (Reverse flow, n=85**) | 5.5 ± 1.4 (2.7 - 9.3) | 12.1 ± 3.5 (5.8 - 30.6) | 193 ± 118 (40 - 620.5) |
Data are expressed as number or mean ± standard deviation (range).
*, Three patients with portal trunk showing bidirectional flow direction were excluded.
**, Twenty-five patients were excluded (15 with no detection of left gastric vein, 5 with bidirectional flow direction, 5 with forward flow direction).
HVPG and Clinical Findings
There were 18 patients (16.4%) with HVPG < 10 mmHg (2.9-9.9, median 8.6) and 92 patients (83.6%) with HVPG ≥ 10 mmHg (10.3-30.3, median 15.1) (Table 3). Liver function reserve showed no difference between the two groups.
The presence of VVC was more frequent in patients with HVPG < 10 mmHg (10/18, 55.6 %) than in those with HGPV ≥ 10 mmHg (19/92, 20.7%; p = 0.0021) (Figure 1). The HVPG was lower in patients with VVC (n = 29; 12.6 ± 5.6 mmHg, 2.9-24.3) than in those without VVC (n = 81; 15.3 ± 4.3 mmHg, 7.4-30.3; p = 0.009). However, there was no difference in the incidence of extrahepatic portosystemic shunt between the two HVPG-related groups (5/18 vs. 30/92; p = 0.69).
As for the variceal findings, there were no differences in the degree of EV, bleeding rate, incidence of cardiac/fundal varices, and PHG between the two groups. However, red sign was more frequent in patients with HVPG < 10 mmHg (16/18, 88.9%) than in those with HVPG ≥ 10 mmHg (45/92, 48.9%; p = 0.0018). There was no significant difference in the bleeding rate in the cohort with red sign between patients with HVPG < 10 mmHg (6/16, 37.5%) and those with HVPG ≥ 10 mmHg (24/45, 53.3%; p = 0.28).
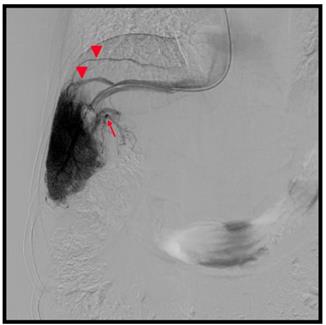
53-year old male, hepatitis C related cirrhosis. Hepatic venography showed hepatic veins (arrow heads) demonstrated via venous-venous communications. The hepatic venous pressure gradient was 9.6 mmHg. (Arrow, intrahepatic portal vein)
Comparison of clinical findings with respect to HVPG
| Hepatic venous pressure gradient | P value | ||
|---|---|---|---|
| < 10 mmHg (2.9-9.9, median 8.6) | 10 mmHg ≤ (10.3-30.3, median 15.1) | ||
| Number | 18 (16.4%) | 92 (83.6%) | |
| Age | 62.4 ± 12.5 (39-78) | 62.4 ± 12.0 (23-86) | 0.99 |
| Sex (Male/Female) | 12 / 6 | 61 / 31 | 0.98 |
| Body mass index (kg/m2) | 22.9 ± 4.3 (14.8-31) | 23.5 ± 3.9 (14.7-35.5) | 0.61 |
| Etiology, HCV/HBV/HBV+HCV/alcohol/NASH/PBC/ PSC/NBNC | 5 / 1 / 0 / 3 / 5 / 3 / 1 / 0 | 26 / 7 / 2 / 20 / 13 / 10 / 2 / 12 | 0.57 |
| Esophageal varices, n (%) | 0.78 | ||
| Small | 2 (11.2%) | 11 (12.0%) | - |
| Medium | 8 (44.4%) | 50 (54.3%) | - |
| Large | 8 (44.4%) | 31 (33.7%) | - |
| Red sign, -/+ | 2 / 16 | 47 / 45 | 0.0018 |
| Bleeding, -/+ | 11 / 7 | 50 / 42 | 0.6 |
| Cardiac varices, -/+ | 4 / 14 | 33 / 59 | 0.26 |
| Gastric fundal varices, -/+ | 16 / 2 | 78 / 14 | 0.65 |
| Portal hypertensive gastropathy, -/+ | 11 / 7 | 60 / 32 | 0.74 |
| Ascites, -/mild/moderate to severe | 11 / 6 / 1 | 57 / 24 / 11 | 0.6 |
| Splenomegaly, -/+ | 2 / 16 | 15 / 77 | 0.58 |
| Hepatocellular carcinoma, -/+ | 15 / 3 | 67 / 25 | 0.35 |
| Portal vein thrombosis, -/+ | 18 / 0 | 88 / 4 | 0.37 |
| VVC, -/+ | 8 / 10 | 73 / 19 | 0.0021 |
| Left gastric vein | (n=16) | (n=69) | |
| Diameter | 6.1 ± 1.5 (3.6-9.1) | 5.5 ± 1.2 (3.05-9.3) | 0.08 |
| Velocity | 13.4 ± 3.2 (5.85-19.25) | 12.0 ± 3.5 (5.8-30.6) | 0.13 |
| Flow volume | 251.9± 150.6 (77.5-620.5) | 181 ± 100.5 (40-497.5) | 0.02 |
| Extrahepatic portosystemic shunt, -/+ | 13 / 5* | 62 / 30** | 0.69 |
| Blood test | |||
| Platelet count (×109/L) | 63.7 ± 33.1 (29-176) | 89.1 ± 58.4 (19-391) | 0.08 |
| Aspartate aminotransferase (IU/L) | 43.6 ± 14.5 (27-87) | 51.7 ± 46 (3-420) | 0.46 |
| Alanine aminotransferase (IU/L) | 29.2 ± 11.6 (2-56) | 36.4 ± 51.2 (9-501) | 0.55 |
| Albumin (g/dL) | 3.6 ± 0.5 (2.6-4.5) | 3.3 ± 0.5 (1.8-4.8) | 0.06 |
| Total bilirubin (mg/dL) | 1.5 ± 0.6 (0.9-2.7) | 1.6 ± 1.1 (0.4-8.2) | 0.7 |
| Prothrombin time (%) | 81.5 ± 11.9 (64-106) | 79.7 ± 16.5 (38-128) | 0.66 |
| Child-Pugh score | 6.3 ± 1.1 (5-9) | 6.8 ± 1.7 (5-13) | 0.2 |
| Child-Pugh classification A/B/C | 11 / 7 / 0 | 50 / 34 / 8 | 0.43 |
Data are expressed as number or mean ± standard deviation (range).
HCV hepatitis C virus, HBV hepatitis B virus, NASH non-alcoholic steatohepatitis, PBC primary biliary cholangitis, PSC primary sclerosing cholangitis, NBNC, non B non C, VVC venous-venous communications (assessment by hepatic venogram).
*, splenorenal shunt 4 and short gastric vein 1.
**, splenorenal shunt 19, short gastric vein 10 (1 with splenorenal shunt), and inferior mesenteric vein 4 (1 with splenorenal shunt, 1 with short gastric vein).
Correlation between HVPG and LGV parameters. The diameter and velocity in the LGV showed no correlation with HVPG. However, the flow volume in the LGV and the HVPG showed mild negative correlation (r = -0.21, p = 0.05). A, Diameter and HVPG (r = -0.13, p = 0.24). B, Velocity and HVPG (r = -0.18, p = 0.98). C, Flow volume and HVPG (r = -0.21, p=0.05). HVPG, hepatic venous pressure gradient; LGV, left gastric vein.

Relationship between HVPG and LGV Parameters With Respect to Clinical Findings
The LGV was successfully detected in 95 patients (95/110, 86.4%); 85 with reverse flow direction, 5 with bidirectional flow, and 5 with forward flow direction. Therefore, the LGV hemodynamics was assessed in the 85 patients with LGV showing reverse flow direction (52/85 with positive red sign on EV). The LGV flow volume showed difference between patients with HVPG < 10 mmHg (251.9 ± 150.6 mL/min) and those with HVPG ≥10 mmHg (181 ± 100.5 mL/min; p = 0.02), although the diameter and the velocity showed no difference (Table 3). The HVPG and the flow volume in the LGV showed mild negative correlation (r = -0.21) with marginal difference (p = 0.05; Figure 2).
Comparison of clinical findings between patients with and without red sign
| Red sign | P value | ||
|---|---|---|---|
| - N=49 | + N=61 | ||
| Esophageal varices Small/Medium/Large | 10/31/8 | 3/27/31 | 0.0003 |
| Bleeding, -/+ | 30 / 19 | 31 / 30 | 0.28 |
| VVC, -/+ | 39 / 10 | 42 / 19 | 0.2 |
| HVPG (mmHg) | 16.2 ± 4.6 (3.7-30.3) | 13.3 ± 4.5 (2.9-22.4) | 0.001 |
| Left gastric vein | (n=33) | (n=52) | |
| Diameter | 5.4 ± 1.3 (3.05-9.3) | 5.7 ± 1.3 (3.5-9.1) | 0.38 |
| Velocity | 10.9 ± 2.3 (5.85-16.1) | 13.2 ± 3.8 (5.8-30.6) | 0.002 |
| Flow volume | 160.1± 83.1 (40-453) | 217.5 ± 126.6 (40-620.5) | 0.02 |
| Child-Pugh score | 6.9 ± 1.9 (5-13) | 6.6 ± 1.4 (5-12) | 0.4 |
| Child-Pugh classification A/B/C | 27 / 16 / 6 | 34 / 25 / 2 | 0.17 |
Data are expressed as number or mean ± standard deviation (range).
VVC, venous-venous communications (assessment by hepatic venogram).
HVPG, hepatic venous pressure gradient.
There was a significant difference in the degree of EV (p = 0.0003) between patients with and without red sign (Table 4). The patients with red sign had lower HVPG (13.3 ± 4.5 mmHg) but advanced LGV hemodynamics (velocity 13.2 ± 3.8 cm/s; flow volume 217.5 ± 126.6 mL/min), whereas those without red sign had higher HVPG (16.2 ± 4.6 mmHg, p = 0.001) but poorer LGV hemodynamics (10.9 ± 2.3, p = 0.002; 160.1 ± 83.1, p = 0.02), although the LGV diameter showed no difference (5.4 ± 1.3 mm vs. 5.7 ± 1.3 mm; p = 0.38). There were no differences in the clinical background, variceal findings, or portal parameters between bleeders and nonbleeders (Table 5).
Comparison of clinical findings between bleeders and non-bleeders
| Bleeding | P value | ||
|---|---|---|---|
| - (n=61) | + (n=49) | ||
| Esophageal varices Small/Medium/Large | 7/30/24 | 6/28/15 | 0.63 |
| Red sign, -/+ | 30 / 31 | 19 / 30 | 0.28 |
| VVC, -/+ | 43 / 18 | 38 / 11 | 0.4 |
| HVPG (mmHg) | 14.7 ± 5.1 (2.9-30.3) | 14.4 ± 4.4 (3.7-23.5) | 0.7 |
| Left gastric vein | (n=51) | (n=34) | |
| Diameter | 5.7 ± 1.3 (3.5-9.3) | 5.5 ± 1.4 (3.05-9.1) | 0.55 |
| Velocity | 12.0 ± 3.9 (5.8-30.6) | 12.7 ± 2.6 (7.65-18.35) | 0.4 |
| Flow volume | 192.8± 108 (40-497.5) | 197.6 ± 124.9 (40-620.5) | 0.85 |
| Child-Pugh score | 6.6 ± 1.7 (5-13) | 6.9 ± 1.5 (5-11) | 0.3 |
| Child-Pugh classification A/B/C | 37 / 19 / 5 | 24 / 22 / 3 | 0.33 |
Data are expressed as number or mean ± standard deviation (range).
VVC, venous-venous communications (assessment by hepatic venogram).
HVPG, hepatic venous pressure gradient.
Discussion
Doubtlessly, the HVPG is a popular and representative marker for the severity of portal hypertension. However, as shown in the previous reports, there is a certain incidence of patients with EV showing lower HVPG [19-21]. The present study may be the first to focus on the clinical features of high-risk EV with sub-CSPH condition, which showed 16.4% incidence with median HVPG value of 8.6 mmHg. Because of the high detectability of VVC (55.6%), although it is unavoidable with hepatic venous catheterization, the HVPG may be underestimated in these patients.
Our study demonstrated a unique hemodynamic feature in patients with high-risk sub-CSPH EV, which is an increased flow volume in the LGV, which may compensate for the severity of portal hypertension against lower HVPG. The close linkage between the LGV hemodynamics and the development of red sign has not been discussed elsewhere and may support the presence of risky varices even under the lower HVPG condition. A significant difference in the flow volume between the two HVPG-related groups may suggest the substantial hemodynamic effect of the LGV because it reflects both the diameter and velocity according to the calculation formula. Needless to say, the fundamental research base of the present work is the scientific evidence of more than 80% detectability of the LGV by US in patients with EV [22]. The present study may enhance the practical application of Doppler sonography as a noninvasive tool to predict patients with high-risk EV accompanied with red sign.
The incidence of red sign is reported to be 45.4% with small varices, 65.0% with mid-size varices, and significant factors showing a close correlation with red sign are the number of varices, size of varices, platelet count, and alpha-fetoprotein level [23]. However, because no study had been performed regarding the influence of hemodynamics on the red sign, the present study first demonstrates the substantial effects of velocity and flow volume in the LGV on the development of red sign.
A red sign is considered to be related to wall thickness, which determines the wall tension of varices [1]. Vascular response to the shared stress caused by a change in velocity is a well-known physiological phenomenon [24, 25], and increased flow velocity results in endothelial damage in the arterial system [26]. However, there may be some common pathophysiologic conditions in the arterial and the portal venous system that result in the formation of red sign. In addition, the present study presented a trade-off like relationship between HVPG and LGV hemodynamics with respect to the presence of red sign, which may suggest the importance of local but not systemic factors for the formation of red sign.
Variceal bleeding occurs when the tension exerted by the variceal wall exceeds the rupture point [1], and it is unlikely to occur unless the HVPG exceeds 12 mmHg [27, 28]. However, there is an argument about the relationship between HVPG and variceal bleeding because HVPG did not differ between patients with previous bleeds and those without bleeds with HCV-related or alcoholic cirrhosis reported by Bellis et al [29]. The present study also showed no significant difference in the HVPG between bleeders and nonbleeders. However, the data may not deny the role of HVPG because all subjects in our study were candidates for prophylactic treatment, which is, having a potentially severe condition of portal hypertension. A bleeding and/or red sign of EV may occur with additional factors, even in patients with HVPG less than 10 mmHg; advanced hemodynamics in the LGV may be one of the factors.
The major limitation of our study is that there is a potential bias regarding the patient cohort. That is, the study required HVPG data as an important parameter for severity of portal hypertension. Because hepatic venous catheterization is the standard procedure before variceal treatment in our institution, the study did include patients with advanced varices alone. Further studies may be necessary to elucidate our data in a larger cohort, including patients with small EV without red sign who are not candidates for prophylactic treatment. Another limitation is that the study did not examine the relationship between the liver stiffness and the EV with lower HVPG, which needs to be clarified sometime soon.
In conclusion, there was 16.4% incidence of high-risk EV with sub-CSPH condition. Although low HVPG may be underestimated by the presence of VVC, the increased LGV hemodynamics compensate for the severity of portal hypertension, which may contribute to the development of red sign. The actual incidence and natural history of such patients in the cirrhosis cohort need to be elucidated in the future.
Abbreviations
EV: esophageal varices; HVPG: hepatic venous pressure gradient; CSPH: clinically significant portal hypertension; VVC: venous-venous communications; LGV: left gastric vein; US: ultrasound; CT: computed tomography; MRI: magnetic resonance imaging; HCC: hepatocellular carcinoma; PHG: portal hypertensive gastropathy; SD: standard deviation.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sanyal AJ, Bosch J, Blei A, Arroyo V. Portal hypertension and Its complications. Gastroenterology. 2008;134:1715-28
2. Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-61
3. Turon F, Casu S, Hernandez-Gea V, Garcia-Pagán JC. Variceal and other portal hypertension related Bleeding. Best Pract. Res. Clin Gastroenterol. 2013;27:649-64
4. D'Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis. 1999;19:475-505
5. Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823-32
6. Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-35
7. Ripoll C, Groszmann R, Garcia-Tsao G. et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481-8
8. Abraldes JG, Villanueva C, Banares R. et al. Hepatic venous pressure gradient and prognosis in patients with acute variceal bleeding treated with pharmacologic and endoscopic therapy. J Hepatol. 2008;48:229-36
9. Ripoll C, Bañares R, Rincón D. et al. Influence of hepatic venous pressure gradient on the prediction of survival of patients with cirrhosis in the MELD era. Hepatology. 2005;42:793-801
10. de Franchis R. Revising consensus in portal hypertension: Report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762-8
11. Keiding S, Vilstrup H. Intrahepatic heterogeneity of hepatic venous pressure gradient in human cirrhosis. Scand J Gastroenterol. 2002;37:960-4
12. Osada Y, Kanazawa H, Narahara Y, Mamiya Y, Natsuhisa N, Sakamoto C. Wedged hepatic venous pressure does not reflect portal pressure in patients with cirrhosis and heaptic veno-venous communications. Dig Dis Sci. 2008;53:7-13
13. Sakaguchi T, Suzuki S, Inaba K. et al. Analysis of intrahepatic venovenous shunt by hepatic venography. Surgery. 2010;147:805-10
14. Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519-24
15. Futagawa S, Fukazawa M, Musha H. et al. Hepatic venography in noncirrhotic idiopathic portal hypertension. Comparison with cirrhosis of the liver. Radiology. 1981;141:303-9
16. Maruyama H, Kamezaki H, Kondo T. et al. Sonographic and clinical features of collateral vessels at the splenic hilum in cirrhosis. Clin Radiol. 2014;69:e140-5
17. Tajiri T, Yoshida H, Obara K. et al. General rules for recording endo-scopic findings of esophagogastric varices (2nd edition). Dig Endosc. 2010;22:1-9
18. Bosch J, Abraldes JG, Berzigotti A. et al. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573-82
19. Wadhawan M, Dubey S, Sharma BC, Sarin SK. Hepatic venous pressure gradient in cirrhosis: correlation with the size of varices, bleeding, ascites, and Child's status. Dig Dis Sci. 2006;51:2264-9
20. Colecchia A, Montrone L, Scaioli E. et al. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology. 2012;143:646-54
21. Cucchetti A, Cescon M, Golfieri R. et al. Hepatic venous pressure gradient in the preoperative assessment of patients with resectable hepatocellular carcinoma. J Hepatol. 2016;64:79-86
22. Maruyama H, Kobayashi K, Kiyono S. et al. Left gastric vein-based noninvasive test for esophageal varices: a same-day comparison of portal hemodynamic assessment with endoscopic appearance. Clin Transl Gastroenterol. 2018;9:154
23. Murachima N, Ikeda K, Kobayashi M. et al. Incidence of the appearance of the red color sign on esophageal varices and its predictive factors: long-term observations of 359 patients with cirrhosis. J Gastroenterol. 2001;36:368-74
24. Rodbard S. Vascular caliber. Cardiology. 1975;60:4-49
25. Kamiya A, Togawa T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol. 1980;239:H14-H21
26. Fry DL. Acute vascular endothelial changes associated with increased blood velocity gradient. Circ Res. 1968;22:165-97
27. Groszmann RJ, Garcia-Tsao G, Bosch J. et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254-61
28. Garcia-Tsao G, Groszmann RJ, Fisher RL, Conn HO, Atterbury CE, Glickman M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology. 1985;5:419-24
29. Bellis L, Castellacci R, Montagnese F, Festuccia F, Corvisieri P, Puoti C. Hepatic venous pressure gradient determination in patients with hepatitis C virus-related and alcoholic cirrhosis. Eur J Gastroenterol Hepatol. 2003;15:1085-9
Author contact
![]() Corresponding author: Hitoshi Maruyama, Department of Gastroenterology, Juntendo University, 2-1-1, Hongo, Bunkyo-ku, Tokyo, 113-8421, Japan. TEL: +81-3-38133111; Fax: +81-3-56845960; E-mail: h.maruyama.twac.jp
Corresponding author: Hitoshi Maruyama, Department of Gastroenterology, Juntendo University, 2-1-1, Hongo, Bunkyo-ku, Tokyo, 113-8421, Japan. TEL: +81-3-38133111; Fax: +81-3-56845960; E-mail: h.maruyama.twac.jp

 Global reach, higher impact
Global reach, higher impact