Impact Factor
ISSN: 1449-1907
Int J Med Sci 2019; 16(9):1260-1270. doi:10.7150/ijms.37322 This issue Cite
Research Paper
Fluoxetine ameliorates dysbiosis in a depression model induced by chronic unpredicted mild stress in mice
1. State Key Laboratory of Cancer Biology, National Clinical Research Center for Digestive Diseases and Xijing Hospital of Digestive Diseases, The Fourth Military Medical University, Xi'an, China
2. Department of Clinical Nutrition, Xijing Hospital, The Fourth Military Medical University, Xi׳an, China
3. Department of Psychiatry, Xijing Hospital, The Fourth Military Medical University, Xi'an, China
4. Department of Radiology, Tangdu Hospital, The Fourth Military Medical University, Xi'an, China
# These authors contributed equally to this work
Abstract
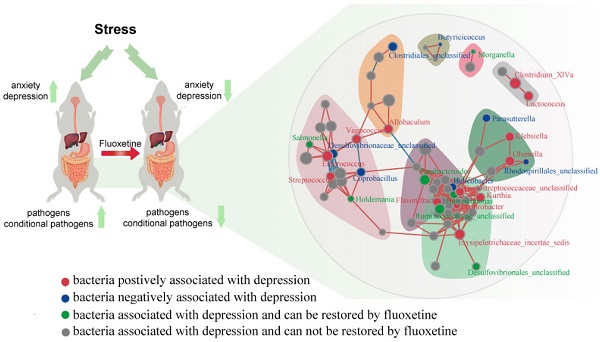
Background: Accumulating evidence has shown that neuropsychiatric disorders are associated with gut microbiota through the gut-brain axis. However, the effects of antidepressant treatment on gut microbiota are rarely studied. Here, we investigated whether stress led to gut microbiota changes and whether fluoxetine plays a role in microbiota alteration.
Methods: We investigated changes in gut microbiota in a depression model induced by chronic unpredicted mild stress (CUMS) and a restoration model by applying the classic antidepressant drug fluoxetine.
Results: We found that stress led to low bacterial diversity, simpler bacterial network, and increased abundance of pathogens, such as Escherichia/Shigella, and conditional pathogens, such as Enterococcus, Vagococcus, and Aerococcus. However, these changes were attenuated by fluoxetine directly and indirectly. Furthermore, the correlation analysis indicated strong correlations between gut microbiota and anxiety- and depression-like behaviors.
Conclusions: This study revealed that fluoxetine led to restoration of dysbiosis induced by stress stimulation, which may imply a possible pathway through which one CNS target drug plays its role in reshaping the gut microbiota.
Keywords: fluoxetine, depression, gut-brain axis, stress, microbiota

 Global reach, higher impact
Global reach, higher impact