Impact Factor
ISSN: 1449-1907
Int J Med Sci 2018; 15(14):1658-1666. doi:10.7150/ijms.28411 This issue Cite
Research Paper
RKIP-Mediated NF-κB Signaling is involved in ELF-MF-mediated improvement in AD rat
1. Department of Experimental Pathology, Beijing Institute of Radiation Medicine, Beijing, China
2. Beijing Key Laboratory of Bioelectromagnetism, Institute of Electrical Engineering, Chinese Academy of Sciences, Beijing, China.
*These authors contributed equally to this work.
Received 2018-7-9; Accepted 2018-10-12; Published 2018-11-5
Abstract
In a previous study, we reported the positive effects of extremely low frequency electromagnetic field (ELF-MF) exposure on Alzheimer's disease (AD) rats; however, the underlying mechanism remains unclear. In addition, we found that Raf-1 kinase inhibitor protein (RKIP) was downregulated by microwave exposure in the rat hippocampus. Our hypothesis was that RKIP-mediated NF-κB pathway signaling is involved in the effect of ELF-MF on the AD rat. In this study, D-galactose intraperitoneal (50 mg/kg/d for 42 d) and Aβ25-35 hippocampal (5 μL/unilateral, bilateral, single-dose) injection were implemented to establish an AD rat model. Animals were exposed to 50 Hz and 400 µT ELF-MF for 60 continuous days. The spatial memory ability of the rat was then tested using the Morris water maze. Protein expression and interaction were detected by western blotting and co-immunoprecipitation for RKIP-mediated NF-κB pathway factors. The results showed that ELF-MF exposure partially improved the cognitive disorder, upregulated the levels of RKIP, TAK1, and the RKIP/TAK1 interaction, but downregulated p-IKK levels in AD rats. These results indicated that RKIP-mediated NF-κB pathway signaling plays an important role in the ELF-MF exposure-mediated improvements in the AD rat. Our study suggested that ELF-MF exposure might have a potential therapeutic value for AD. Further in depth studies are required in the future.
Keywords: ELF-MF, AD, rat, RKIP, NF-κB pathway
Introduction
Alzheimer's disease (AD) is an age-related progressive neurodegenerative disease, characterized by progressive memory loss and decline of cognitive function [1]. In 2015, a report from Alzheimer's Disease International (ADI) showed that over 46,000,000 people worldwide have suffered the agonies of dementia, most of which suffered from AD, and this number is likely to double roughly every twenty years [2]. However, the etiology of AD is not entirely clear and there is no effective therapy for AD.
Extremely low frequency electromagnetic field (ELF-MF) is generated mostly by electric equipment, such as high voltage transmission lines, transformer substations, motors, and household appliances, with a frequency of 50 Hz (China, Europe) or 60 Hz (USA) [3]. The possible association between ELF-MF and childhood leukemia was first reported by Wertheimer and Leeper, in 1979 [4]. Thereafter, the effects of ELF-MF on health were extensively studied. Increasing evidence shows that different parameters of ELF-MF may lead to different neurobiological effects [5,6,7]. Generally, low-intensity and short-term ELF-MF exposure has no significant influence on brain structure and learning-memory ability; however, long-term, especially occupational, exposure can cause some health problems [8,9]. Notably, various positive effects of ELF-MF exposure on neural differentiation and rehabilitation have been reported in recent years [10,11,12].
Few studies have investigated the relationship between ELF-MF exposure and AD development, most of which were limited to epidemiological investigations. It was reported that long-standing occupational ELF-MF exposure might be a predictive risk factor for increased AD development [13]; however, no correlation was found in other reports [14,15]. For example, Zhang et al. found that ELF-MF exposure (50 Hz, 100 µT, 12 w) had no effect on the pathogenesis of AD in aluminum-overloaded rats [6]. Our previous study showed that ELF-MF exposure (50 Hz, 100 µT, 60 d) partially improved the cognitive and pathological symptoms of AD rats [16]; however, the underlying mechanism remains unclear and no reasonable explanation is available currently.
Raf-1 kinase inhibitor protein (RKIP) is a member of the phosphatidylethanolamine-binding protein family (PEBP), which are involved in many neural physiological actions, such as proliferation and apoptosis [17]. Studies have revealed that there is a lower expression of RKIP in the brain of patients with AD patients and AD rats [18,19]. In a previous study, we found that downregulation of RKIP in rat hippocampus after microwave radiation [20]. This prompted us to investigate the mechanism of the effect of ELF-MF exposure on AD.
RKIP can inhibit the nuclear factor kappa B (NF-κB) signaling pathway [21]. NF-κB pathway shows both inhibitory and promotional effects on AD development [22]. On the one hand, moderate activation of the NF-κB pathway can protect neurons from apoptosis by increasing the expression of anti-apoptotic factors [23]. On the other hand, excessive and persistent activation of the NF-κB pathway can aggravate β-amyloid protein (Aβ)-induced neuronal injury by increasing the expression of inflammatory factors [24].
We postulated that RKIP-mediated NF-κB pathway signaling is involved in the effect of ELF-MF on AD rats. Therefore, this study combined behavioral and molecular detection to investigate the role of RKIP-mediated NF-κB pathway signaling in the effect of ELF-MF exposure on AD.
Material and Methods
Animal Grouping
One hundred and twenty male Wistar rats aged 8 weeks (body weight 218.3 ± 11.5 g) were provided by the Experimental Animal Center of the Academy of Military Medical Science. The Morris water maze (MWM) training session (refer to the section entitled “Morris water maze”) was implemented to measure the average escape latency (AEL) of each rat before grouping. The outliers were identified based on the Grubbs criterion [25]. 24 corresponding rats were eliminated. The remaining 96 rats were randomly divided into the control group (Con), magnetic field group (MF), Alzheimer's disease group (AD), and complex model group (AD+MF). Rats fed with food and water ad libitum, under an environment of constant temperature (23.0 ± 0.5 °C) and humidity (65-75%) with a 12 h light/dark cycle (light on from 8:00-20:00). The animal experiment protocol was approved by the Animal and Human Use in Research Committee of the Academy of Military Medical Science.
ELF-MF exposure
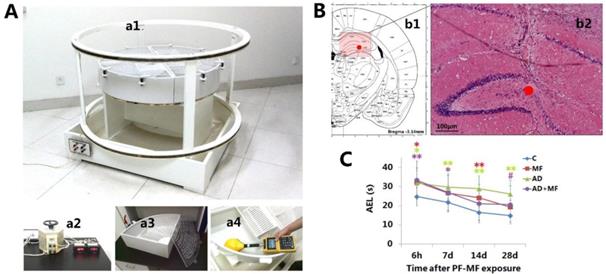
The ELF-MF animal exposure system (Fig. 1, A) was established by the Institute of Electrical Engineering, Chinese Academy of Sciences, and consisted of voltage-regulator and two parallel coaxial circular coils (Fig. 1, a1) of 70 cm in height and 140 cm in diameter. Two modular breeding devices were used. Each one comprised four individual boxes (Fig. 1, a3) with a height of 20 cm and radius of 50 cm at the quadrant bottom. Twelve rats were kept in each box. The MF and AD+MF groups were placed in room 1, which was installed with the ELF-MF exposure system. When the input current (Fig. 1, a2) was 50 Hz and 1.65 A, a Gauss Meter (EFA300, Narda Safety Test Solutions, Pfullingen, Germany) (Fig. 1, a4) showed that the interior ELF-MF intensity in the breeding device was attenuated ringwise but was unchanged longitudinally along the axis of the coils. The ELF-MF was 50 Hz and about 420 μT at the vertex, 400 μT at 25 cm to the vertex, and 380 μT at the edge of the box.
Rats were continuously raised in the device, and exposed to ELF-MF for 24 h/d for 60 d. The Con and AD groups were placed in room 2, which was on the same floor and with the same ventilation system as room 1, but without the ELF-MF exposure system. The humidity, light, temperature, and air quality of the two rooms were the same. When the exposure system started in room 1, the background magnetic field of room 2 (involved ELF-MF created by electrical appliances, such as electric light, air conditioning etc.) was measured using a Gauss Meter and was identified as below 400 nT.
Modeling
Based on our previous study [16], ELF-MF exposure combined with D-galactose intraperitoneal and Aβ25-35 hippocampal injection was implemented in this study to establish a complex rat model. Briefly, D-galactose (G0750, Sigma, St. Louis, MO, USA) and Aβ25-35 (A4559, Sigma) were dissolved in 0.9% NaCl saline to reach a concentration of 3 g/L and 1 μg/μL, respectively. Aβ25-35 was aggregated by in vitro incubation at 37 °C for 7 d and then stored at 4 °C. From the first day of ELF-MF exposure, rats of the AD and AD+MF groups received D-galactose (50 mg/kg) intraperitoneal injection once per day for 42 d. The Con and MF groups were injected with the same volume of saline. On the 43rd day, the rats were anesthetized by intraperitoneal injection with 2% pentobarbital sodium (60 mg/kg), and subsequently received hippocampal injection (5 μL/unilateral, bilateral) of Aβ25-35 (AD and AD+MF groups) or saline (Con and MF groups), through a stereotaxic apparatus (BW-SDA903, Bio-will, Shanghai, China) and microinjection system (SYS-Micro4, WPI, Sarasota, FL, USA). Hippocampal injection was performed in the CA3 region (Fig. 1, B), and the injection location was 3.5 mm behind the bregma, 3 mm beside the sagittal suture, and 3.5 mm under the surface of skull. The injection speed was 1 µL/min. After surgery, the rats were given intramuscular injection of penicillin sodium (50,000 U) once per day for 3 d, and then continue to be raised until ELF-MF exposure termination.
Morris water maze
The Morris water maze (MWM) test is a behavioral procedure widely used in behavioral neuroscience to study spatial learning and memory [26]. In this study, the MWM system (SLY-WMS, Shuolinyuan, Beijing, China) comprised a circular swimming pool of 180 cm in diameter and a computerized tracking system. The pool was filled to a depth of 30 cm with water and surrounded by a blackout curtain; the water temperature was kept at 25.0 ± 0.5 °C. The water surface was divided into four quadrants, and an escape platform of 8 cm in diameter was placed at the center of quadrant I. The computerized tracking system and software (SLY-WMS, Shuolinyuan, Beijing, China) was used to record the movements of the rats.
The MWM training session: This session was performed once a day for three consecutive days before ELF-MF exposure. In this session, rat was put into the water facing the wall from quadrant I to IV, in turn. Rat was allowed to swim to search for the platform for 60 s. When they boarded the platform, rats were kept on it for 20 s, and were then put into next quadrant until they had finished training for all four quadrants. Finally, the rats were dried with a towel and returned to the breeding device. The time take by a rat to find and board the platform was recorded as the escape latency (EL). If the rat could not find the platform in 60 s, the EL was recorded as 60 s, and the rat was put onto the platform for 20 s. The average escape latency (AEL) of the four quadrants was calculated for animal screening by the Grubbs test (refer to section “Animal Elimination and Grouping”).
The MWM test session: This session was performed at 6 h, 7 d, 14 d, and 28 d after ELF-MF exposure. This session was similar to the training session mentioned above, except that the rats did not need to stay on the platform for 20 s. If the rat could not find the platform in 60 s, the EL was recorded as 60 s, and the rat was allowed to continue to the next quadrant test. The AEL of each rat was recorded for statistical analysis.
Hippocampal protein extraction
Five rats from each group were decapitated after deeply anesthesia using 2% pentobarbital sodium (60 mg/kg i.p.), at 6 h, 7 d, 14 d, and 28 d after the termination of ELF-MF exposure. Hippocampi were collected and placed immediately in 4 °C Radioimmunoprecipitation assay buffer (RIPA buffer) lysis solution (P0013B, Beyotime, Shanghai, China) containing a protease inhibitor cocktail (04693159001, Roche, Nutley, NJ, USA), homogenized using an ultrasonic disruptor (20 kHz, 3 s×4) (BL99-IIDL, Voshin Instruments, Wuxi, China), and incubated on ice for 30 min. The homogenates (200 mg/mL) were centrifuged at 5000 rpm (CR-22E, HITACHI, Tokyo, Japan) for 15 min at 4 °C, and the supernatants were collected as protein samples and stored at -80 °C until use.
The protein concentration of each sample was determined using a Micro BCA Protein Assay Kit (P0012, Beyotime) with the following steps: albumin standard samples with a gradient concentration (0-2 μg/μL) were prepared, and samples were diluted 10-fold. A BCA working reagent was prepared and 25 μL of each standard or sample was pipetted into a well of a 96-well plate with replications. Two hundred microliters of the working reagent was added to each well and mixed thoroughly. The plate was incubated at 37 °C for 30 min, cooled to room temperature, and the absorbance at 562 nm was measured on a plate reader (Model 550, BIO-RAD, Hercules, CA, USA).
Western blotting
Samples were diluted to 5 μg/μL with 4× Loading Buffer (P0015, Beyotime), incubated at 95 °C for 10 min, and then electrophoresed on 10% SDS-PAGE gels (P0052, Beyotime) with electrophoresis buffer (P0014, Beyotime) and electrophoresis system (1658001, BIO-RAD). The quantity of loaded protein was 50 μg. After electrophoresis, gels were blotted onto polyvinylidene difluoride membranes (E578, Amresco, Solon, OH, USA) using electrophoretic transfer (1703930, Bio-Rad) for 4 h with 120 mA constant current and transfer buffer (P0021, Beyotime). The membranes were treated with TBS-T (pH 7.4) for 3 × 2 min, 5% skimmed milk for 30 min at room temperature, and incubated overnight at 4 °C with primary antibodies. The anti-RKIP (P30086), p-IKK (BS4597), p-NFκB (BS4737), and GAPDH (AP0063) primary antibodies were bought from Bioworld Technology (St. Louis Park, MN, USA). The anti-TAK1 (sc-7967) antibody was bought from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The membranes were washed 3×15 min in TBS-T and incubated with secondary antibody for 1 h at room temperature. The Goat anti-Rabbit IgG-HRP (BS13278) and Goat anti-Mouse IgG-HRP (BS12478) secondary antibodies were bought from Bioworld Technology. After washing for 3×15 min in TBS-T, the membranes were treated by ECL chemiluminescent HRP substrate (BLH01S100, Bioworld), and then scanned using an imaging system (SCA/FluorCh, Alpha Innotech, San Francisco, CA, USA). The integral optical density of each protein band was quantified using the imaging system software. Relative quantification was calculated based on the expression of GAPDH as internal control.
Co-immunoprecipitation
Samples were diluted to 1 μg/μL with lysis solution and incubated with the anti-RKIP antibody for 1 h at room temperature, followed by incubation overnight at 4 °C with Protein A+G Agarose Beads (P2012, Beyotime) (500 μL of sample, 5 μL of antibody, and 20 μL of agarose beads). The beads were then washed with lysis solution three times, incubated at 95 °C for 5 min with loading buffer, and subjected to western blotting analysis with the anti-TAK1 antibody.
Statistical analysis
Data were expressed as the mean ± standard deviation, and analyzed by the Statistical Package for the Social Sciences (SPSS), Version 17.0 (SPSS Inc., Chicago, IL, USA). Repeated measure analysis of variance (ANOVA) was used for the AEL data analysis, with “time” as the within variable, and “MF” and “AD” as the between variables. Three-factor analysis of variance, with “MF”, “AD”, and “time” as the factors, was used for the western blot and co-immunoprecipitation data analysis. Within-group variability was analyzed using Levene's test for homogeneity of variances. In all cases, P < 0.05 was considered statistically significant.
Results
ELF-MF exposure improved the spatial learning disorder of AD rats
For the MWM test, rats were trained to remember the location of the platform, and the repetitive training helped to establish spatial memory. Usually, AEL tends to decrease with increased training time. Thus, the AEL has been considered as being prognostic with regard to spatial learning disorders [26].
In this study, the MWM test results (Fig. 1, C) showed that the AEL of the AD group was prolonged significantly compared with the Con group, at 6 h-28 d (6 h P = 0.025, 7 d P = 0.005, 14 d P = 0.000, 28 d P = 0.000) after the termination of ELF-MF exposure. Significantly prolonged AEL in the MF and AD+MF groups was observed at 6 h (P = 0.014) / 14 d (P = 0.003) and 6 h (P = 0.007) / 7d (P = 0.046) respectively. Meanwhile, the AEL of the AD group was prolonged significantly compared with that of the AD+MF group at 28 d (P = 0.038).
The ELF-MF exposure system, hippocampal microinjection, and changes in average escape latency in rats. A: Long-term ELF-MF exposure system for rats. a1 Parallel coaxial circular coils, a2 Voltage-regulator, a3 Breeding device, a4 Dosimetry and calibration with Gauss Meter. B: Hippocampal stereotactic Aβ25-35 injection. b1 Transection of rat brain (AP, -3.5 mm). b2 Hippocampal morphology showing the microinjection site (7 d after Aβ25-35 microinjection, hematoxylin and eosin staining; Scale bar = 100 μm, ●: injection site). C: Changes in the average escape latency (AEL) in rats after ELF-MF (50 Hz, 400 µT, 60 d) exposure (vs. Con, *P < 0.05; vs. AD, #P < 0.05).
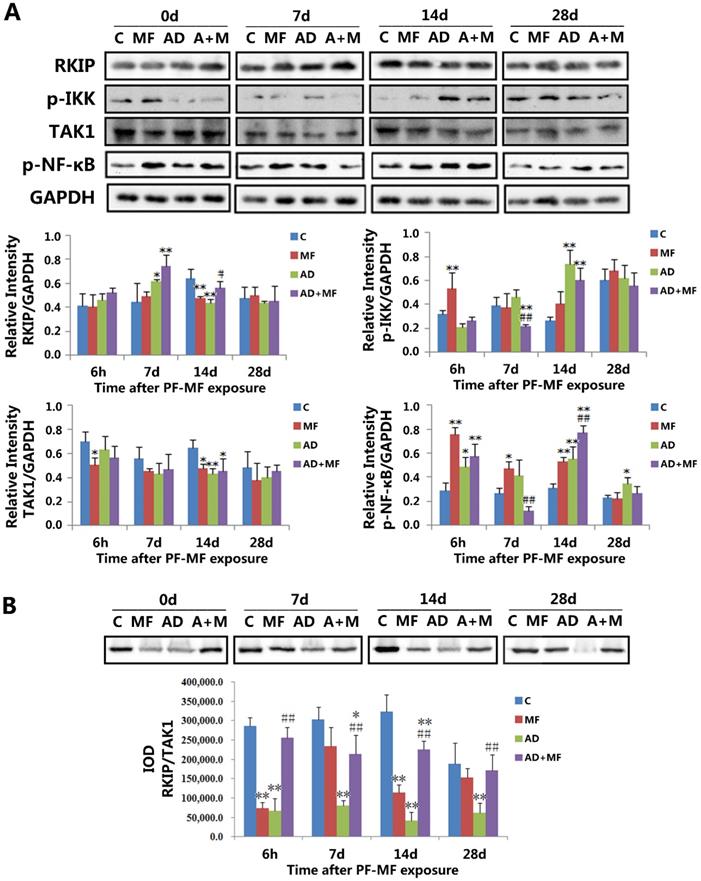
The changes of RKIP, p-IKK, TAK1, and p-NFκB levels and the RKIP/TAK1 interaction in rat hippocampus after ELF-MF (50 Hz, 400 µT, 60 d) exposure. A: The changes and quantification results of RKIP, p-IKK, TAK1, and p-NFκB levels. B: The changes and quantification result of RKIP/TAK1 interaction. (vs. Con, *P < 0.05; vs. AD, #P < 0.05.)
Moreover, repeated measure ANOVA showed a significant interaction between ELF-MF exposure and AD modeling (P = 0.002), which indicated that ELF-MF exposure significantly improved the spatial learning disorder of AD rats. In addition, there was a significant interaction between ELF-MF and time (P = 0.028), suggesting that the effect of ELF-MF on the AEL was recoverable within a certain time period. No significant interaction was observed between AD modeling and time (P = 0.606).
The effect of ELF-MF exposure on the levels of RKIP, TAK1, p-IKK, and p-NFκB in hippocampus of AD rats
The western blotting results (Fig. 2 A) showed downregulation of RKIP in the MF group (P = 0.004) at 14 d after ELF-MF exposure, while AD modeling induced the upregulation and downregulation of RKIP in the AD group at 7 d (P = 0.049) and 14 d (P = 0.001). Moreover, the level of RKIP was significantly increased in the AD+MF group (P = 0.017) compared with the AD group at 14 d after ELF-MF exposure. Meanwhile, downregulation of TAK1 was observed in the MF group at 6 h (P = 0.033) and 14 d (P = 0.017), and in both the AD group (P = 0.005) and the AD+MF group (P = 0.010) at 14 d after ELF-MF exposure. There was no significance between the AD and AD+MF groups in terms of the TAK1 level.
In addition, the phosphorylation of IKK (p-IKK) was upregulated in the MF group (P = 0.006) at 6 h, and in both the AD group (P = 0.000) and the AD+MF group (P = 0.003) at 14 d after ELF-MF exposure; however, it was downregulated at 7 d in the AD+MF group compared with both the Con group (P = 0.023) and AD group (P = 0.004). Furthermore, the phosphorylation of NF-κB (p-NFκB) was upregulated by ELF-MF exposure (6 h P = 0.000, 7 d P = 0.012, 14 d P = 0.003) and AD modeling (6 h P = 0.047, 14 d P = 0.002, 28 d P = 0.016), and significance was observed between the AD and AD+MF groups at 7 d (P = 0.002) and 14 d (P = 0.003).
Altogether, our results suggested that ELF-MF exposure influenced the expression of RKIP, p-IKK, and p-NFκB in the hippocampus of AD rats, but had no effect on the expression of TAK1. RKIP-regulated NF-κB pathway signaling may play an important role in the effect of ELF-MF exposure on AD development.
ELF-MF exposure increased the interaction between RKIP and TAK1 in the hippocampus of AD rats
To investigate the regulation of the NF-κB pathway by RKIP, the interaction between RKIP and TAK1 was examined using co-immunoprecipitation. The results (Fig. 2 B) showed that, for the MF group, a significant difference was observed at 6 h (P = 0.000) and 14 d (P = 0.000) after ELF-MF exposure, compared with the Con group. The RKIP/TAK1 interaction in the AD group decreased significantly compared with the Con group at each time point in this study (6 h P = 0.000, 7 d P = 0.000, 14 d P = 0.000, 28 d P = 0.000). These results confirmed the regulation of the NF-κB pathway by RKIP via interaction with TAK1. Both ELF-MF exposure and AD modeling induced RKIP downregulation and a decrease in the RKIP/TAK1 interaction, resulting in increases in p-IKK and p-NFκB levels. The RKIP/TAK1 interaction in the AD+MF group was always significantly larger than that in the AD group (6 h P = 0.000, 7 d P = 0.003, 14 d P = 0.000, 28 d P = 0.008), but was still decreased compared with the Con group, especially at 7 d (P = 0.025) and 14 d (P = 0.003).
Moreover, three-factor analysis of variance also showed that AD modeling (P = 0.000) could cause a decrease in the RKIP/TAK1 interaction, and there was a significant interaction between ELF-MF exposure and AD modeling (P = 0.002). This result further indicated the important role of NF-κB pathway regulation by RKIP in the effect of ELF-MF exposure on AD development.
Discussion
AD modeling
Alzheimer's disease (AD) is an age-related progressive neurodegenerative disease characterized by progressive memory loss and a decline in cognitive function. The characteristic pathological changes of AD include differing degrees of neuronal loss or apoptosis, senile plaques (SP) formed by extracellular deposits of amyloid-β (Aβ), and intracellular neurofibrillary tangles (NFT) comprising hyperphosphorylated microtubule-associated protein tau (Tau) in the brain [1].
Over the past few years, various AD animal models have been established, based on the possible mechanisms of AD. However, because of the long duration and complex pathogenesis of AD, it is difficult to construct an animal model that can completely simulate the pathological, physiological, and behavioral changes of AD [27,28]. The intracerebral injection of Aβ25-35 peptide fragments can induce AD-like clinicopathological features [29], and the intraperitoneal injection of D-galactose can cause premature aging and organ decline, and learning- memory disorder [30]. In a previous study, we found that the AD rat model established by D-galactose peritoneal injection, combined with Aβ25-35 hippocampus intracerebral injection, could simulate both the learning-memory disorder and the characteristic pathological changes of AD [16]. Using the above AD animal model, we continued to investigate the mechanisms of AD improvement by ELF-MF exposure in the present study.
ELF-MF parameters
It has been reported that the biological effects of ELF-MF is related to the frequency, flux density, and exposure duration of the magnetic field. In China, the power source of electric appliances is 220 V and 50 Hz alternating current, so people are most frequently exposed to 50 Hz ELF-MF during daily life. Exposure to 100 μT and 50 Hz for 12 weeks had no effect on the learning and memory ability of rats [6]. Moreover, acute exposure to 0.5 mT and 50 Hz for 20 min showed no effect on the social and fear behavior of rats, but might have increased their passivity and situational anxiety [31]. Furthermore, depressive-like behavior was found following 28-42 days of continuous exposure [32]. In addition, 8 mT and 50 Hz exposure for 20 min might impair the consolidation of spatial memory in rats [5], and exposure for 90 min [33] or 4 h [34] showed devastating effects on the memory function of mice. According to the ICNIRP guidelines (2010), the occupational and public reference exposure threshold for 50 Hz ELF-MF is 1 mT and 200 µT [35]. In our preliminary experiment, no obvious behavioral and pathological effect was observed in rats after 200 μT and 50 Hz ELF-MF exposure for 60 days. Previously, we reported the positive effects of 400 μT and 50 Hz ELF-MF exposure on AD rats [16]. Therefore, in this study, we continued to combine 400 μT and 50 Hz ELF-MF exposure with AD modeling, to explore the underlying molecular mechanisms of the effects of ELF-MF exposure on AD.
The effects of ELF-MF exposure on AD rats
In vitro studies proved that ELF-MF exposure could influence the physiological function of neural cells, such as the induction of oxidative stress [36], the activation of neuronal ion channels and membrane receptors [37,38], the inhibition of proliferation, and the promotion of apoptosis [39]. Epidemiological investigations identified that ELF-MF exposure could cause a total decline in sleep quality [40], and some neurological symptoms, such as depression, anxiety, fatigue, and memory decline [41]. Other studies have suggested the potential association between ELF-MF and AD development. Schulte et al. found that AD occurs more frequently in some occupations than in others [42]. Another study further indicated an increased risk of AD among employees with occupational exposure to ELF-MF [43]. Huss et al. found that there was a dose-response relationship with respect to years of residence in the immediate vicinity of power lines and AD [13]. These results suggested that ELF-MF exposure was involved in AD development; however, the underlying mechanism has not been determined.
With regard to the unclear etiology of AD, ELF-MF exposure might serve as an important factor for AD development. Nevertheless, the biological effects of ELF-MF depend on the exposure parameters and duration. Various positive effects of magnetic field exposure have been reported in recent years. For example, ELF-MF exposure (60 Hz, 0.7 mT, 21 d) improved neurological scores, enhanced neurotrophic factor levels, and reduced both oxidative damage and neuronal loss in a Huntington's disease rat model [12]. 2 mT, 50 Hz, 1 h/d exposure from postnatal day 23-35 improved the spatial learning acquisition and memory retention of early adolescent male mice [44]. ELF-MF exposure of 5 mT, 50 Hz, and 60 min/d for 12 d exposure facilitated bone marrow stroma stem cell differentiation into neural cells [10]. Similarly, exposure at 80-150 µT, 40 Hz, 20 min/day, 5 d/w, for 4 w had positive effects on peripheral nerve regeneration [45]. In a previous study, we found that ELF-MF exposure (400 µT, 50 Hz, 60 d) partially improved the cognitive and clinicopathological symptoms of AD rats [16].
However, it remains unknown how AD develops, and how ELF-MF interacts with living material. This study used a rat model to simulate AD development after ELF-MF exposure, and revealed that inhibition of RKIP-mediated NF-kB pathway signaling by ELF-MF exposure is involved in the pathophysiological development of AD, and that ELF-MF exposure inhibits the development of AD disease. This result indicated the potential value of controlled ELF-MF exposure for AD therapy. Further studies are needed to determine the details of the interaction between ELF-MF and AD.
The role of RKIP mediated NF-κB pathway signaling in the effects of ELF-MF exposure on AD rats
NF-κB (nuclear factor κ-light-chain-enhancer of activated B cells) is a transcription factor that regulates many genes, and participates in cellular proliferation, apoptosis, invasion, and differentiation. Under normal physiological conditions, NF-κB is complexed with an endogenous inhibitor, IκB (inhibitor of NF-κB), and remains inactive. When cells are stimulated by certain damage factors, the NF-κB/IκB complex can be decomposed by IKK (inhibitor of nuclear factor kappa-B kinase), and the dissociated NF-κB enters nucleus where it exerts it effects [22]. Many studies have suggested that the NF-κB pathway participates in the occurrence and development of neurodegenerative diseases. Recent studies have shown that the NF-κB pathway has a two-phase effect on AD: On the one hand, moderate activation of the NF-κB pathway can inhibit neuronal apoptosis that is induced by Aβ, through upregulating anti-apoptosis genes [24]. On the other hand, continuous and excessive activation of the NF-κB pathway can increase the expression of inflammatory factors, which aggravate the neuronal injury of AD [46,47].
RKIP exists extensively within both eukaryotic and prokaryotic cells, and plays an important role in different signal pathways. Recent studies have found that RKIP is mainly expressed around senile plaques, which are formed by β-amyloid decomposition [19], and the expression of RKIP mRNA and protein decreased correspondingly in the hippocampus of AD patient [18] and an AD mouse [19]. Further study found that RKIP was sensitive to hippocampal damage induced by electromagnetic radiation [20]. In addition, RKIP has also been recognized as an inhibitor of the NF-κB pathway. When combined with TAK1, RKIP can inhibit the phosphorylation of IKK, which has a negative regulatory role in the decomposition of the NF-κB/IκB complex [46].
In this study, we found that both ELF-MF exposure (50 Hz, 400 μT, 60 d) and AD modeling could result in the downregulation of RKIP, a decrease in the RKIP/TAK1 interaction, and upregulation of p-IKK and p-NF-κB. The above changes showed a recoverable trend at 28 d after exposure in the MF group. Correspondingly, the learning and memory disfunction of the MF group recovered to close to the control group at 28 d after ELF-MF exposure. However, the above changes in RKIP-mediated NF-κB pathway signaling were not reversible in the AD group, especially the expression of p-NFκB and the RKIP/TAK1 interaction. Moreover, ELF-MF exposure significantly inhibited RKIP-mediated NF-κB pathway signaling in the AD rats. With regard to the improvement in spatial learning induced by ELF-MF exposure in the AD rats, RKIP-mediated NF-κB pathway signaling might play an important role in this process.
Conclusion
Our findings indicated that certain conditions of ELF-MF exposure (50 Hz, 400 μT, 60 d) could partially improve the cognitive symptoms of AD in a rat model. RKIP-mediated NF-κB pathway signaling is involved in this process, and might play an important role in the effect of ELF-MF exposure on AD. This study also suggested the potential therapeutic value of ELF-MF exposure for AD. Further studies are required in the future.
Acknowledgements
We sincerely thank Dr. Peilan Zhou and Prof. Tao Song for their excellent technical assistance and guidance of intracerebral injection and ELF-MF exposure calibration.
Grant information
We (including all authors) agree to authorize this manuscript entitled “RKIP-Mediated NF-κB Signaling is Involved in ELF-MF-mediated improvement in AD rat” to be edited and published by International Journal of Medical Sciences. We agree to authorize International Journal of Medical Sciences exclusive right to edit, publish, and distribute the above manuscript, and declare that this authorization will not invade other's rights or benefits. Any infringement dispute or loss caused by the use of the above right shall be borne by us.
This work was sponsored by the National Natural Science Foundation of China [grant numbers 51307181, 81472951, 51037006].
Conflict of interest statement
We (including all authors) declare that we have no financial and personal relationships with other people or organizations that could inappropriately influence our work. There is no professional or other personal interest of any nature or kind in any product, service, and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled, “RKIP-Mediated NF-κB Signaling is involved in ELF-MF-mediated improvement in AD rat”.
References
1. Dong S, Duan Y, Hu Y. et al. Advances in the pathogenesis of Alzheimer's disease: a re-evaluation of amyloid cascade hypothesis. Transl Neurodegener. 2012;1(1):18
2. Prince M, Wimo A, Guerchet M. et al. World Alzheimer Report, Alzheimers Disease International (ADI), London. 2015.
3. National Energy Administration of China. DL/T799, 7-2010 Labour environment monitoring technological specification of electric power industry. China Electric Power Press, Beijing. 2011
4. Wertheimer N, Leeper E. Electrical wiring configurations and childhood cancer. Am J Epidemiol. 1979;109(3):273-284
5. Jadidi M, Firoozabadi SM, Rashidy-Pour A. et al. Acute exposure to a 50 Hz magnetic field impairs consolidation of spatial memory in rats. Neurobiology of Learning and Memory. 2007;88(4):387-392
6. Zhang C, Li Y, Wang C. et al. Extremely low-frequency magnetic exposure appears to have no effect on pathogenesis of Alzheimer's disease in aluminum-overloaded rat. Plos One. 2013;8(8):e71087
7. Lai J, Zhang Y, Liu X. et al. Effects of extremely low frequency electromagnetic fields (100μT) on behaviors in rats. Neurotoxicology. 2016;52:104-113
8. Barsam T, Monazzam MR, Haghdoost AA. et al. Effect of extremely low frequency electromagnetic field exposure on sleep quality in high voltage substations. Iranian J Environ Health Sci Eng. 2012;9(1):15
9. Huang J, Tang T, Hu G. et al. Association between exposure to electromagnetic fields from high voltage transmission lines and neurobehavioral function in children. Plos One. 2013;8(7):e67284
10. Bai WF, Xu WC, Feng Y. et al. Fifty-Hertz electromagnetic fields facilitate the induction of rat bone mesenchymal stromal cells to differentiate into functional neurons. Cytotherapy. 2013;15(8):961-970
11. Jung IS, Kim HJ, Noh R. et al. Effects of extremely low frequency magnetic fields on NGF induced neuronal differentiation of PC12 cells. Bioelectromagnetics. 2014;35(7):459-469
12. Tasset I, Medina FJ, Jimena I. et al. Neuroprotective effects of extremely low-frequency electromagnetic fields on a Huntington's disease rat model: effects on neurotrophic factors and neuronal density. Neuroscience. 2012;209:54-63
13. Huss A, Spoerri A, Egger M. et al. Swiss National Cohort Study. Residence near a power lines and mortality from neurodegenerative diseases: longitudinal study of the Swiss population. Am J Epidemiol. 2009;169(2):167-175
14. Frei P, Poulsen AH, Mezei G. et al. Residential Distance to High-voltage Power Lines and Risk of Neurodegenerative Diseases: a Danish Population-based Case-Control Study. Am J Epidemiol. 2013;177(9):970-978
15. Vergara X, Heifets L, Greenland S. et al. Occupational exposure to extremely low-frequency magnetic fields and neurodegenerative disease: a meta-analysis. J Occup Environ Med. 2013;55(2):135-146
16. Liu X, Zuo HY, Wang DW. et al. Improvement of Spatial Memory Disorder and Hippocampal Damage by Exposure to Electromagnetic Fields in an Alzheimer's Disease Rat Model. Plos One. 2015;10(5):e0126963
17. Al-Mulla F, Bitar MS, Taqi Z. et al. RAF kinase inhibitory protein (RKIP) modulates cell cycle kinetics and motility. Mol Biosyst. 2011;7(3):928-994
18. Maki M, Matsukawa N, Yuasa H. et al. Decreased expression of hippocampal cholinergic neurostimulating peptide precursor protein mRNA in the hippocampus in Alzheimer disease. J Neuropathol Exp Neurol. 2002;61(2):176-185
19. George AJ, Gordon L, Beissbarth T. et al. A Serial Analysis of Gene Expression Profile of the Alzheimer's Disease Tg2576 Mouse Model. Neurotox Res. 2010;17:360-379
20. Zuo HY, Lin T, Wang DW. et al. RKIP regulates neural cell apoptosis induced by exposure to microwave radiation partly through the MEK/ERK/CREB pathway. Mol Neurobiol. 2015;51(3):1520-1529
21. Yeung KC, Rose DW, Dhillon AS. et al. Raf Kinase Inhibitor Protein Interacts with NF-κB-Inducing Kinase and TAK1 and Inhibits NF-κB Activation. Mol Cell Biol. 2001;21(21):7207-7217
22. Chaisson ML, Brooling JT, Ladiges W. et al. Hepatocyte-specific inhibition of NF-kappaB leads to apoptosis after TNF treatment, but not after partial hepatectomy. J Clin Invest. 2002;110:193-202
23. Barger SW, Hörster D, Furukawa K. Tumor necrosis factors alpha and beta protect neurons against amyloid beta-peptide toxicity: evidence for involvement of a kappa B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc Natl Acad Sci. 1995;92:9328-9332
24. Srinivasan M, Lahiri DK. Significance of NF-κB as a pivotal therapeutic target in the neurodegenerative pathologies of Alzheimer's disease and multiple sclerosis. Expert Opin Ther Targets. 2015;19:471-487
25. Lemeshko BY, Lemeshko SB. Extending the Application of Grubbs-Type Tests in Rejecting Anomalous Measurements. Measurement Techniques. 2005;48:536-547
26. Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848-858
27. Woodruff-Pak DS. Animal models of Alzheimer's disease: therapeutic implications. Journal of Alzheimers Disease. 2008;15(4):507-521
28. Laferla FM, Green KN. Animal Models of Alzheimer Disease. Cold Spring Harbor Perspectives in Medicine. 2012;2(11):705-709
29. Yin Y, Ren Y, Wu W. et al. Protective effects of bilobalide on Aβ(25-35) induced learning and memory impairments in male rats. Pharmacol Biochem Behav. 2013;106:77-84
30. Aydın S, Yanar K, Atukeren P. et al. Comparison of oxidative stress biomarkers in renal tissues of D-galactose induced, naturally aged and young rats. Biogerontology. 2012;13(3):251-260
31. Balassa T, Szemerszky R, Bárdos G. Effect of short-term 50Hz electromagnetic field exposure on the behavior of rats. Acta Physiol Hung. 2009;96(4):437-448
32. Szemerszky R, Zelena D, Barna I. et al. Stress-related endocrinological and psychopathological effects of short- and long-term 50Hz electromagnetic field exposure in rats. Brain Res Bull. 2010;81(1):92-99
33. Foroozandeh E, Naeini MS, Ahadi H. Effects of 90min Exposure to 8mT Electromagnetic Fields on Memory in Mice. Journal of American Science. 2011;7:58-61
34. Foroozandeh E, Derakhshan-Barjoei P, Jadidi M. Toxic effects of 50 Hz electromagnetic field on memory consolidation in male and female mice. Toxicol Ind Health. 2013;29(3):293-299
35. International Commission on Non-Ionizing Radiation Protection. Guidelines for limiting exposure to time-varying electric and magnetic fields (1 Hz to 100 kHz). Health Phys. 2010;99(6):818-836
36. Morabito C, Guarnieri S, Fanò G. et al. Effects of acute and chronic low frequency electromagnetic field exposure on PC12 cells during neuronal differentiation. Cell Physiol Biochem. 2010;26(6):947-958
37. He YL, Liu DD, Fang YJ. et al. Exposure to extremely low-frequency electromagnetic fields modulates Na+ currents in rat cerebellar granule cells through increase of AA/PGE2 and EP receptor-mediated cAMP/PKA pathway. Plos One. 2013;8(1):e54376
38. Piacentini R, Ripoli C, Mezzogori D. et al. Extremely low-frequency electromagnetic fields promote in vitro neurogenesis via upregulation of Ca(v)1-channel activity. J Cell Physiol. 2008;215(1):129-139
39. Yi C, Sun HY, Cui YH. et al. Effects of exposure to 50 Hz magnetic field of 1 mT on the performance of detour learning task by chicks. Brain Res Bull. 2007;74(1-3):178-182
40. Barsam T, Monazzam MR, Haghdoost AA. et al. Effect of extremely low frequency electromagnetic field exposure on sleep quality in high voltage substations. Iranian J Environ Health. 2012;9(1):15
41. Huang J, Tang T, Hu G. et al. Association between exposure to electromagnetic fields from high voltage transmission lines and neurobehavioral function in children. Plos One. 2013;8(7):e67284
42. Schulte PA, Burnett CA, Boeniger MF. et al. Neurodegenerative diseases: occupational occurrence and potential risk factors, 1982 through 1991. Am J Public Health. 1996;86(9):1281-1288
43. Håkansson N, Gustavsson P, Johansen C. et al. Neurodegenerative diseases in welders and other workers exposed to high levels of magnetic fields. Epidemiology. 2003;14(4):420-428
44. Wang X, Zhao K, Wang D. et al. Effects of exposure to a 50 Hz sinusoidal magnetic field during the early adolescent period on spatial memory in mice. Bioelectromagnetics. 2013;34(4):275-284
45. Suszyński K, Marcol W, Szajkowski S. et al. Variable spatial magnetic field influences peripheral nerves regeneration in rats. Electromagn Biol Med. 2013;33(3):198-205
46. Hayden MS, Ghosh S. NF-κB in immunobiology. Cell Res. 2011;21(2):223-244
47. Karunaweera N, Raju R, Gyengesi E. et al. Plant polyphenols as inhibitors of NF-κB induced cytokine production-a potential anti-inflammatory treatment for Alzheimer's disease? Front Mol Neurosci. 2015;16:8-24
Author contact
![]() Corresponding author: Department of Experimental Pathology, Beijing Institute of Radiation Medicine, 27 Taiping Road, Haidian District, Beijing, 100850, China. Tel: +86-10-66932218. Fax: +86-10-68214653. E-mail: zuohy2005com
Corresponding author: Department of Experimental Pathology, Beijing Institute of Radiation Medicine, 27 Taiping Road, Haidian District, Beijing, 100850, China. Tel: +86-10-66932218. Fax: +86-10-68214653. E-mail: zuohy2005com

 Global reach, higher impact
Global reach, higher impact