Impact Factor
ISSN: 1449-1907
Int J Med Sci 2019; 16(11):1504-1509. doi:10.7150/ijms.38605 This issue Cite
Research Paper
Rhabdomyolysis Associations with Antibiotics: A Pharmacovigilance Study of the FDA Adverse Event Reporting System (FAERS)
1. Pharmacotherapy Division, College of Pharmacy, The University of Texas at Austin, San Antonio, TX, USA
2. Pharmacotherapy Education and Research Center, Long School of Medicine, The University of Texas Health Science Center at San Antonio, San Antonio, TX, USA
3. Health Outcomes Division, College of Pharmacy, The University of Texas at Austin, Austin, TX, USA
4. South Texas Veterans Health Care System, San Antonio, TX, USA
5. University Health System, San Antonio, TX, USA
Received 2019-7-21; Accepted 2019-9-5; Published 2019-10-15
Abstract
Introduction: Daptomycin, macrolides, trimethoprim-sulfamethoxazole, linezolid, fluoroquinolones, and cefdinir are known to be associated with rhabdomyolysis. Other antibiotics may also lead to rhabdomyolysis, but no study has systemically compared rhabdomyolysis associations for many available antibiotics.
Objectives: The objective of this study was to evaluate the association between rhabdomyolysis and many available antibiotics using the FDA Adverse Event Report System (FAERS).
Methods: FAERS reports from January 1, 2004 to December 31, 2017 were included in the study. The Medical Dictionary for Regulatory Activities (MedDRA) was used to identify rhabdomyolysis cases. Reporting Odds Ratios (RORs) and corresponding 95% confidence intervals (95%CI) for the association between antibiotics and rhabdomyolysis were calculated. An association was considered statistically significant when the lower limit of the 95%CI was greater than 1.0.
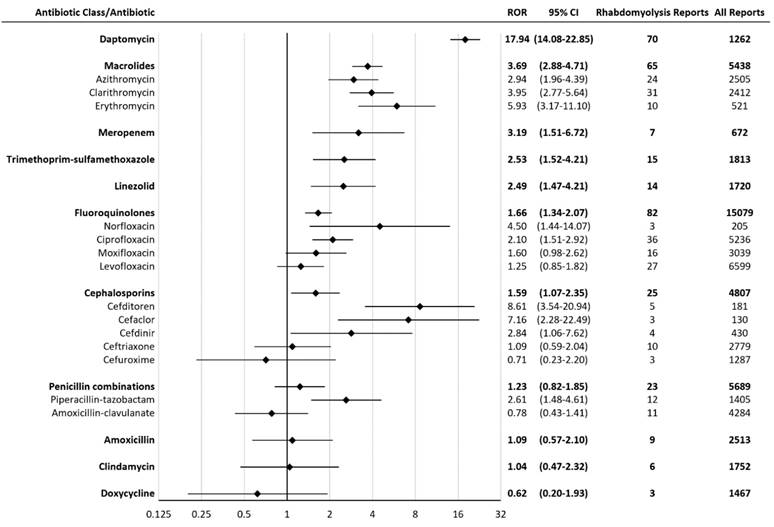
Results: A total of 2,334,959 reports (including 7,685 rhabdomyolysis reports) were considered, after inclusion criteria were applied. Daptomycin had the greatest proportion of rhabdomyolysis reports, representing 5.5% of all daptomycin reports. Statistically significant rhabdomyolysis RORs (95% CI) for antibiotics were (in descending order): daptomycin 17.94 (14.08-22.85), cefditoren 8.61 (3.54-20.94), cefaclor 7.16 (2.28-22.49), erythromycin 5.93 (3.17-11.10), norfloxacin 4.50 (1.44-14.07), clarithromycin 3.95 (2.77-5.64), meropenem 3.19 (1.51-6.72), azithromycin 2.94 (1.96-4.39), cefdinir 2.84 (1.06-7.62), piperacillin-tazobactam 2.61 (1.48-4.61), trimethoprim-sulfamethoxazole 2.53 (1.52-4.21), linezolid 2.49 (1.47-4.21), ciprofloxacin 2.10 (1.51-2.92).
Conclusions: This study confirms prior evidence for rhabdomyolysis associations with daptomycin, macrolides, trimethoprim-sulfamethoxazole, linezolid, fluoroquinolones, and cefdinir. This study also identifies previously unknown rhabdomyolysis associations with meropenem, cefditoren, cefaclor, and piperacillin-tazobactam.
Keywords: rhabdomyolysis, adverse drug events, antibiotics, antimicrobial stewardship
Introduction
Rhabdomyolysis is a clinical syndrome of skeletal muscle injury associated with myoglobinuria, electrolyte abnormalities, and acute kidney injury [1]. The severity of rhabdomyolysis can range from an asymptomatic elevation in creatinine kinase (CK) to severe life-threatening symptoms associated with high CK levels and renal failure. The most common presenting symptoms include myalgia, weakness, and tea-colored urine.
Some antibiotics have been shown to play a role in developing rhabdomyolysis. Multiple case reports have shown an association between daptomycin and rhabdomyolysis [2-4]. The use of macrolides alone or in combination with statins was reported to be related to rhabdomyolysis [5,6]. A review of 53 cases showed rhabdomyolysis associated with concomitant use of statins and azithromycin [7]. Additionally, case reports indicated that trimethoprim-sulfamethoxazole induced rhabdomyolysis occurred in both immunocompetent and immunocompromised patients [8,9]. There have been case reports linking the use of linezolid to serious cases of rhabdomyolysis [10,11]. Fluoroquinolones have also been shown to be associated with rhabdomyolysis in case reports when fluoroquinolones were used alone or in combination with statins [12,13]. Rhabdomyolysis is listed as an adverse drug reaction (ADR) of cefdinir in its United States Food and Drug Administration (FDA) label [14]. Rhabdomyolysis was also associated with certain medications, such as statins [15].
Antibiotics are frequently prescribed home medications, so it is imperative to know which classes and specific agents within classes are more likely to be associated with rhabdomyolysis. Although some antibiotics are known to be associated with rhabdomyolysis, no study has systematically compared rhabdomyolysis associations for common antibiotics. The objective of this study was to evaluate the association between rhabdomyolysis and common antibiotics using the FDA Adverse Event Report System (FAERS).
Methods
Data Source
FAERS is a publicly available database, which is composed of adverse event reports that were submitted to the FDA [16]. FAERS data contain drug information (drug name, active ingredient, route of administration, the drug's reported role in the event) and reaction information. Each report has a primary suspected drug with one or more ADRs and may include other drugs taken by the patient.
Study Design
The methods of data mining were previously used in other published FAERS studies [17-19]. FAERS data from January 1, 2004 to December 31, 2017 were included in the study. Some reports were submitted to FDA multiple times with updated information. Therefore, duplicate reports were removed by case number, with only the most recently submitted version included in the study. Another step of removing duplicate reports was performed by matching age, sex, event date, and reporter country.
Drug Exposure Definition
Each antibiotic was identified in FAERS by generic and brand names listed in the Drugs@FDA Database [17-20]. Drugs with a reported role coded as “PS” (Primary Suspect Drug) were evaluated for inclusion. Antibiotics with less than three ADR reports were excluded from data analysis [21].
Adverse Drug Reaction Definition
FAERS defines ADRs using Preferred Terms (PT) from the Medical Dictionary for Regulatory Activities (MedDRA) [22]. Preferred Term “Rhabdomyolysis” was used to identify rhabdomyolysis cases. Standardised MedDRA Queries (SMQs) are groupings of MedDRA terms, usually at the PT level, which relate to an adverse drug reaction. Hepatic disorders (SMQ) was used to identify cases with hepatic disorders. Acute renal failure (SMQ) and the term “Renal failure acute” were used to identify acute kidney injury cases.
Statistical Analysis
A disproportionality analysis was conducted by computing Reporting Odds Ratios (ROR) and corresponding 95% confidence intervals (95%CI) for the association between rhabdomyolysis and each antibiotic class or individual antibiotic or each statin [23]. ROR was calculated as the ratio of the odds of reporting rhabdomyolysis versus all other ADRs for a given drug, compared with this reporting odds for all other drugs present in FAERS [23]. An association was considered to be statistically significant if the lower limit of 95%CI was above 1.0 [23]. A higher ROR suggested a stronger association between the antibiotic and rhabdomyolysis. An adjusted ROR was calculated after removing reports of potentially confounding statins from the data analysis. These drugs include atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, and simvastatin. Rhabdomyolysis RORs for meropenem reports with hepatic disorders and without hepatic disorders were calculated. Rhabdomyolysis RORs for piperacillin-tazobactam reports with acute kidney injury and without acute kidney injury were computed. Data analysis was performed using Microsoft Access 2016, Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA), SAS 9.4, and JMP Pro 13.2.1 (SAS Institute, Cary, NC).
Results
A total of 2,334,959 reports (including 7,685 rhabdomyolysis reports) were considered, after inclusion criteria were applied. Daptomycin had the greatest proportion of rhabdomyolysis reports, representing 5.5% of all daptomycin reports. Statistically significant rhabdomyolysis RORs (95% CI) for antibiotics were (in descending order): daptomycin 17.94 (14.08-22.85), cefditoren 8.61 (3.54-20.94), cefaclor 7.16 (2.28-22.49), erythromycin 5.93 (3.17-11.10), norfloxacin 4.50 (1.44-14.07), clarithromycin 3.95 (2.77-5.64), meropenem 3.19 (1.51-6.72), azithromycin 2.94 (1.96-4.39), cefdinir 2.84 (1.06-7.62), piperacillin-tazobactam 2.61 (1.48-4.61), trimethoprim-sulfamethoxazole 2.53 (1.52-4.21), linezolid 2.49 (1.47-4.21), ciprofloxacin 2.10 (1.51-2.92) (Figure 1). Meropenem had seven rhabdomyolysis reports, including four reports with hepatic disorders. Rhabdomyolysis RORs (95% CI) for meropenem reports with hepatic disorders and without hepatic disorders were 9.77 (3.61-26.46) and 1.68 (0.54-5.23), respectively. Cefditoren had five rhabdomyolysis reports, including one report of 5-year-old girl with decreased carnitine level. Cefaclor had three rhabdomyolysis reports, including two reports on concomitant Nonsteroidal Anti-Inflammatory Drugs (NSAIDS), which were indomethacin and flurbiprofen. Piperacillin-tazobactam had twelve rhabdomyolysis reports, including three reports with acute kidney injury. Rhabdomyolysis RORs (95% CI) for piperacillin-tazobactam reports with acute kidney injury and without acute kidney injury were 4.91 (1.57-15.37) and 2.26 (1.17-4.35), respectively.
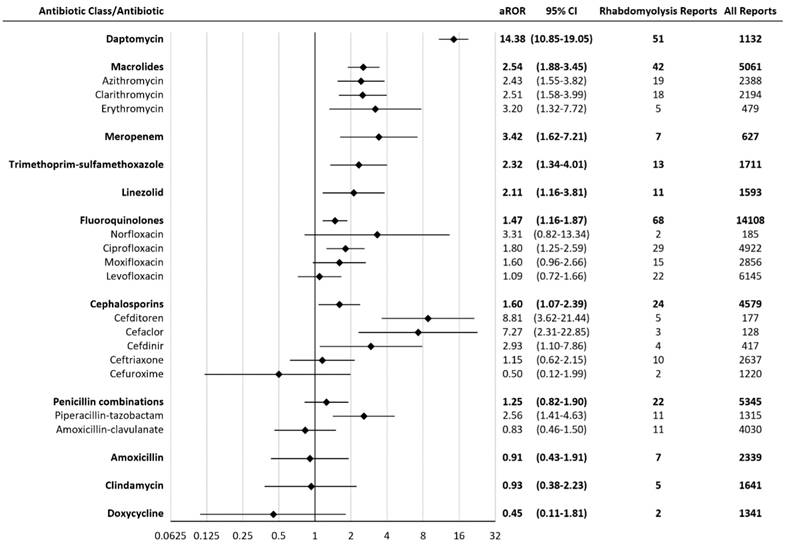
An adjusted ROR was calculated after removing reports of concomitant statins. The adjusted RORs for agents with a significant association with rhabdomyolysis were: daptomycin 14.38 (10.85-19.05), cefditoren 8.81 (3.62-21.44), cefaclor 7.27 (2.31-22.85), meropenem 3.42 (1.62-7.21), erythromycin 3.20 (1.32-7.72), cefdinir 2.93 (1.10-7.86), piperacillin-tazobactam 2.56 (1.41-4.63), clarithromycin 2.51 (1.58-3.99), azithromycin 2.43 (1.55-3.82), trimethoprim-sulfamethoxazole 2.32 (1.34-4.01), linezolid 2.11 (1.16-3.81), ciprofloxacin 1.80 (1.25-2.59) (Figure 2). The ROR association rank had little change when adjusted to exclude statins.
All statins were significantly associated with rhabdomyolysis. RORs (95% CI) for statins were (in descending order): simvastatin 76.29 (70.92-82.06), lovastatin 50.95 (34.59-75.05), pitavastatin 38.92 (27.86-54.35), fluvastatin 24.51 (16.89-35.58), pravastatin 23.66 (17.83-31.40), rosuvastatin 22.94 (20.75-25.37), atorvastatin 15.09 (13.77-16.53).
Reporting Odds Ratios (RORs) for rhabdomyolysis with antibiotics. CI = confidence interval.
Adjusted Reporting Odds Ratios (aRORs) for rhabdomyolysis with antibiotics. CI = confidence interval. All statins were significantly associated with rhabdomyolysis. Rhabdomyolysis RORs (95% CI) for statins range from atorvastatin 15.09 (13.77-16.53) to simvastatin 76.29 (70.92-82.06).
Discussion
Our results demonstrated significant rhabdomyolysis associations (from strongest to weakest) with daptomycin, cefditoren, cefaclor, erythromycin, norfloxacin, clarithromycin, meropenem, azithromycin, cefdinir, piperacillin-tazobactam, trimethoprim-sulfamethoxazole, linezolid, and ciprofloxacin. Our results confirmed previously known rhabdomyolysis associations with daptomycin, macrolides, trimethoprim-sulfamethoxazole, linezolid, fluoroquinolones, and cefdinir [2-14]. Meropenem, cefditoren, cefaclor, and piperacillin-tazobactam were found to be associated with rhabdomyolysis, which were not reported in the literature. In a case report, meropenem induced prolonged severe hypokalemia and hypomagnesaemia, leading to chronic muscle weakness [24]. Meropenem-induced hypokalemia and hypomagnesaemia might play a role in meropenem-induced muscle injury and rhabdomyolysis. In our study, four out of seven meropenem-associated rhabdomyolysis reports had hepatic disorders. Rhabdomyolysis ROR for meropenem reports with hepatic disorders was higher than those without hepatic disorders. Since meropenem is hepatically metabolized [25], hepatic disorders might be a risk factor for meropenem-induced rhabdomyolysis.
A case report demonstrated that cefditoren pivoxil induced hypocarnitinemia and hypoglycemia in a 6-year-old girl with Fukuyama-type congenital muscular dystrophy [26]. In our study, a 5-year-old girl on cefditoren had both rhabdomyolysis and decreased carnitine level. Although there were commonalities between these two cases, more data would be necessary prior to making any conclusions between hypocarnitemia and rhabdomyolysis. In addition, hypocarnitemia may be a sign of Long-Chain 3-Hydroxyacyl-Coenzyme A Dehydrogenase (LCHAD) deficiency which can cause rhabdomyolysis. In this respect, it is possible that the cefditoren did not cause the rhabdomyolysis, but these patients had underlying illness that caused them to have rhabdomyolysis.
There were three cefaclor-associated rhabdomyolysis reports in our study. Two of these reports had concomitant NSAIDS. NSAIDS are known to be associated with acute kidney injury [27] and acute kidney injury might increase the cefaclor exposure which led to rhabdomyolysis. However, since acute kidney injury was used to diagnose rhabdomyolysis, the association between rhabdomyolysis and cefaclor was confounded by acute kidney injury.
In our study, twelve piperacillin-tazobactam reports listed rhabdomyolysis as an ADR. Three of these reports had acute kidney injury as well. Rhabdomyolysis ROR for piperacillin-tazobactam reports with and without acute kidney injury were statistically similar.
Limitations
A causal relationship between a drug and an ADR cannot be determined by FAERS. Significant bias may occur because of the spontaneous and voluntary reporting of ADRs. Media attention and recent publication of an ADR in the literature might affect the reporting behaviors. The association between a drug and an ADR is confounded by comorbidities and concomitant drugs [19]. Despite the limitations, FAERS has a large sample size and is suitable for discovering new and rare drug-ADR associations.
Conclusions
This study confirms prior evidence for rhabdomyolysis associations with daptomycin, macrolides, trimethoprim-sulfamethoxazole, linezolid, fluoroquinolones, and cefdinir. This study also identifies previously unknown rhabdomyolysis association with meropenem, cefditoren, cefaclor, and piperacillin-tazobactam. Results obtained from FAERS should be interpreted with caution in the context of data limitations. Antibiotic stewardship is needed to prevent rhabdomyolysis and to improve health outcomes. Monitoring of baseline and weekly CK levels for daptomycin and other antibiotics which were associated rhabdomyolysis is warranted.
Abbreviations
ADR: adverse drug reaction; CK: creatinine kinase; CI: confidence interval; FDA: Food and Drug Administration; FAERS: FDA Adverse Event Reporting System; MedDRA: Medical Dictionary for Regulatory Activities; NSAIDS: Nonsteroidal Anti-Inflammatory Drugs; PT: Preferred Term; ROR: Reporting Odds Ratio; SMQ: Standardised MedDRA Queries.
Acknowledgements
No funding was sought for this research study. Dr. Frei was supported, in part, by a NIH Clinical and Translational Science Award (National Center for Advancing Translational Sciences, UL1 TR001120, UL1 TR002645, and TL1 TR002647) while the study was being conducted. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs, the National Institutes of Health, or the authors' affiliated institutions.
Authors' contributions
Study concept and design: Teng, Frei. Statistical analysis: Teng. Interpretation of data: All authors. Drafting of the manuscript: Teng. Critical revision of the manuscript for important intellectual content: All authors. Study supervision: Frei.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Zimmerman JL, Shen MC. Rhabdomyolysis. Chest. 2013;144(3):1058-65
2. Kazory A, Dibadj K, Weiner ID. Rhabdomyolysis and acute renal failure in a patient treated with daptomycin. J Antimicrob Chemother. 2006;57(3):578-9
3. Papadopoulos S, Ball AM, Liewer SE, Martin CA, Winstead PS, Murphy BS. Rhabdomyolysis during therapy with daptomycin. Clin Infect Dis. 2006;42(12):e108-10
4. Patel SJ, Samo TC, Suki WN. Early-onset rhabdomyolysis related to daptomycin use. Int J Antimicrob Agents. 2007;30(5):472-4
5. Brener ZZ, Bilik I, Khorets B, Winchester JF, Bergman M. Rhabdomyolysis following clarithromycin monotherapy. Am J Med Sci. 2009;338(1):78
6. Patel AM, Shariff S, Bailey DG, Juurlink DN, Gandhi S, Mamdani M. et al. Statin toxicity from macrolide antibiotic coprescription: a population-based cohort study. Ann Intern Med. 2013;158(12):869-76
7. Strandell J, Bate A, Hagg S, Edwards IR. Rhabdomyolysis a result of azithromycin and statins: an unrecognized interaction. Br J Clin Pharmacol. 2009;68(3):427-34
8. Ainapurapu B, Kanakadandi UB. Trimethoprim-sulfamethoxazole induced rhabdomyolysis. Am J Ther. 2014;21(3):e78-9
9. Jen SP, Sharma R. Trimethoprim-sulphamethoxazole-associated rhabdomyolysis in an HIV-infected patient. Int J STD AIDS. 2011;22(7):411-2
10. Carroll MW, Choi H, Min S, Hwang S, Park H, Song T. et al. Rhabdomyolysis in a patient treated with linezolid for extensively drug-resistant tuberculosis. Clin Infect Dis. 2012;54(11):1624-7
11. Lechner AM, Past E, Porsche U, Kern JM, Hoppe U, Pretsch I. Two cases of serious rhabdomyolysis during linezolid treatment. Infection. 2017;45(4):563-6
12. John F, Oluronbi R, Pitchumoni CS. Levofloxacin-induced rhabdomyolysis: a case report. J Med Case Rep. 2016;10(1):235
13. Goldie FC, Brogan A, Boyle JG. Ciprofloxacin and statin interaction: a cautionary tale of rhabdomyolysis. BMJ Case Rep. 2016:2016
14. Cefdinir [package insert]. Morris Plains, NJ: Parke-Davis; 1999. Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/1999/50739S2LBL.PDF. Accessed May 26. 2019
15. Omar MA, Wilson JP. FDA adverse event reports on statin-associated rhabdomyolysis. Ann Pharmacother. 2002;36(2):288-95
16. Food and Drug Administration. FDA Adverse Event Reporting System (FAERS). Available from https://www.fda.gov/drugs/surveillance/fda-adverse-event-reporting-system-faers. Accessed May 26. 2019
17. Evoy KE, Teng C, Encarnacion VG, Frescas B, Hakim J, Saklad S. et al. Comparison of quetiapine abuse and misuse reports to the FDA Adverse Event Reporting System with other second-generation antipsychotics. Subst Abuse. 2019;13:1178221819844205
18. Teng C, Reveles KR, Obodozie-Ofoegbu OO, Frei CR. Clostridium difficile infection risk with important antibiotic classes: an analysis of the FDA Adverse Event Reporting System. Int J Med Sci. 2019;16(5):630-5
19. Teng C, Walter EA, Gaspar DKS, Obodozie-Ofoegbu OO, Frei CR. Torsades de pointes and QT prolongation associations with antibiotics: a pharmacovigilance study of the FDA Adverse Event Reporting System. Int J Med Sci. 2019;16(7):1018-22
20. Food and Drug Administration. Drugs@FDA: FDA Approved Drug Products. Available from https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed May 26. 2019
21. Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001;10(6):483-6
22. MedDRA MSSO. Introductory Guide for Standardised MedDRA Queries (SMQs) Version 21.0. Available from https://www.meddra.org/sites/default/files/guidance/file/smq_intguide_21_0_english.pdf. Accessed May 26. 2019
23. Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18(6):427-36
24. Margolin L. Impaired rehabilitation secondary to muscle weakness induced by meropenem. Clin Drug Investig. 2004;24(1):61-2
25. Craig WA. The pharmacology of meropenem, a new carbapenem antibiotic. Clin Infect Dis. 1997;24(Suppl 2):S266-75
26. Ito M, Fukuda M, Suzuki Y, Wakamoto H, Ishii E. Carnitine-related hypoglycemia caused by 3 days of pivalate antibiotic therapy in a patient with severe muscular dystrophy: a case report. BMC Pediatr. 2017;17(1):73
27. Zhang X, Donnan PT, Bell S, Guthrie B. Non-steroidal anti-inflammatory drug induced acute kidney injury in the community dwelling general population and people with chronic kidney disease: systematic review and meta-analysis. BMC Nephrol. 2017;18(1):256
Author contact
![]() Corresponding author: Chengwen Teng, Pharm.D., M.S., Adjoint Assistant Professor, Pharmacotherapy Education and Research Center, Long School of Medicine, The University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Dr., MSC-6220, San Antonio, TX 78229; email: tengc3uthscsa.edu
Corresponding author: Chengwen Teng, Pharm.D., M.S., Adjoint Assistant Professor, Pharmacotherapy Education and Research Center, Long School of Medicine, The University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Dr., MSC-6220, San Antonio, TX 78229; email: tengc3uthscsa.edu

 Global reach, higher impact
Global reach, higher impact