3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2018; 15(9):937-943. doi:10.7150/ijms.22224 This issue Cite
Research Paper
Susceptibility to serious skin and subcutaneous tissue disorders and skin tissue distribution of sodium-dependent glucose co-transporter type 2 (SGLT2) inhibitors
1. Department of Pharmacokinetics, Kyoto Pharmaceutical University, Kyoto 607-8414, Japan
2. Department of Medical Informatics and Bioinformatics, Kobe University Hospital, Kobe 650-0017, Japan
3. Department of Regulatory Science, Nagoya City University Graduate School of Pharmaceutical Sciences, Nagoya 467-8603, Japan
4. Department of Hospital Pharmacy, Otsu City Hospital, Otsu 520-0804, Japan
Received 2017-8-4; Accepted 2018-5-27; Published 2018-6-13
Abstract
Objectives: In Japan, sodium-glucose co-transporter type 2 (SGLT2) inhibitors have been reported to be associated with serious skin and subcutaneous tissue disorders. A post-marketing surveillance (PMS) study suggested that the association was specific for ipragliflozin and, to a lesser extent for dapagliflozin. These studies were performed to confirm the association of 6 SGLT2 inhibitors with serious skin disorders in a clinical setting, to elucidate the role of melanin in serious skin disorders and to understand the underlying mechanisms.
Methods: The latest PMS records were retrieved from the Japanese Adverse Drug Event Report (JADER) database, and the associations were analyzed by data mining techniques. In silico 3-D docking simulation of SGLT2 inhibitors with melanin was performed using the MOE software. The skin tissue distribution of SGLT2 inhibitors was evaluated using albino rats after oral administration at clinical doses.
Results: The adjusted reporting odds ratio (95% confidential limit) was 1.667 (1.415, 1.963) for ipragliflozin, 0.514 (0.317, 0.835) for dapagliflozin, 0.149 (0.048, 0.465) for tofogliflozin, 0.624 (0.331, 1.177) for luseogliflozin, 0.590 (0.277, 1.257) for canagliflozin and 0.293 (0.073, 1.187) for empagliflozin, when drugs other than the SGLT2 inhibitors were referred, and the association was detected only for ipragliflozin in clinical use. In silico 3-D docking simulation suggested the influence of melanin in ipragliflozin-specific serious skin disorders. The skin tissue-to-plasma concentration ratio of ipragliflozin was 0.45 ± 0.20 (±SD) at 1 hr after administration and increased in a time-dependent manner to 5.82 ± 3.66 at 24 hr (p<0.05), but not in case of other SGLT2 inhibitors.
Conclusions: Serious skin disorders were suggested to be specific for ipragliflozin. Interaction with melanin might be implicated in ipragliflozin-specific serious skin disorders. Ipragliflozin was retained in the skin tissue, which suggested its interaction with the skin tissue in serious skin disorders.
Keywords: Sodium-glucose co-transporter type 2 (SGLT2), skin and subcutaneous tissue disorders, ipragliflozin, dapagliflozin
Introduction
Sodium-glucose co-transporters (SGLTs) are responsible for renal reabsorption of filtered glucose. The majority of glucose reabsorption is controlled by a low-affinity, high-capacity SGLT2 expressed predominantly in the S1 segment of proximal tubule and the remaining by a high-affinity, low-capacity SGLT1 in the later part S2/S3 [1-4]. The inhibition of SGLT2 was proposed as a novel strategy for the treatment of type 2 diabetes mellitus (T2DM); several inhibitors, including dapagliflozin, canagliflozin, and empagliflozin, are readily available in the world [5]. They alleviate hyperglycemia by decreasing reabsorption and thereby increasing urinary excretion of glucose. Clinical investigations have proven that SGLT2 inhibitors reduce glycated hemoglobin, with a minimal risk of hypoglycemia [6-8]. Commonly observed adverse events include urogenital infection, polyuria, and dehydration [6-8]. Recently, a randomized clinical trial with 7020 patients at a high risk for cardiovascular events (the EMPA-REG OUTCOME trial) demonstrated that empagliflozin lowered the rate of primary composite cardiovascular outcome and death from any cause when it was added to standard care [9]. In addition to secondary prevention of cardiovascular events, a study has indicated that canagliflozin is useful for primary prevention (the CANVAS program) [10, 11]. Potential reasons for this result include weight loss, blood pressure reduction, uricosuric effect, anti-inflammatory effect, osmotic diuresis and anti-arrhythmic effect, in addition to anti-hyperglycemic effect [12, 13].
In Japan, serious adverse events were reported immediately after the first inhibitor, ipragliflozin, was introduced into clinical practice in 2014 [14]. Based on the 3-month post-marketing surveillance (PMS) data in Japan, Yabe et al. suggested that the incidence of serious adverse events was higher with ipragliflozin than with dapagliflozin, tofogliflozin and luseogliflozin, which were introduced after ipragliflozin, [14]. Unexpectedly, serious skin and subcutaneous tissue disorders were conspicuous and suggested to be specific for ipragliflozin and, to a lesser extent for dapagliflozin [14]. Serious skin disorders included serious generalized rash, eruption, urticaria, erythema, and eczema, and the symptoms were usually observed within 2 weeks of treatment initiation, but sometimes on the first day [14]. They are not notable in countries other than Japan and this may be explained by the fact that ipragliflozin is available only in Japan [14]; however, the association with dapagliflozin suggested an inter-species difference in susceptibility.
In this study, the latest PMS data retrieved from the Japanese Adverse Drug Event Report (JADER) database managed by the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan were used to compare SGLT2 inhibitors in terms of association with serious skin disorders. Moreover, in silico 3-D docking simulation of SGLT2 inhibitor with melanin was performed to elucidate the role of melanin in serious skin disorders. The skin tissue distribution of SGLT2 inhibitors was also evaluated using albino rats to understand the mechanisms underlying serious skin disorders.
Methods
Materials
Ipragliflozin, dapagliflozin, canagliflozin, and empagliflozin were obtained from Med-Chemexpress Co., Ltd. (New Jersey, USA). Tofogliflozin was kindly provided by Kowa Company Ltd. (Tokyo, Japan). Luseogliflozin was extracted from commercially available tablets (brand name: Lusefi®, Taisho Pharmaceutical Co., Ltd., Tokyo, Japan). Its purity was confirmed by 1H-NMR and acceptable for the standard analyte. All other reagents were of analytical grade and were used without further purification.
JADER data mining
The JADER dataset was downloaded from the PMDA's homepage (http://www.pmda.go.jp/) on May 22, 2017. It includes 4 tables: 1) patient demographic data (gender, age, weight, etc.), 2) drug information (drug name, etc.), 3) adverse events, and 4) medical history. This database structure complies with the international safety reporting guidelines, ICH E2B. In this study, 691359 records from the first quarter of 2004 to the fourth quarter of 2016 were used. The records without data on age and gender were excluded. The records with 2 or more SGLT2 inhibitors were also excluded. To analyze the associations between SGLT2 inhibitors and serious skin disorders, the reporting odd ratio (ROR) and its two-sided 95% confidence limit (CI) were calculated from two-by-two contingency table [15]. Given the report number with a drug and an adverse event of interest is n11, that with a drug and without an adverse event is n10, that without a drug and with an adverse event is n01, and that without a drug and without an adverse event is n00, the ROR is defined as (n11 n00)/(n10 n01) [15]. Considering the effects of age and gender, ROR was adjusted by logistic regression analysis using the equation: log (ROR) = β0 + β1*A + β2*G + β3*D, where β1, β2 and β3 are partial regression coefficients, A is age, B is gender, and D is intake of any SGLT2 inhibitor. Adjusted ROR was calculated as exp (β3).
Definition of serious skin disorders in datamining
Seventy-seven skin-related adverse events were defined as serious skin disorders according to the MedDRA ver.19.1, including preferred terms of 1) dermatitis acneiform, 2) rash pruritic, 3) dermatitis allergic, 4) viral rash, 5) Stevens Johnson reaction, 6) Stevens-Johnson syndrome, 7) dermatitis due to drugs and medicines taken internally, 8) dermatitis due to drugs and medicaments taken internally, 9) papule, 10) rash popular, 11) acute generalised exanthematous pustulosis, 12) perivascular dermatitis, 13) blood blister, 14) oral mucosa erosion, 15) oral mucosal eruption, 16) oral mucosal blistering, 17) lip erosion, 18) lip blister, 19) oral papule, 20) eosinophilic pustular folliculitis, 21) erythema, 22) rash erythematous, 23) eczema, 24) blister, 25) dermatitis bullous, 26) genital rash, 27) adult onset Still's disease, 28) dermatitis contact, 29) rash generalised, 30) herpes zoster, 31) dermatitis medicamentosa, 32) toxic epidermal necrolysis, 33) necrolysis epidermal toxic (Lyell type), 34) toxic epidermal necrolysis, 35) necrolysis epidermal toxic (Lyell type), 36) dandruff, 37) solar dermatitis, 38) mucosa vesicle, 39) enanthema, 40) impetigo, 41) pustular psoriasis, 42) rash pustular, 43) exfoliative rash, 44) dermatitis exfoliative, 45) rash, 46) rash maculo-papular, 47) rash macular, 48) subcutaneous haematoma, 49) haemorrhage subcutaneous, 50) subcutaneous abscess, 51) eczema asteatotic, 52) skin erosion, 53) skin test positive, 54) dermatitis, 55) skin necrosis, 56) dry skin, 57) skin fissures, 58) skin swelling, 59) skin disorder, 60) skin warm, 61) oculomucocutaneous syndrome, 62) mucocutaneous rash, 63) skin exfoliation, 64) skin lesion, 65) skin discomfort, 66) skin oedema, 67) skin degenerative disorder, 68) pain of skin, 69) epidermal necrosis, 70) epidermal necrolysis, 71) epidermolysis bullosa, 72) epidermolysis, 73) rubella, 74) dermatitis medicamentosa, 75) drug eruption, 76) scab and 77) urticaria.
In silico 3-D docking simulation of SGLT2 inhibitor with melanin
The 3-D structure of melanin monomer was constructed by using the MOE software (Chemical Computing Group Inc., Montreal, QC, Canada). The monomer structure was assembled into a planar tetramer unit and four layers of which were stacked in accordance with a previous report [16]. This model structure of melanin was subjected to molecular mechanics (MM) calculations using MOE with the MMFF94x force field and with explicit water molecules until the root mean square gradient was 0.01 kcal/mol/Å. After 250 ps heating process to attain 310 K as starting temperature, 5000 ps production run of molecular dynamic (MD) simulation was performed at 310 K using NAMD software [17]. The 3-D structures of SGLT2 inhibitors were obtained from the ChemIDPlus (Register number: ipragliflozin, 761423-87-4; luseogliflozin, 898537-18-3; dapagliflozin, 461432-26-8; tofogliflozin, 903565-83-3; canagliflozin, 842133-18-0; empagliflozin, 864070-44-0). Hundred docking runs for the model structure of melanin with each SGLT2 inhibitor were performed by using the MOE-Dock program. The docking sites were defined as the overall the molecular surface of the melanin structure. The docking results were clustered for each complex using group average clustering method using R software (https://www.R-project.org/) based on the difference of atomic coordinates. The number of clusters was determined by the upper tail method [18].
Skin tissue, kidneys, and small intestine distribution in rats
All the animal studies were performed after the experimental protocol was approved by an institutional review board of the Kyoto Pharmaceutical University, Japan, and were in accordance with the Kyoto Pharmaceutical University Guidelines for Animal Experimentation. Male Wistar rats (10 weeks of age) were purchased from Nippon SLC Co., Ltd. (Hamamatsu, Japan). All rats were housed in a temperature-controlled facility with a 12-h light/dark cycle. Food and water were made available continuously. The rats were randomly assigned to 5 treatment groups (n = 9 or 10 for ipragliflozin, n = 4 for other SGLT2 inhibitors). After fasting overnight with free access to water, rats were orally administered with 1.0 mg/kg ipragliflozin, 0.1 mg/kg dapagliflozin, 0.4 mg/kg tofogliflozin, 0.05 mg/kg luseogliflozin, or 2.0 mg/kg canagliflozin prepared in 1% carboxymethyl-cellulose sodium in distilled water (2 mL/kg). The dose was decided on the basis of the clinical daily dose in Japan; 50 mg, 5 mg, 20 mg, 2.5 mg, and 100 mg, respectively. Blood samples (250 μL) were collected from the external left jugular vein at 1, 8, and 24 h after the administration and transferred to heparinized centrifuge tubes. The blood samples were centrifuged at 12,000 rpm for 15 min, and the obtained plasma samples were stored at -80 degrees until analyzed. Immediately thereafter, the rats were euthanized by cervical dislocation and their kidneys were perfused with pH 7.4 phosphate-buffered saline (PBS). The abdominal skin tissue (with hair removed), kidneys, and small intestine were removed, washed with PBS, and blotted with filtered paper. They were homogenized in 9-fold volume of PBS of each sample weight by using a homogenizer (PT10-35 GT, Kinematica AG, Switzerland). After centrifugation at 3,000 x g for 15 min, the supernatant fractions were stored at -80 degrees until analysis. The concentrations were determined using the liquid chromatography-tandem mass spectrometry according to previous reports [19-23]. The lower limit of quantification was 10 ng/mL, 2.5 ng/mL, 0.5 ng/mL, 0.5 ng/mL, and 10 ng/mL for plasma, and 40 ng/g, 5.0 ng/g, 5.0 ng/g, 5.0 ng/g, and 5.0 ng/g for skin tissue, kidneys and small intestine, respectively.
Adjusted reporting odds ratio of SGLT2 inhibitors for serious skin and subcutaneous tissue disorders
| Drugs other than SGLT2 inhibitors | T2DM drugs other than SGLT2 inhibitors | |
|---|---|---|
| Ipragliflozin | 1.667 (1.415, 1.963) | 2.395 (2.019, 2.840) |
| Dapagliflozin | 0.514 (0.317, 0.835) | 0.703 (0.432, 1.144) |
| Tofogliflozin | 0.149 (0.048, 0.465) | 0.200 (0.064, 0.623) |
| Luseogliflozin | 0.624 (0.331, 1.177) | 0.843 (0.447, 1.592) |
| Canagliflozin | 0.590 (0.277, 1.257) | 0.792 (0.371, 1.690) |
| Empagliflozin | 0.293 (0.073, 1.187) | 0.398 (0.098, 1.617) |
The reference was drugs or T2DM drugs other than SGLT2 inhibitors.
The reporting odds ratio was adjusted by logistic regression analysis, and shown with 95% confidence limit in the parentheses.
Statistical analysis
The normal distribution was assumed for the tissue distribution data, and all values reported are the mean ± standard deviation (SD). The unpaired Student's t-test or one-way ANOVA was used for group comparisons, and P values of less than 0.05 were considered significant.
Results
JADER data mining
A total number of 660915 records were used, of which 2673 records included one of 6 the SGLT2 inhibitors. Serious skin disorders were included in 44977 records. Table 1 presents the adjusted ROR values. The adjusted ROR (95% CI) values were 1.667 (1.415, 1.963) for ipragliflozin, 0.514 (0.317, 0.835) for dapagliflozin, 0.149 (0.048, 0.465) for tofogliflozin, 0.624 (0.331, 1.177) for luseogliflozin, 0.590 (0.277, 1.257) for canagliflozin and 0.293 (0.073, 1.187) for empagliflozin, when drugs other than the SGLT2 inhibitors were referred. With reference to T2DM drugs other than the SGLT2 inhibitors, the values were 2.395 (2.019, 2.840), 0.703 (0.432, 1.144), 0.200 (0.064, 0.623), 0.843 (0.447, 1.592), 0.792 (0.371, 1.690) and 0.398 (0.098, 1.617), respectively.
In silico 3-D docking simulation of SGLT2 inhibitor with melanin
Table 2 indicates the result of cluster analyses of 3-D docking simulation. A total of 13 clusters were suggested for 6 SGLT2 inhibitors. Of them, clusters 1, 2, and 9 and clusters 3, 5, and 6 were the same regarding the 3-D features. The docking scores (sums of docking scores) in clusters 1, 2 and 9 were -7.35 ± 0.35 (-191.10) for ipragliflozin, -6.60 ± 0.91 (-85.80) for dapagliflozin, -6.10 ± 0.36 (-61.00) for tofogliflozin, -7.36 ± 0.30 (-36.80) for luseogliflozin, -7.50 ± 1.02 (-270.00) for canagliflozin, and -6.95 ± 0.80 (-132.05) for empagliflozin. Figure 1 shows typical docking forms in cluster 2.
Typical docking forms in cluster 2 for melanin with SGLT2 inhibitors. Green: Melanin in the four layers of planar tetramers, purple: SGLT2 inhibitors. No docking form was suggested for empagliflozin.
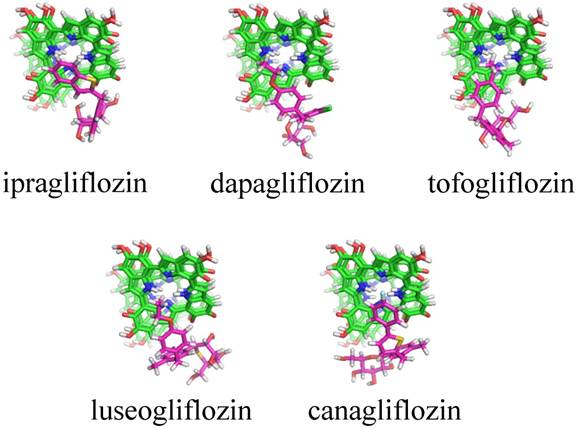
Cluster analyses of 3-D docking results of the SGLT2 inhibitors with melanin
| Cluster | Ipragliflozin | Dapagliflozin | Tofogliflozin | Luseogliflozin | Canagliflozin | Empagliflozin |
|---|---|---|---|---|---|---|
| 1, 2, 9 | -7.35±0.35 (26) | -6.60±0.91 (13) | -6.10±0.36 (10) | -7.36±0.30 (5) | -7.50±1.02 (36) | -6.95±0.80 (19) |
| 3, 5, 6 | -5.54±0.61 (29) | -5.58±0.51 (86) | -5.12±0.59 (3) | -5.80±0.52 (42) | -6.03±0.50 (66) | |
| 4 | -5.34±0.46 (45) | -5.31 (2) | -5.54±0.45 (52) | -5.65 (2) | ||
| 7 | -4.04 (1) | |||||
| 8 | -5.57±0.40 (85) | |||||
| 10 | -5.74 (1) | |||||
| 11 | -6.17±0.54 (64) | |||||
| 12 | -7.57±0.69 (9) | |||||
| 13 | -5.70±0.40 (4) |
The values were mean ± SD of docking score with the number of complex (cluster size) in parentheses.
SGLT2 inhibitor concentrations in skin tissue (ng/g) in rats
| 1 h | 8 h | 24 h | |
|---|---|---|---|
| Ipragliflozin | 151.7±53.4 (0.45±0.20) | 168.2±28.7 (2.15±2.55) | 90.6±38.9 (5.82±3.66) * |
| Dapagliflozin | 42.6±24.7 (0.74±0.30) | 13.0±5.0 (0.20±0.05) * | ND ( - ) |
| Tofogliflozin | 103.4±29.2 (0.59±0.16) | 27.1±3.2 * (0.77±0.21) | - a) ( - ) a) |
| Luseogliflozin | ND ( - ) | ND ( - ) b) | ND ( - ) b) |
| Canagliflozin | 37.0±18.5 (0.18±0.18) | 82.6±22.9 (0.17±0.03) | 20.1±11.6 (0.26±0.20) |
The values are mean ± SD of the concentrations with their ratios to plasma concentrations in the parentheses.
* P < 0.05, compared with the data at 1hr.
ND: below the limit of detection.
a) The data included 1 or more data of ND.
b) The plasma concentration was below the limit of detection.
SGLT2 inhibitor concentrations in kidneys (ng/g) in rats
| 1 h | 8 h | 24 h | |
|---|---|---|---|
| Ipragliflozin | 1419.5±405.2 (5.18±2.85) | 1091.1±455.8 (10.45±7.51) | 301.0±86.9 * (16.81±10.85) |
| Dapagliflozin | 1034.1±520.8 (25.83±22.88) | 530.8±191.0 (8.39±2.02) | 170.1±40.2 * (15.63±4.76) |
| Tofogliflozin | 848.5±115.8 (5.11±1.82) | 492.0±101.0 * (13.68±2.88) * | 200.8±42.9 * (98.62±35.07) * |
| Luseogliflozin | 94.9±74.3 (43.96±34.79) | 80.1±51.7 ( - ) b) | - a) ( - ) b) |
| Canagliflozin | 802.7±269.2 (3.69±2.84) | 1014.1±306.0 (2.11±0.54) | 636.2±222.5 (7.55±2.62) |
The values are mean ± SD of the concentrations with their ratios to plasma concentrations in the parentheses.
* P < 0.05, compared with the data at 1hr.
a) The data included 1 or more data of ND (below the limit of detection.).
b) The plasma concentration was below the limit of detection.
SGLT2 inhibitor concentrations in small intestine (ng/g) in rats
| 1 h | 8 h | 24 h | |
|---|---|---|---|
| Ipragliflozin | 729.5±377.2 (2.63±1.48) | 347.6±104.8 (3.89±3.73) | - a) ( - ) a) |
| Dapagliflozin | 37.9±10.3 (0.71±0.35) | 19.7±6.7 (0.32±0.13) | ND ( - ) |
| Tofogliflozin | 1356.0±512.6 (7.48±0.97) | 250.1±114.5 * (7.07±3.41) | - a) ( - ) a) |
| Luseogliflozin | 250.8±178.4 (115.53±82.81) | - a) ( - ) b) | - a) ( - ) b) |
| Canagliflozin | 2925.2±1074.2 (12.93±9.88) | 440.1±117.2 * (0.92±0.20) | 325.9±218.0 * (4.05±2.83) |
The values are mean ± SD of the concentrations with their ratios to plasma concentrations in the parentheses.
* P < 0.05, compared with the data at 1hr.
ND: below the limit of detection.
a) The data included 1 or more data of ND.
b) The plasma concentration was below the limit of detection.
Skin tissue, kidneys, and small intestine distribution in rats
Table 3 lists the SGLT2 inhibitor concentrations in skin tissue after oral administration to rats. The skin tissue concentration of ipragliflozin was 151.7 ± 53.4 ng/g at 1 hr, which was higher than that of the other inhibitors. At 24 hr, ipragliflozin and canagliflozin were detected, but the other inhibitors were not detected. The skin tissue-to-plasma concentration ratio was 0.45 ± 0.20 for ipragliflozin at 1 hr. The ratio increased in a time-dependent manner to 5.82 ± 3.66 at 24 hr, but this phenomenon was not observed for other inhibitors. Tables 4 and 5 show the data for the kidneys and small intestine. The kidney-to-plasma concentration ratio increased time-dependently for tofogliflozin and tended to increase for ipragliflozin. There was no time-dependent increase in the ratio for the small intestine for any of the inhibitors.
Discussion
The information obtained by data mining of spontaneous reports is only a signal, and no causal relationship can be demonstrated [24]. The World Health Organization defines a “signal” as “reported information on a possible causal relationship between an adverse event and a drug, the relationship being unknown or incompletely documented previously” [25]. In the case of ROR, a signal is detected when the lower limit of 95% CI exceeds 1 [24, 25]. Thus, the data mining of JADER database suggested that only ipragliflozin was associated with serious skin disorders, whereas the other 5 SGLT2 inhibitors were not associated. In the report by Yabe et al. [14], dapagliflozin also showed a causal relationship; however, this was not observed in our analysis. In our study, dapagliflozin and tofogliflozin were associated with serious skin disorders to a lesser extent (Table 1). The sampling size or period of PMS data may have caused this difference. A recently published comprehensive evaluation of dapagliflozin confirmed that it was not associated with serious skin disorders in Asian patients [26].
In silico 3-D docking simulation is now widely used worldwide, especially by pharmaceutical companies, for the discovery of seed or lead compounds and/or optimization of the chemical structure of candidate compounds (lead optimization). The 3-D docking model between SGLT2 and SGLT2 inhibitors was recently reported by Liu et al. [27]. This novel technology was used to determine the anti-diabetic mechanisms of aspalathin and nothofagin found in rooibos (Aspalathus linearis) [27]. An inhibitor, dapagliflozin, was used as a positive control and the results indicated that SGLT2 might be the target of aspalathin and nothofagin [27]. We also obtained the same model (data not shown). Recently, we used this methodology to analyze the association between a non-steroidal anti-inflammatory derivative and transthyretin [28] and that between anti-IL-13 monoclonal antibodies and IL-13 [29]. In this study, the 3-D docking simulation was performed to determine the association of SGLT2 inhibitors and melanin based on the assumption that there was inter-species difference in the susceptibility. The docking score indicates the stability of a SGLT2 inhibitor-melanin complex; lower value indicates a more stable complex. The sums of docking scores were -592.00 for ipragliflozin, -569.90 for dapagliflozin, -560.42 for tofogliflozin, -574.22 for luseogliflozin, -665.30 for canagliflozin, and -632.08 for empagliflozin, which was inconsistent with the PMS data published by Yabe et al. [14] or our data (Table 1). However, the sum of the docking scores for clusters 1, 2, and 9 was relatively low for ipragliflozin and canagliflozin compared with that of the other 4 SGLT2 inhibitors. Although the data for canagliflozin was inconsistent, the simulation suggested the possible role of melanin in ipragliflozin-specific serious skin disorders reported in Japan, although it may be explained by the fact that ipragliflozin is not available in countries other than Japan [14].
Various toxins, drugs, and chemicals are bound to melanin and retained in pigmented tissues, including the skin, eyes, and pigmented part of the brain for long periods of time [30-32]. Although the role of this phenomenon has not been clarified, melanin might act as a protective molecular barrier against exogenous toxic compounds [30-32]. In other words, such toxic compounds can be released into surrounding tissues after the addition of a new compound that more strongly binds to melanin, such as ipragliflozin. Additionally, recent basic investigations suggested that melanin exhibited a variety of biological activities, including anti-oxidant activities, anti-inflammatory effects, anti-carcinogenic effects, and modulation of the immune system via alteration of cytokine production [32]. Ipragliflozin-specific serious skin disorders might be related to the breakdown of skin tissue homeostasis. In contrast, dapagliflozin and tofogliflozin do not have such effects, because the sum of docking scores in clusters 1, 2, and 9 was relatively high, indicating they do not interact with melanin.
The data for canagliflozin from the in silico 3-D docking simulation implicated some factors other than melanin in serious skin disorders. Therefore, the skin tissue distribution was examined using albino rats. The skin tissue concentrations and their time profiles varied among SGLT2 inhibitors, reflecting variations in doses and biological fates. Thus, the skin tissue-to-plasma concentration ratio was calculated to clarify the difference in skin tissue distribution properties. The ratio of tofogliflozin and canagliflozin was constant until 24 h after administration, whereas that of ipragliflozin increased in a time-dependent manner. This indicates that the skin tissue distribution of tofogliflozin and canagliflozin was under rapid equilibrium across the vascular wall, whereas ipragliflozin was retained in the skin tissue despite of a decrease in plasma concentration. The rats are albino, and this retention of ipragliflozin indicates the binding to components other than melanin in the skin tissue. A time-dependent increase of the kidney-to-plasma concentration ratio was observed only for tofogliflozin. This can be explained by its lower plasma protein binding, although the mechanisms remain unclear.
In conclusion, serious skin disorders were suggested to be specific for ipragliflozin. Interaction with melanin may be involved in ipragliflozin-specific serious skin disorders. Ipragliflozin was retained in the skin tissue, which suggested that the serious skin disorders can be explained by local interaction in the skin tissue.
Acknowledgements
The study was supported by Kyoto Pharmaceutical University Fund for the Promotion of Collaborative Research. In silico 3-D docking simulation performed in Kobe University, Japan, was supported in part by Kowa Pharmaceutical Company Ltd. (Tokyo, Japan).
Competing Interests
See Acknowledgements.
References
1. Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol. Rev. 2011;91:733-794
2. Jabbour SA, Goldstein BJ. Sodium glucose co-transporter 2 inhibitors: blocking renal tubular reabsorption of glucose to improve glycaemic control in patients with diabetes. Int. J. Clin. Pract. 2008;62:1279-1284
3. Chao EC, Henry RR. SGLT2 inhibition-a novel strategy for diabetes treatment. Nat. Rev. Drug Discov. 2010;9:551-559
4. Fujita Y, Inagaki N. Renal sodium glucose cotransporter 2 inhibitors as a novel therapeutic approach to treatment of type 2 diabetes: Clinical data and mechanism of action. J. Diabetes Investig. 2014;5:265-275
5. Madaan T, Akhtar M, Najmi AK. Sodium glucose cotransporter 2 (SGLT2) inhibitors: Current status and future perspective. Eur. J. Pharm. Sci. 2016;93:244-252
6. Hasan FM, Alsahli M, Gerich JE. SGLT2 inhibitors in the treatment of type 2 diabetes. Diabetes Res. Clin. Pract. 2014;104:297-322
7. Scheen AJ. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75:33-59
8. Scheen AJ. SGLT2 inhibitors: Benefit/risk balance. Curr. Diab. Rep. 2016;16:92
9. Zinman B, Wanner C, Lachin JM. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015;373:2117-2128
10. Neal B, Perkovic V, Mahaffey KW. et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017;377:644-657
11. Mahaffey KW, Neal B, Perkovic V. et al. Canagliflozin for Primary and Secondary Prevention of Cardiovascular Events: Results From the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation. 2017 in press
12. Solini A. Role of SGLT2 inhibitors in the treatment of type 2 diabetes mellitus. Acta Diabetol. 2016;53:863-870
13. Kalra S. Sodium-glucose cotransporter 2 (SGLT2) inhibitors and cardiovascular disease: A systematic review. Cardiol. Ther. 2016;5:161-168
14. Yabe D, Nishikino R, Kaneko M. et al. Short-term impacts of sodium/glucose co-transporter 2 inhibitors in Japanese clinical practice: considerations for their appropriate use to avoid serious adverse events. Expert Opin. Drug Saf. 2015;14:795-800
15. van Puijenbroek EP, Bate A, Leufkens HG. et al. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol. Drug Saf. 2002;11:3-10
16. Kaxiras E, Tsolakidis A, Zonios G. et al. Structural model of eumelanin. Phys. Rev. Lett. 2006;97:218102
17. Phillips JC, Braun R, Wang W. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005;26:1781-1802
18. Mojena R. Hierarchical grouping methods and stopping rules: An evaluation. Comput. J. 1977;20:359-363
19. Kobuchi S, Ito Y, Yano K. et al. A quantitative LC-MS/MS method for determining ipragliflozin, a sodium-glucose co-transporter 2 (SGLT-2) inhibitor, and its application to a pharmacokinetic study in rats. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015;1000:22-28
20. Aubry AF, Gu H, Magnier R. et al. Validated LC-MS/MS methods for the determination of dapagliflozin, a sodium-glucose co-transporter 2 inhibitor in normal and ZDF rat plasma. Bioanalysis. 2010;2:2001-2009
21. Kobuchi S, Matsuno M, Fukuda E. et al. Development and validation of an LC-MS/MS method for the determination of tofogliflozin in plasma and its application to a pharmacokinetic study in rats. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016;1027:227-233
22. Kobuchi S, Matsuno M, Kawamoto M. et al. A simple and rapid LC-MS/MS method for quantitation of luseogliflozin in rat plasma and its application to a PK study. Bioanalysis. 2017;9:163-171
23. Kobuchi S, Yano K, Ito Y. et al. A validated LC-MS/MS method for the determination of canagliflozin, a sodium-glucose co-transporter 2 (SGLT-2) inhibitor, in a lower volume of rat plasma: application to pharmacokinetic studies in rats. Biomed. Chromatogr. 2016;30:1549-1555
24. Sakaeda T, Tamon A, Kadoyama K. et al. Data mining of the public version of the FDA adverse event reporting system. Int. J. Med. Sci. 2013;10:796-803
25. Lindquist M. The need for definitions in pharmacovigilance. Drug Saf. 2007;30:825-830
26. Mellander A, Billger M, Johnsson E. et al. Hypersensitivity events, including potentially hypersensitivity-related skin events, with dapagliflozin in patients with type 2 diabetes mellitus: A pooled analysis. Clin. Drug Investig. 2016;36:925-933
27. Liu W, Wang H, Meng F. In silico modeling of aspalathin and nothofagin against SGLT2. J. Theor. Comput. Chem. 2015;14:1550056
28. Qiang L, Guan Y, Li X. et al. CSP-1103 (CHF5074) stabilizes human transthyretin in healthy human subjects. Amyloid. 2017;24:42-51
29. Nakamura Y, Sugano A, Ohta M. et al. Docking analysis and the possibility of prediction efficacy for an anti-IL-13 biopharmaceutical treatment with tralokinumab and lebrikizumab for bronchial asthma. PLoS One. 2017;12:e0188407
30. Karlsson O, Lindquist NG. Melanin affinity and its possible role in neurodegeneration. J. Neural. Transm (Vienna). 2013;120:1623-1630
31. Karlsson O, Lindquist NG. Melanin and neuromelanin binding of drugs and chemicals: toxicological implications. Arch. Toxicol. 2016;90:1883-1891
32. ElObeid AS, Kamal-Eldin A, Abdelhalim MAK. et al. Pharmacological properties of melanin and its function in health. Basic Clin. Pharmacol. Toxicol. 2017;120:515-522
Author contact
![]() Corresponding author: Toshiyuki Sakaeda, Ph.D., Department of Pharmacokinetics, Kyoto Pharmaceutical University, Kyoto 607-8414, Japan, Tel: +81-75-595-4625, Fax: +81-75-595-4751, e-mail: sakaedatkyoto-phu.ac.jp
Corresponding author: Toshiyuki Sakaeda, Ph.D., Department of Pharmacokinetics, Kyoto Pharmaceutical University, Kyoto 607-8414, Japan, Tel: +81-75-595-4625, Fax: +81-75-595-4751, e-mail: sakaedatkyoto-phu.ac.jp

 Global reach, higher impact
Global reach, higher impact