3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2018; 15(11):1143-1152. doi:10.7150/ijms.26450 This issue Cite
Review
T Cell Immunity To Enterovirus 71 Infection In Humans And Implications For Vaccine Development
Centre for Virus and Vaccine Research, School of Science and Technology, Sunway University, Bandar Sunway, Kuala Lumpur, Selangor 47500, Malaysia.
Received 2018-4-2; Accepted 2018-6-5; Published 2018-7-25
Abstract
Enterovirus 71 (EV-A71) is one of the major pathogens causing hand, foot and mouth disease (HFMD). Some strains can lead to neurological disease and fatality in children. Up to date, there is no FDA-approved vaccine to prevent severe HFMD and mortality. Although the inactivated vaccine has advanced to production in China, lack of long-term protection and the requirement of multiple boosters have necessitated the development of other types of vaccines. Recent studies indicate that cellular and not humoral immunity determines the clinical outcome of EV-A71 infections. High levels of cytokines such as IL-1β, IL-6, IL-10 and IFN-γ tend to correlate with clinical severity in patients with pulmonary edema and encephalitis. The live attenuated vaccine may serve as the preferred choice as it can induce excellent humoral and cellular immunity as well as live-long immunity. Expression of certain HLA alleles such as TNF-α promoter type II (-308 allele), HLA-A33 and HLA-DR17 responses have been linked to severe HFMD. However, the high variability of MHC genes could restrict T cell recognition and be a major obstacle in the design of peptide vaccines. Hence, the development of a T cell universal vaccine (incorporating both CD4+ and CD8+ T cell epitopes) that induces broad, multifunctional and cross-reactive CD8+ T cell responses maybe desirable.
Keywords: Hand, foot and mouth disease, enterovirus 71, cellular immunity, immunogenicity.
Introduction
Vaccines against the Hand, Foot and Mouth Disease (HFMD) are highly desirable as HFMD has evolved to become a life-threatening epidemic, ravaging lives of young children in cyclical epidemics in the Asia Pacific. In 2017, a total of 1,952,435 cases of HFMD were reported in China [1]. With rising concern about the virulence of EV-A71, there is an urgent need for a vaccine against EV-A71 to be produced that is approved by the FDA. Pharmaceutical companies such as CAMS, Vigoo and Sinovac have obtained drug certificates and production licenses by China's FDA for the inactivated vaccine (IV) against sub-genotype C4a [2].
Sinovac reported that the efficacy of their inactivated vaccine (IV) against EV-A71-associated hospitalization and HFMD cases with neurologic complications were both 100% [3]. Another study also showed an efficacy rate of 95.1% against EV-A71-associated HFMD for the second year post-vaccination and an overall efficacy rate of 94.7% at two years [4]. It would be interesting to perform phase IV studies to assess the types of immune response and correlates of protection against all EV-A71 genotypes/sub-genotypes. Although the IV induces good humoral immunity, it may be lacking in a strong cellular response, which is needed for long-term protection. Therefore, there is a need to develop other types of vaccines which can induce robust humoral and cellular immunity. The live attenuated vaccine (LAV) is an attractive candidate against severe EV-A71 as it can induce both humoral and cellular immunity, and conferring livelong immunity. However, LAVs face potential problems such as the risk of reversion to wild type virulence. This has been observed in countries which carried out vaccination with the Sabin Oral Polio Vaccine. In addition, LAVs can be excreted from vaccine candidates and this would be dangerous to immunocompromised individuals who have not received the polio vaccine [5].
As there have been concerns about genetic stability of LAV, recent studies have focused on another type of experimental vaccine that is devoid of genetic material. They are the virus-like particles (VLPs) that resemble the authentic, native virus in morphology, capsid protein and protein composition. However, they do not contain any nucleic acid material and would allay fears of genetic stability and risk of virulent revertants. Bivalent VLPs that replaced the SP70 epitope within the VP1 capsid protein of EV-A71 with that of CV-A16 have been designed. These ChiEV-A71 VLPs produced in Saccharomyces cerevisiae demonstrated similarities in morphology and protein composition as the EV-A71 VLPs. BALB/c neonatal mice immunized with the ChiEV-A71 VLPs showed strong cellular immunity as indicated by the enhanced production of IFN-γ, IL-2, IL-4, and IL-6 in splenocytes. In addition, passive immunization with anti-ChiEV-A71 VLP sera conferred full protection against lethal challenges with both CV-A16 and EV-A71 in neonatal mice [6].
Three-dimensional bivalent EV-A71/CV-A16 VLPs utilizing a baculovirus-insect cell expression system have been reconstructed and these structures resembled natural empty particles of EV-A71 and 135S-like expanded particles of CV-A16. The cryo-electron microscopy results also showed that the linear neutralizing epitopes and conformational epitopes were well preserved in the bivalent VLPs. In addition, immunogenicity tests were carried out in mice with the monovalent EV-A71 VLPs, monovalent CV-A16 VLPs and bivalent EV-A71/CV-A16 VLPs. Mice immunized with the VLP bivalent/composite-adjuvant (alum and CpG-oligodeoxynucleotides) vaccine was able to induce high NtAb titers ranging from 1:160 to 1:320 against four strains of EV-A71 (804232Y, 8052303F, 804251Y, 8061001Y) and two strains of CV-A16 viruses (705212F, 705213F) [7].
Nevertheless, there remains considerable interest as to which HFMD-causing pathogen should be included in a bivalent or trivalent vaccine. As EV-A71, CV-A6, CV-A10 and CV-A16 were found to co-circulate during HFMD outbreaks and most of the cases were due to EV-A71 and CV-A16, it would be desirable for a bivalent EV-A71/CV-A16 vaccine to be produced [8]. In addition, the bivalent vaccine should be able to protect against the EV-A71 sub-genotype C4 or B4 as these sub-genotypes were the predominant ones causing fatal HFMD. Moreover, neutralizing antibodies (NtAbs) elicited by C4 and B4 have been found to cross neutralize against other EV-A71 sub-genotypes [9, 10]. This greatly simplifies the choice of vaccine strain for EV-A71 as immunization with one sub-genotype could potentially cross-protect against all other sub-genotypes. This is in contrast to the polio vaccine whereby 3 serotypes were required to construct the oral polio vaccine. In addition, the chosen strain should be able to grow to high titers in a FDA approved cell line. These studies could provide a reference to the design of future multivalent vaccines against EV-A71 and other Coxsackieviruses as a safe and cost-effective EV-A71 vaccine with broad cross-protection.
T Cell Immunity against EV-A71
Research has indicated that cellular and not humoral immunity determines the clinical outcome of EV-A71 infections. There was no difference in the level of NtAb titers between mild, severe and fatal HFMD cases [11]. There remains concern about the development of vaccines that do not elicit cellular immunity but only induces humoral immunity. For example, the IV is often a poor inducer of T cell responses. There is no viral replication in an IV and therefore, there is reduced antigen to sustain an extended antigen response [12]. IVs are deemed partially successful as it is based solely on antibody-mediated protection. Although NtAbs are very efficient in preventing the progression of viral infections, there may be too many surface proteins of viruses that will evolve on a continual basis and this could cause viral escape [13]. The research and development of successful vaccines would need the production of strong and robust T cell responses since cytotoxic CD8+ T cell responses are responsible for the clearance of viral infection [14].
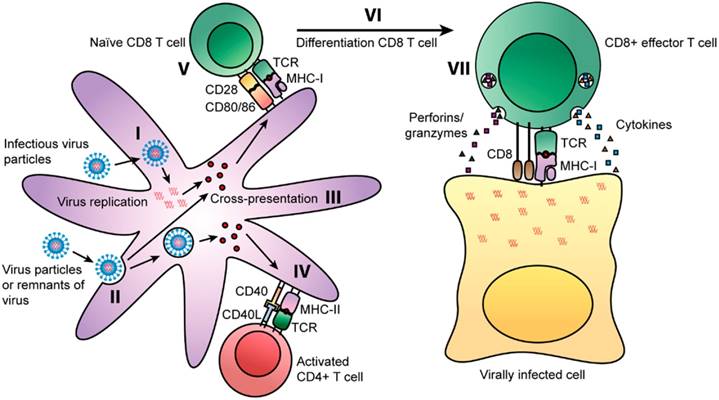
T cells can decrease or eradicate viral multiplication upon infection. In particular, CD4+ T cell memory can be life-long and it is intriguing that after in vitro re-stimulation, there was detection of CD4+ T cells for up to 50 years after smallpox vaccination [15]. Viruses can enter cells via two routes whereby some can infect cells directly. In the first route, antigen presenting cells (APCs) like dendritic cells (DCs) and macrophages are able to process viral proteins into peptide fragments that would be presented by MHC I molecules to naïve CD8+ T cells [Fig. 1(I)]. In the alternative route for viral entry, DCs can also uptake viral particles or remnants of viral-infected cells [Fig. 1(II)]. Viral antigens during proteolytic processing can be loaded onto MHC I molecules via the cross presentation pathway [Fig. 1(III)]. This pathway would mean that such peptides could also be loaded onto MHC II molecules [13].
Some CD4+ T cell subsets such as T follicular helper (TFH), Th1, Th17 and cytotoxic CD4+ T cells have vital antiviral roles. Such antiviral functions range from the production of cytokines/chemokines (IFN-γ, TNF-α) to direct cytotoxic effects on viral infected cells. Memory CD4+ T cells have an added protective advantage over naïve cells as they can elicit an early innate inflammatory response in tissues that are involved in viral control such as faster activation of B cells. This would result in a faster and more robust antiviral immune response. In addition, secondary effectors derived from memory CD4+ T cell precursors have more direct antiviral potential than primary effectors originating from naïve CD4+ T cells. Therefore, a synergistic mechanism of intricate CD4+ T cell subsets would indeed provide an impressive level of viral control. More ex vivo/in vivo studies should be carried out to comprehend the full potential of memory CD4+ T cells in the design of better vaccines [16].
Routes of presentation of viral peptides on APCs [13]. Some viruses can infect cells and replicate prior to being degraded into peptide fragments. Some fragments will be presented on MHC class I molecules to naïve CD8+ T cells. Binding of the TCR to the MHC class I-peptide complex activates the CD8+ T cells and releases cytotoxic granules and cytokines (TNF-α and IFN-γ). Some viral peptides can be presented on MHC class II molecules in parallel by APCs. The TCR of naïve CD4+ T cells can recognize MHC class II-peptide complexes on APCs and differentiate into Th1 and Th2 cells. Copyright 2014. Frontiers Media SA. Permission attained on May 25, 2017 by author Rosendahl Huber and Frontiers in Immunology, in accordance with the Creative Common Attribution licence (https://creativecommons.org/licenses/by/4.0/). Disclaimer: All software used on the site, and the copyright in the code constituting such software, is the property of or is licensed to Frontiers and its use is restricted in accordance with the Frontiers Terms and Conditions. All copyright, and all rights therein, are protected by national and international copyright laws.
The role of TFH cells in EV-A71 infected children has not been explored in depth. Elevated levels of circulating TFH cells with high inducible costimulatory (ICOS) and programmed death-1 (PD-1) expression were significantly correlated with serum-specific NtAb-EV-A71 levels, in addition to high concentrations of IL-21 and IL-6 in EV-A71 infected patients. In addition, the IL-6 and IL-21 mRNA expression in the peripheral blood mononuclear cells (PBMCs) were also significantly greater as compared to the healthy volunteers. This implies that TFH cells and associated cytokines are vital in the regulation of humoral responses in severe HFMD infection [17]. Up to date, little is known about the protective T cell immunity in EV-A71 infected individuals. An ex vivo IFN-γ ELISPOT assay was performed in EV-A71 infected children and in healthy adult controls of the CD4+ and CD8+ T cell responses to overlapping peptides encompassing the VP1 and 3Dpol regions. Most of the severe HFMD patients showed 3Dpol CD4-dependent responses, whereas there was a small group of infected children who had VP1 and 3Dpol CD8-dependent responses. The authors discovered that no VP1-specific CD4-dependent T cell response was detected in 30% of the adult controls. They also characterized 24 peptides consisting of potential CD4+ T cell epitopes [18]. Their observations surmised that it would be predominantly the 3Dpol CD4-dependent T cell responses that were vital in protective immunity against EV-A71 infections.
Cellular Immunity and Vaccine Design
Cellular cytokines such as interferon-gamma-inducible protein-10 (IP-10), IFN-γ and monokine induced by gamma interferon (Mig) have been found to decrease viral load in tissues of EV-A71 infected mice. In severe EV-A71 infection, high levels of IP-10 elevated expressions of Mig, IFN-γ and CD8 T cell infiltrated the murine brain. They deduced that IP-10 was vital for viral clearance in tissues and increased survival rate of mice [19, 20]. There were also increased levels of IL-1β, IL-10, MIP-2, TNF-α, IFN-γ and IL-6 in immuno-deficient AG129 mice that were afflicted with severe HFMD caused by EV-A71 [21].
Ideally, a vaccine should contain multiple CD4+ and CD8+ epitopes that are specific for different MHC-molecules [13]. This would be beneficial as the CD4+ epitopes would provide co-stimulation during CD8+ T cell priming and this would lead to the formation of memory CD8+ T cells [22]. The presence of CD4+ T cells optimizes expression of CD8+ T cells and enhances the level of NtAbs [14]. Stimulation of CD4+ T cell responses have been detected after infections by viruses such as the poliovirus [23], influenza [24], dengue [25], and Epstein Barr viruses [26]. Other studies found that the protection conferred by these vaccines did not require the presence of NtAbs but only occurred in a unique T-cell mediated condition [27]. Existing cytotoxic CD4+ T cells induced in response to influenza H3N2 or H1N1 were linked with milder illness in the absence of NtAbs.
There are a few requirements for an effective immune response to be elicited against viruses. Firstly, there must be an ample number of cytotoxic CD8+ T cells to kill virus-infected cells. This was exemplified in chimpanzees that were completely devoid of CD8+ T cells which could not clear the HBV. However, when the CD8+ T cells were re-introduced into the primates, the chimpanzees were able to completely clear the virus [28]. This shows that CD8+ T cells are vital for optimizing the efficacy of a vaccine. In addition, a broad T cell response would be beneficial. Administration of a vaccine against influenza virus that contained multiple epitopes (3LP; a mixture of the M1, PA, and NS1 lipopeptides) was more effective than a vaccine with a single epitope (either M1-LP, PA-LP, or NS1-LP) [29]. Another requirement for an efficient antiviral response would be the involvement of poly-functional Th1 and Th2 cytokines. As CD8+ T cells are the primary cell types in clearing virus infections, they are characterized by the Th1 cytokines being produced (IFN-γ, IL-2, TNF-α, MIP-1β). IFN-γ has been found to increase the cytotoxic function of CD8+ T cells, whereas TNF-α causes apoptosis of viral-infected cells. Such poly-functional responses are known to elicit more extensive T cell dissemination and protection against viral infection [14].
In addition, increasing number of studies are illustrating that EV-A71 inhibits production of IFNs at different points in the cell signaling pathways. For example, the EV-A71 3Cpro inhibited IFN synthesis in mice, by inducing cleavage of IRF7 that inactivated IFN production [30, 31]. The EV-A71 2A proteinase could also decrease production of IFN but through a different mechanism. It was postulated that this proteinase could directly cleave the Mitochondrial Antiviral signaling protein (MAV) into 3 cleavage fragments that were not able to activate type 1 IFN production [32]. Interestingly, the 2A proteinase could also degrade IFN-1 receptor (IFNAR1), thereby inhibiting IFN-mediated phosphorylation of Janus activated kinase (Jak)-signal transducers and activators of transcription (STAT) [33]. However, contrasting results were attained by Liu et al. (2014) when they deduced that EV-A71 did not inhibit IFNAR1, but inhibited JAK1-STAT signaling through the down regulation of JAK1 [34]. Hence, further studies should be carried out to explore the effect of EV-A71 towards IFNAR1 and downstream signaling pathways.
It has been demonstrated that EV-A71 has the capability to infect CD14+ cells and to migrate to other target tissues such as the cardiovascular and lymphatic tissues. This could occur through the circulation of the virus in the blood and lymphatic systems. CD14 is a pattern recognition molecule and is a major hallmark of immature denditric cells and monocytes. CD14+ cells could stimulate the replication of T cells, hence modulated the cytokine expression profiles after EV-A71 infection. With the back-transfusion of EV-A71-infected CD14+ cells in donor rhesus monkeys, an adaptive immune response was elicited. There was significant increase in the levels of functional cytokines such as IFN-γ, IL-6 and TNF-α. The authors hypothesized that the immune responses activated by EV-A71-infected CD14+ cells was indicative of a skewed Th2 response [35].
A panel of 120 immune factors in HFMD cases was screened and the macrophage inflammatory protein (MIP-1β), granulocyte-macrophage colony stimulating factor (GM-CSF), granulocyte-colony stimulating factor (G-CSF), monocyte chemoattractant protein-1 (MCP-1), IL-2, IL-23, and IL-33 secretions were significantly elevated in severe/very severe patients who had neurological damage in comparison to healthy subjects. Hence, these chemokines could serve as potential predictors for EV-A71-afflicted brainstem encephalitis and pulmonary edema [36]. The elevation of IL-23 and IL-33 in severe patients was not surprising as these cytokines have been associated with cell-mediated immune responses such as Th2 cell polarization [37]. Interestingly, out of the 7 elevated immune mediators in severe patients, G-CSF and MCP-1 were markedly increased in the CSF than in the plasma. These chemokines could be dominant mediators elicited during neurological damage in the CSF as chemokines are usually elicited within a few hours after injury in the central nervous system (CNS) cells to activate mononuclear phagocytes [38]. Hence, these chemokines are integral components of the adaptive immunity to decrease severe tissue damage.
In another study measuring 50 cytokines/chemokines in the serum samples of HFMD patients and controls, there were elevated levels of TNF-α, IL-12p40, IL-3 and IL-6 in patients who displayed relatively mild HFMD symptoms, indicative of systemic inflammation. For patients who did not exhibit any cardiopulmonary symptoms, there was a reduction in other biomarkers such as soluble ICAM-1, CXCL-1, IL-1Ra, IL-8, IL-16, and CCL27. There was no change in expression levels of HFMD biomarkers such as CD-107a, IFN-γ or IL-2 after patients were treated with intravenous methylprednisolone. Instead, they registered an increase in expression levels of IL-17A which was unexpected as this cytokine was not a HFMD-associated biomarker [39]. There was significant correlation between cytokines such as TNF-α, IL-10, IL-4, IL-6, and IFN-γ with HFMD severity. Higher levels of these HFMD-associated biomarkers were detected compared to patients who showed mild symptoms, especially during the 2-5th day after disease onset. Their findings were indeed implicative that TNF-α, IL-4, IL-6, IL-10 and IFN-γ were involved in disease severity [40]. Another study concurred that there were elevated levels of IL-10, IL-13, and IFN-γ in patients presenting symptoms of brainstem encephalitis [41].
Hence, a simple parameter to evaluate a good vaccine-elicited response is the measurement of multifunctional cytokines such as IFN-γ, TNF-α, IL-2 and IL-6. Particularly, TNF-α and IFN-γ have been shown to mediate clearing of a wide range of microorganisms, from viruses, parasites, fungi to bacteria [42, 43]. IL-2 is vital to promote the expansion of CD8+ and CD4+ T cells, either in a paracrine or autocrine way, which could increase CD8+ T cell memory function [44]. IL-6, in particular has been closely associated with patients with pulmonary edema and encephalitis [45]. In addition, the measurement of CD107a could also be an indirect gauge of the levels of degranulation, indicative of the cytolytic activity of CD8+ T cells via release of granzymes. This is because CD107a is expressed on CD8+ T cell surface, following activation with a corresponding peptide, in concordance with the production of intracellular IFN-γ [46]. Hence, the measurement of the recurrence of multi-functional CD8+ T cells that elicit 2 or more of such cytokines are invaluable. In fact, improved control of viral diseases such as influenza, Hepatitis C and HIV has been attributed to the increase in multifunctional T cells [47].
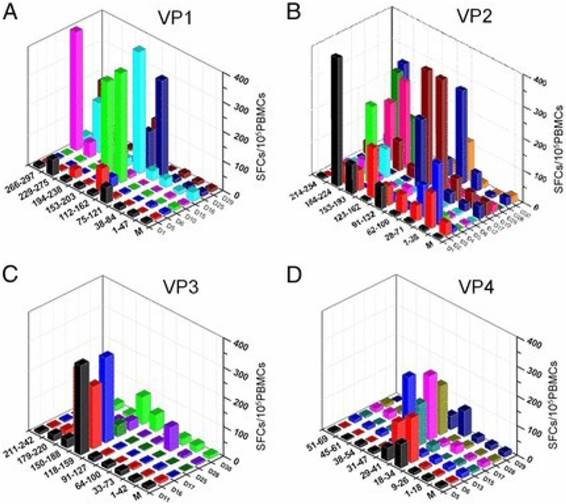
It is increasingly clear that there is a need to elicit both quality CD4+ and CD8+ T cell responses in vaccine development. In the first comprehensive study of EV-A71-responsive cellular immunity in healthy human volunteers, majority of the broad T cell responses (approximately 93%) were predominated by antigens (Ag) from the VP2 as compared with Ags from VP1, VP3 and VP4 after in vitro expansion, consistent with the high NtAb levels (Fig. 2). Interestingly, these cellular responses were mainly CD4+ T cell-dependent. Upon further investigation, the broad EV-A71-responsive cellular responses were mainly by IFN-γ-secreting cells and not by the IL-2-secreting cells. The authors utilized a panel of 110 overlapping peptides encompassing the 4 structural Ags of EV-A71 to investigate existing T cell epitope profiles of volunteers in a HLA-independent restricted methodology [48]. Hence, the dominant immunogenicity of the VP2 Ag provides vital connotations in the growing knowledge of EV-A71 cellular immune responses as intensive research has been conducted on the VP1 Ag as well as some HLA-class II-restricted T cell epitopes [49].
In a LAV, knowledge of the location of CD4+ and CD8+ epitopes are important. For example, characterization of T cell epitope reactivity towards the dengue virus (DENV) genome has been carried out and 30 novel T cell epitopes were discovered in Asian dengue patients, out of which 21 were CD8+ epitopes and the remaining were CD4+ epitopes. In addition, they found that the CD4+ T cells recognize the same viral proteins as the B cells (envelope, capsid), whereas the CD8+ T cells preferred the nonstructural proteins NS3 and NS5. The differential recognition of viral proteins by the CD4+ and CD8+ T cells could possibly be attributed to the dosage and timing of viral protein production [50]. The authors hypothesize that peptide recognition by CD4+ T cells are in the context of antigen-specific B cells. Such information is vital in vaccine design as it may be necessary to include nonstructural proteins, particularly NS3 and NS5 in their DENV vaccine formulations to elicit robust CD8+ T cell response [51].
Although NtAbs are vital in the prevention of infection, studies have shown that it would be the CD8+ T cells that are able to contain viral load and prevent disease progression into more fatal cases [14]. It can be observed that in the most fatal EV-A71infections with pulmonary oedema, blood samples showed higher levels of cellular IL-1β, IL-6 and tumor necrosis factor-α (TNF- α) [11]. High CSF levels of IL-6 have been shown to be correlated with disease severity. This explains why fatal HFMD infections generally afflict the very young as they would have weaker cellular immunity although they may have some maternal EV-A71 NtAbs. This has led to an increased understanding about disease progression of EV-A71 post-infection, which range from an absence of symptoms (~71%) to fatality (~0.05%) [52]. In fact, polymorphism of the cytotoxic T lymphocyte protein-4 (CTLA-4) has been linked to changes in cellular immunity which is correlated to disease severity. The CTLA-4 polymorphism could contribute towards a higher frequency of G/G allele in patients with meningoencephalitis, compared to those without this condition. They postulated that children with CTLA-4 polymorphism, in combination with an altered cellular but not humoral response, could be associated with severe EV-A71 [53].
Diverse distribution of VP2-specific T cell immunogenic regions [48]. The T cell responses were specified to a region of 40-50 aa for VP1 (A), VP2 (B), VP3 (C), and VP4 (D). The y-axis displays the strength of responses as represented by SFCs per 105 PBMCs. The x- and z-axes show the peptide spanning region and subjects, respectively. The magnitude of responses to VP2 were more diversely distributed along the Ag (B), whereas there was an intensely focused distribution of responses to the central region in VP1, VP3, and VP4. Copyright 2013. The American Association of Immunologists, Inc. Permission obtained on April 12, 2017 from the American Association of Immunologists, Inc.
HLA-DR variation and peptide vaccine design
The peptide vaccine is an emerging experimental vaccine worldwide due to its low cost, easy production and is a well-defined epitope-based vaccine. Although peptide vaccines can elicit production of antigen-specific T cells, it has low immunogenicity which could pose as a major challenge. Hence, it could be formulated with FDA-approved adjuvants or delivered with nanoparticles. The immunogenicity of peptide vaccines against influenza virus could be increased by augmenting the affinity for the MHC molecules though exchanging amino acids in wild type sequences with non-proteogenic amino acids. This in turn increased the level of CD8+ T cell response. Specifically, HLA-A*0201 epitopes GILGFVFTL, FMYSDFHFI and NMLSTVLGV were chosen and for each epitope, chemically altered peptide ligands (CPLs) that showed greater binding affinity than their wild type was manufactured. They showed that 50% CPLs of each epitope demonstrated elevated IFN-γ levels in the splenocytes of HLA-A*0201 transgenic mice that were inoculated with the CPLs. The authors demonstrated that this strategy could be extended to other alleles and could effectively increase the immunogenicity and range of preventive T cell-targeted peptide vaccines [54].
Hence, it was a natural progression in the design of peptide vaccines that strong T cell epitopes were introduced in its construction. Researchers identified T cell epitopes in the C terminal of the CS protein that were exposed by multiple MHC class II molecules which could be recognized by CD4+ T cells from humans and mice [55]. That was remarkable as conventionally, one MHC molecule could only at most bind to a few peptides [56]. The uniqueness of such an approach led to the characterization of many more epitopes displaying similar properties [57]. Hence, these epitopes were referred to as universal CD4+ T cell epitopes. With such universal epitopes, there could be strong humoral and cellular immunity being induced in individuals with diverse MHC haplotypes. This was exemplified by a second generation malaria vaccine that included a universal CD4+ epitope and the CS protein that could elicit broad-based immunity [58]. With the existence of universal CD4+ T cell epitopes, this has greatly overcome the problem of the restriction of T cell recognition of antigens due to the high variability of human MHC genes.
Several human leukocyte antigen (HLA) alleles are correlated with more severe disease and weaker CD8+ responses. HLA-A*0101 and A*2402 restricted responses had the lowest anti-DENV response, whereas HLA-B*35:01 restricted responses displayed the highest magnitude of anti-DENV response [59]. It has been reported that HLA-A and not HLA-B is linked to Dengue Hemorrhagic Fever (DHF) [60]. This might explain why individuals who carry non-protective alleles (HLA-A) and disease-exacerbating antibodies developed DHF with secondary DENV infection. HLA A*24:02 and A*02:01 were associated with lower anti-DENV response [59]. Their results were compared with blood donors from a Sri Lankan population and there was a strong relationship between the magnitude of CD8+ T cell responses and HLA allele restriction. This implies the effect of HLA genes on the nature of cellular responses. The poignant observation was that uniquely, the B*35:01 DENV-specific T cells were linked with elevated levels of the programmed death 1 protein (PD-1). PD-1 is a member of the CD28 superfamily and is a marker on virus-specific CD8+ T cells to measure the degree of T cell activation. It is believed that PD-1 may prevent excessive tissue damage while preserving anti-viral function. The expression of certain HLA alleles significantly influenced the spread and intensity of CD8+ T cell responses [61].
A vital factor in the design of peptide vaccines would be HLA-DR variation. Some notable examples would be that for Hepatitis B. About 5-10% of vacinees had poorly induced vaccine responses which possibly could be attributed to the presence of HLA-DRB1*0701 and DQB*0202 haplotypes in the poor responders [62]. As for the measles vaccine, the predominant limiting factor for robust T cell response towards the measles virus phosphoprotein (MV-P) and nucleoprotein (MV-N) would be the presence of HLA-DRB1 in vacinees who respond weakly towards the vaccination [63]. Hence, it can be surmised that varying MHC class II variants have different peptide binding preferences and this would aid in the prediction of CD4+ T cell responses to vaccine challenge. Therefore, it is vital to define targets of CD4+ T cell responses that are induced by vaccination. Studies to investigate MHC specificity are of paramount importance as peripheral blood mononuclear cells (PBMCs) from varied individuals typically express multiple MHC class II proteins. In addition, epitope validation studies such as isolation of epitope-specific T cell lines and research on cross-reactivity with epitopes processed from viruses can contribute towards knowledge on MHC specificity [64].
The importance of CD8+ T cell responses have been illustrated by numerous studies that HIV escape mutations frequently happen at HLA-binding sites specific for CD8 epitopes. For example, certain HLA-alleles are correlated with protection from HIV disease development and there exists a transient relationship between an increase in CD8+ T cells and reduction in viral titer [65, 66]. In addition, a univariate analysis of 219 EV-A71 infections were discovered and TNF-α promoter type II (-308 allele), HLA-A33 and HLA-DR17 were linked to complicated severe cases. Amongst the 3 alleles, patients with HLA-A33 were significantly most susceptible to EV-A71 infection when compared to patients carrying the rest of the alleles. Interestingly, HLA-A33 is a rare genotype among Caucasians but is a common genotype among Asians [67], hence the study increases our understanding on the frequent occurrence of EV-A71 cyclical epidemics affecting the Asia Pacific region. Indeed, immune factors such as varying clinical and histopathological CD4+ T responses were able to mediate tissue damage caused by virulent EV-A71 strains [68]. It is intriguing that different EV-A71 genotypes can produce mild or severe symptoms in different individuals, dependent on the genetic makeup and immune status of individuals.
Although many studies have elucidated that VP1 is the major target of NtAb in mice, Tan et al. (2013) showed that it was the VP2 Ag that is the predominant antigen in eliciting T cell responses in humans. Consistent with their study, Wei and colleagues (2012) utilized an in silico-predicted peptide pool comprising amino acids 176-193 from VP2-24, and was able to characterize a highly conserved T cell epitope in the VP2. In their study, 15 epitopes were identified and three (A3, A8 and A14) of them were dominant in eliciting CD4+ T cell response. Amongst the three, A3 was the most predominant epitope and was highly conserved among coxsackieviruses that cause HFMD, specifically CV-A4, CV-A6, and CV-A16, and it has the ability to attach to multiple HLA-DR (antigen D Related) alleles. Interestingly, this A3 epitope, which is located in the VP2 capsid, was also highly conserved amongst other HFMD-unrelated coxsackieviruses, echoviruses and polioviruses. The authors also discovered that the CD4+ T cells specific for EV-A71, A3 could cross-react with PV A3v epitope, implicating the possibility for immune responses that are cross-reactive and related to PV immunization or other enteroviruses in early childhood. There could be potential immuno-regulation of subsequent CD4+ T cell response to EV-A71 infection or vaccination [69].
As the LAV has been very successful in reducing rate of poliomyelitis worldwide, studies have shown that the LAV against EV-A71 was able to induce a strong immune response. Similar to an infection with PV, infection with EV-A71 can be easily preventable by LAVs that can induce both cellular and humoral immunity as well. There were high levels of CD69+ and CD3+ T cells in mice administered with the EV-A71 LAV upon challenge with the wild type virus. As EV-A17 and CV-A16 share many common biological and molecular characteristics, the authors repeated the immunization experiments with a CV-A16 LAV. It was expected that this would yield similar results as exemplified by an elevated expression of CD69+ and CD3+ T cells in the CV-A16-immunized mice after EV-A71 challenge [70].
There remains considerable interest in understanding the varying cellular responses in individuals vaccinated with the LAV against mumps. A research group carried out HLA genotyping of 346 healthy children who had been administered with 2 doses of the mumps LAV. Interestingly, there was significant immune response variations of some HLA class II alleles to the mumps LAV. Examples of such HLA alleles would be the DQA1 (*0101, *0105, *0401, and *0501) and DRB1 (*0101, *0301, *0801, *1001, *1201, and *1302). There were significant cellular and humoral immune responses being elicited, specifically by minor alleles for four single nucleotide polymorphisms (SNPs) within the IL-12RB and IL-10RA genes. Their findings highlighted the association between HLA variations and its varying effects on the immune response induced by the mumps LAV [71].
Conclusion
The use of the IV could prevent mild infections caused by EV-A71 but there remains a need for further research to evaluate the efficacy of the IV in comparison with the VLPs and LAVs to confer protection against severe HFMD. Having the IV as the only vaccine candidate that is protective against mild HFMD may not be optimal. In addition, phase IV clinical trials should be conducted to gauge long-term efficacy and optimization of the IV over a much larger target population. There remains a greater need to understand the role of cellular immunity such as CD4+ and CD8+ T cells in the immuno-protection of EV-A71. Identification of broad-based (cross-reactive) immunogenic CD8+ T cell epitopes that will induce more cytotoxic CD8+ T cell responses has to be carried out. The characterization of universal CD4+ T helper and CD8+ T cell epitopes to elicit broad-based immunity in individuals who express certain MHC allelic variants will contribute to the rational design of an optimal EV-A71 peptide vaccine that could trigger immune responses from both cellular and humoral systems. The link between gene polymorphisms and HLA variations to elicit humoral and cellular immune responses may provide further insights into the design of an ideal vaccine targeting EV-A71 causing severe HFMD.
Abbreviations
EV-A71, Enterovirus-A71; PV, Poliovirus; HFMD, Hand, Foot and Mouth Disease; NtAb, neutralizing antibody; CV-A16, Coxsackievirus-A16, MHC, major histocompatibility complex; HLA, human leukocyte antigen; ag, antigen; APC, antigen presenting cell; nt, nucleotide; DNA, deoxyribonucleic acid; IV, inactivated vaccine; LAV, live attenuated vaccine; VLP, virus-like particle; 3DPol, 3D Polymerase; TNF, Tumour Necrosis Factor; IFN, Interferon; IL, Interleukin; TFH, T follicular helper.
Acknowledgements
We are sincerely grateful for the financial support of the Fundamental Research Grant Scheme (FRGS/2/2014/ST03/SYUC//1) from the Malaysian Ministry of Education, Dr Ranjeet Bhagwan Singh Medical Research Grant (EXT-SST-RCBS-RBS-2018-01) from MOSTI, Malaysia and the Sunway Vice-Chancellor Research Fellowship to Isabel Yee.
Competing Interests
The authors have declared that no competing interest exists.
References
1. WHO Western Pacific Region. Surveillance summary in the Western Pacific Region. 2018.
2. Yi EJ, Shin YJ, Kim JH, Kim TG, Chang SY. Enterovirus 71 infection and vaccines. Clin Exp Vaccine Res. 2017;6:4-14
3. Zhu F, Xu W, Xia J, Liang Z, Liu Y, Zhang X. et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med. 2014;370:818-828
4. Li JX, Song YF, Wang L, Zhang XF, Hu YS, Hu YM. et al. Two-year efficacy and immunogenicity of Sinovac enterovirus 71 vaccine against hand, foot and mouth disease in children. Expert Rev Vaccines. 2016;15:129-137
5. Chumakov K, Ehrenfeld E. New generation of inactivated poliovirus vaccines for universal immunization after eradication of poliomyelitis. Clin Infect Dis. 2008;47:1587-1592
6. Zhao H, Li HY, Han JF, Deng YQ, Zhu SY, Li XF. et al. Novel recombinant chimeric virus-like particle is immunogenic and protective against both enterovirus 71 and coxsackievirus A16 in mice. Sci Rep. 2015;5:7878-7895
7. Gong M, Zhu H, Zhou J, Yang C, Feng J, Huang X. et al. Cryo-electron microscopy study of insect cell-expressed enterovirus 71 and coxsackievirus A16 virus-like particles provides a structural basis for vaccine development. J Virol. 2014;88:6444-6452
8. Liu W, Wu S, Xiong Y, Li T, Wen Z, Yan M. et al. Co-circulation and genomic recombination of coxsackievirus A16 and enterovirus 71 during a large outbreak of hand, foot, and mouth disease in central China. PLoS ONE. 2014;9:e96051
9. Zhang H, An D, Liu W, Mao Q, Jin J, Xu L. et al. Analysis of cross-reactive neutralizing antibodies in human HFMD serum with an EV71 pseudovirus-based assay. PLoS ONE. 2014;9:e100545
10. Chou AH, Liu CC, Chang JY, Lien SP, Guo MS, Tasi HP. et al. Immunological evaluation and comparison of different EV71 vaccine candidates. Clin Dev Immunol. 2012;2012:1-8
11. Chang LY, Hsiung CA, Lu CY, Lin TY, Huang FY, Lai YH. et al. Status of cellular rather than humoral immunity is correlated with clinical outcome of enterovirus 71. Pediatr Res. 2006;60:466-471
12. Zhu FC, Meng FY, Li JX, Li XL, Mao QY, Tao H. et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2013;381:2024-2032
13. Huber RS, van Beek J, de Jonge J, Luytjes W, van Baarle D. T cell responses to viral infections - opportunities for peptide vaccination. Front Immunol. 2014;5:1-12
14. Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247-258
15. Demkowicz WE, Littaua RA, Wang J, Ennis FA. Human cytotoxic T-cell memory: long-lived responses to vaccinia virus. J Virol. 1996;70:2627-2631
16. Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4+ T cells in immunity to viruses. Nat Rev Immunol. 2012;12:136-148
17. Wu J, Cui D, Yang X, Lou J, Lin J, Ye X. et al. Increased frequency of circulating follicular helper T cells in children with hand, foot, and mouth disease caused by enterovirus 71 infection. J Immunol Res. 2014;2014:1-11
18. Dang S, Gao N, Li Y, Li M, Wang X, Jia X. et al. Dominant CD4-dependent RNA-dependent RNA polymerase-specific T-cell responses in children acutely infected with human enterovirus 71 and healthy adult controls. Immunology. 2014;142:89-100
19. Lin YW, Chang KC, Kao CM, Chang SP, Tung YY, Chen SH. Lymphocyte and antibody responses reduce enterovirus 71 lethality in mice by decreasing tissue viral loads. J Virol. 2009;83:6477-6483
20. Shen FH, Tsai CC, Wang LC, Chang KC, Tung YY, Su IJ. et al. Enterovirus 71 infection increases expression of interferon-gamma-inducible protein 10 which protects mice by reducing viral burden in multiple tissues. J Gen Virol. 2013;94:1019-1027
21. Khong WX, Foo DGW, Trasti SL, Tan EL, Alonso S. Sustained high levels of interleukin-6 contribute to the pathogenesis of enterovirus 71 in a neonate mouse model. J Virol. 2011;85:3067-3076
22. Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171-192
23. Wahid R, Cannon MJ, Chow M. Virus-specific CD4+ and CD8+ cytotoxic T-cell responses and long-term T-cell memory in individuals vaccinated against polio. J Virol. 2005;79:5988-5995
24. Bourgault I, Gomez A, Gomard E, Picard F, Levy JP, Gomrad E. A virus-specific CD4+ cell-mediated cytolytic activity revealed by CD8+ cell elimination regularly develops in uncloned human antiviral cell lines. J Immunol. 1989;142:252-256
25. Tai DF, Lin CY, Wu TZ, Chen LK. Recognition of dengue virus protein using epitope-mediated molecularly imprinted film. Anal Chem. 2005;77:5140-5143
26. Martorelli D, Muraro E, Merlo A, Turrini R, Rosato A, Dolcetti R. Role of CD4+ cytotoxic T lymphocytes in the control of viral diseases and dancer. Int Rev Immunol. 2010;29:371-402
27. Bachler BC, Humbert M, Palikuqi B, Siddappa NB, Lakhashe SK, Rasmussen RA. et al. Novel biopanning strategy to identify epitopes associated with vaccine protection. J Virol. 2013;87:4403-4416
28. Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH. et al. CD8+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68-76
29. Tan ACL, La Gruta NL, Zeng W, Jackson DC. Precursor frequency and competition dictate the HLA-A2-restricted CD8+ T cell responses to influenza A infection and vaccination in HLA-A2.1 transgenic mice. J Immunol. 2011;187:1895-1902
30. Lei X, Xiao X, Xue Q, Jin Q, He B, Wang J. Cleavage of interferon regulatory factor 7 by enterovirus 71 3C suppresses cellular responses. J Virol. 2013;87:1690-1698
31. Lee YP, Wang YF, Wang JR, Huang SW, Yu CK. Enterovirus 71 blocks selectively type I interferon production through the 3C viral protein in mice. J Med Virol. 2012;84:1779-1789
32. Wang B, Xi X, Lei X, Zhang X, Cui S, Wang J. et al. Enterovirus 71 protease 2Apro targets MAVS to inhibit anti-viral type I interferon responses. PLoS Path. 2013;9:e1003231
33. Lu J, Yi L, Zhao J, Yu J, Chen Y, Lin MC. et al. Enterovirus 71 disrupts interferon signaling by reducing the interferon receptor 1. J Virol. 2012;86:3767-3776
34. Liu Y, Zhang Z, Zhao X, Yu R, Zhang X, Wu S. et al. Enterovirus 71 inhibits cellular type I interferon signaling by downregulating JAK1 protein expression. Viral Immunol. 2014;27:267-276
35. Wang J, Pu J, Huang H, Zhang Y, Liu L, Yang E. et al. EV71-infected CD14+ cells modulate the immune activity of T lymphocytes in rhesus monkeys. Emerg Microbes Infect. 2013;2:e44
36. Zhang Y, Liu H, Wang L, Yang F, Hu Y, Ren X. et al. Comparative study of the cytokine/chemokine response in children with differing disease severity in enterovirus 71-induced hand, foot, and mouth disease. PLoS ONE. 2013;8:e67430
37. Haraldsen G, Balogh J, Pollheimer J, Sponheim J, Küchler AM. Interleukin-33 - cytokine of dual function or novel alarmin? Trends Immunol. 2009;30:227-33
38. Glabinski AR, Balasingam V, Tani M, Kunkel SL, Strieter RM, Yong VW. et al. Chemokine monocyte chemoattractant protein-1 is expressed by astrocytes after mechanical injury to the brain. J Immunol. 1996;156:4363-4368
39. Zeng M, Zheng X, Wei R, Zhang N, Zhu K, Xu B. et al. The cytokine and chemokine profiles in patients with hand, foot and mouth disease of different severities in Shanghai, China, 2010. PLoS Negl Trop Dis. 2013;7:e2599
40. Duan G, Yang H, Shi L, Sun W, Sui M, Zhang R. et al. Serum inflammatory cytokine levels correlate with hand-foot-mouth disease severity: A nested serial case-control study. PLoS ONE. 2014;9:e112676
41. Wang SM, Lei HY, Huang KJ, Wu JM, Wang JR, Yu CK. et al. Pathogenesis of enterovirus 71 brainstem encephalitis in pediatric patients: Roles of cytokines and cellular immune activation in patients with pulmonary edema. J Infect Dis. 2003;188:564-570
42. Skinner MA, Ramsay AJ, Buchan GS, Keen DL, Ranasinghe C, Slobbe L. et al. A DNA prime-live vaccine boost strategy in mice can augment IFN-γ responses to mycobacterial antigens but does not increase the protective efficacy of two attenuated strains of Mycobacterium bovis against bovine tuberculosis. Immunology. 2003;108:548-555
43. Bloom BR, Flynn J, McDonough K, Kress Y, Chan J. Experimental approaches to mechanisms of protection and pathogenesis in M. tuberculosis infection. Immunobiology. 1994;191:526-536
44. Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890-893
45. Weng KF, Chen LL, Huang PN, Shih SR. Neural pathogenesis of enterovirus 71 infection. Microb Infect. 2010;12:505-510
46. Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M. et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65-78
47. Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol. 2007;81:8468-8476
48. Tan S, Tan X, Sun X, Lu G, Chen CC, Yan J. et al. VP2 dominated CD4+ T cell responses against enterovirus 71 and cross-reactivity against coxsackievirus A16 and polioviruses in a healthy population. J Immunol. 2013;191:1637-1647
49. Foo D, Macary P, Alonso S, Poh C. Identification of human CD4 T-cell epitopes on the VP1 capsid protein of enterovirus 71. Viral Immunol. 2008;21:215-224
50. Moutaftsi M, Bui H-H, Peters B, Sidney J, Salek-Ardakani S, Oseroff C. et al. Vaccinia virus-specific CD4+ T cell responses target a set of antigens largely distinct from those targeted by CD8+ T cell responses. J Immunol. 2007;178:6814-68120
51. Rivino L, Kumaran EAP, Jovanovic V, Nadua K, Teo EW, Pang SW. et al. Differential targeting of viral components by CD4+ versus CD8+ T lymphocytes in dengue virus infection. J Virol. 2013;87:2693-2706
52. Chang LY, King CC, Hsu KH, Ning HC, Tsao KC, Li CC. et al. Risk factors of enterovirus 71 infection and associated hand, foot, and mouth disease/herpangina in children during an epidemic in Taiwan. Pediatrics. 2002;109:e88-e95
53. Yang KD, Yang MY, Li CC, Lin SF, Chong MC, Wang CL. et al. Altered cellular but not humoral reactions in children with complicated enterovirus 71 infections in Taiwan. J Infect Dis. 2001;183:850-856
54. Rosendahl Huber SK, Luimstra JJ, van Beek J, Hoppes R, Jacobi RHJ, Hendriks M. et al. Chemical modification of influenza CD8+ T-cell epitopes enhances their immunogenicity regardless of immunodominance. PLoS ONE. 2016;11:e0156462
55. Sinigaglia F, Guttinger M, Kilgus J, Doran DM, Matile H, Etlinger H. et al. A malaria T-cell epitope recognized in association with most mouse and human MHC class II molecules. Nature. 1988;336:778-780
56. Guillet JG, Lai MZ, Briner TJ, Smith JA, Gefter ML. Interaction of peptide antigens and class II major histocompatibility complex antigens. Nature. 1986;324:260-262
57. Panina-Bordignon P, Tan A, Termijtelen A, Demotz S, Corradin G, Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989;19:2237-2242
58. Nardin EH, Calvo-Calle JM, Oliveira GA, Nussenzweig RS, Schneider M, Tiercy J-M. et al. A totally synthetic polyoxime malaria vaccine containing Plasmodium falciparum B cell and universal T cell epitopes elicits immune responses in volunteers of diverse HLA types. J Immunol. 2001;166:481-489
59. Weiskopf D, Angelo MA, de Azeredo EL, Sidney J, Greenbaum JA, Fernando AN. et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc Natl Acad Sci. 2013;110:e2046-e53
60. Loke H, Bethell DB, Phuong CXT, Dung M, Schneider J, White NJ. et al. Strong HLA class I-restricted T cell responses in dengue hemorrhagic fever: A double-edged sword? J Infect Dis. 2001;184:1369-1373
61. de Alwis R, Bangs DJ, Angelo MA, Cerpas C, Fernando A, Sidney J. et al. Immunodominant dengue virus-specific CD8+ T cell responses are associated with a memory PD-1+ phenotype. J Virol. 2016;90:4771-4779
62. Kruger A, Adams P, Hammer J, Böcher WO, Schneider PM, Rittner C. et al. Hepatitis B surface antigen presentation and HLA-DRB1* - lessons from twins and peptide binding studies. Clin Exp Immunol. 2005;140:325-332
63. Devaux P, Cattaneo R. Measles virus phosphoprotein gene products: Conformational flexibility of the P/V protein amino-terminal domain and C protein infectivity factor function. J Virol. 2004;78:11632-11640
64. Stern LJ, Calvo-Calle JM. HLA-DR: Molecular insights and vaccine design. Curr Pharm Des. 2009;15:3249-3261
65. Goulder P, Price D, Nowak M, Rowland-Jones S, Phillips R, McMichael A. Co-evolution of human immunodeficiency virus and cytotoxic T-lymphocyte responses. Immunol Rev. 1997;159:17-29
66. Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA. et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857-860
67. Chang LY, Chang IS, Chen WJ, Huang YC, Chen GW, Shih SR. et al. HLA-A33 is associated with susceptibility to enterovirus 71 infection. Pediatrics. 2008;122:1271-1276
68. Yue Y, Li P, Song N, Li B, Li Z, Guo Y. et al. Genomic and immunologic factors associated with viral pathogenesis in a lethal EV71 infected neonatal mouse model. Mol Med Rep. 2016;13:4183-4190
69. Wei R, Yang C, Zeng M, Terry F, Zhu K, Yang C. et al. A dominant EV71-specific CD4+ T cell epitope is highly conserved among human enteroviruses. PLoS ONE. 2012;7:e51957
70. Wu TC, Wang YF, Lee YP, Wang JR, Liu CC, Wang SM. et al. Immunity to avirulent enterovirus 71 and coxsackie A16 virus protects against enterovirus 71 infection in mice. J Virol. 2007;81:10310-10315
71. Ovsyannikova IG, Jacobson RM, Dhiman N, Vierkant RA, Pankratz VS, Poland GA. Human leukocyte antigen and cytokine receptor gene polymorphisms associated with heterogeneous immune responses to mumps viral vaccine. Pediatrics. 2008;121:e1091-e1099
Author Biography
Professor Chit Laa Poh graduated with a PhD (Microbiology) from Monash University in 1981. She joined the Department of Microbiology, National University of Singapore (NUS) as an Assistant Professor in 1981 and became an Associate Professor in 1998. She has served as an Adjunct Senior Scientist of the National University Hospital and Senior Research Fellow of the National Eye Research Institute of Singapore from 2005-2007. She has published 100 internationally-refereed papers in journals such as Journal of Biological Chemistry, Journal of Bacteriology, Journal of Clinical Microbiology, PLoS One, and many others. She has been a Premium Member of the American Society of Microbiology and a member of the Japan Society for Biotechnology. Prof Poh has collaborations with Harvard University faculty members on the development of recombinant Herpes Simplex Virus and the study of small RNAs in gene regulation.
Pinn Tsin Isabel Yee was from the University's Scholars Programme in the National University of Singapore (NUS) and she graduated with an Honours in Biochemistry. She is currently undergoing her PhD in Biology and has a MSc. in Life Sciences with Distinction conferred by Sunway University and Lancaster University (UK). She received the Tan Sri Dato' Seri Dr. Jeffrey Cheah Scholastic Award for outstanding academic excellence. She was a Module Coordinator for the Diploma of Medical Biotechnology in Singapore Polytechnic where she received the Most Outstanding Mentor award from Singapore's Ministry of Education. She is currently a Research Fellow from the Centre for Virus and Vaccine Research at Sunway University.
![]() Corresponding author: Chit Laa Poh, Sunway University; pohcledu.my; Tel.: +603-7491-8622 (ext. 7338).
Corresponding author: Chit Laa Poh, Sunway University; pohcledu.my; Tel.: +603-7491-8622 (ext. 7338).

 Global reach, higher impact
Global reach, higher impact