3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2018; 15(7):666-673. doi:10.7150/ijms.23940 This issue Cite
Research Paper
ICOS signal facilitates Foxp3 transcription to favor suppressive function of regulatory T cells
Institute of Biotherapy, School of Laboratory Medicine and Biotechnology, Southern Medical University, Guangzhou, Guangdong, China
# These authors contributed equally to this work.
Received 2017-11-20; Accepted 2018-3-4; Published 2018-4-3
Abstract
Inducible costimulator (ICOS) plays an important role in the suppressive immunity mediated by regulatory T cells (Tregs), but the molecular regulation mechanism is not well known. Here we performed a study to explore the possible mechanism by which ICOS regulates the suppressive functions and survival of Tregs. This study showed that both the ICOS and CD28 signal could promote the survival of Tregs. However, ICOS but not CD28 improved the suppressive function of Tregs. Mechanistic studies demonstrated that ICOS could induce the transcription activity of Foxp3, by facilitating the nuclear factor of activated T cells (NFAT): Foxp3 over NFAT: activator protein 1 (AP-1). The results of Q-PCR showed that AP1 downstream regulatory genes (IL-2 and IL-6) were down-regulated, and Foxp3 downstream regulatory genes (IL-4, IL-10 and TGF-β) were up-regulated. Further, ICOS promoted anti-apoptosis may be by activating protein kinase B (Akt) signal. These findings demonstrated that ICOS signal could facilitate Foxp3 transcription in favor of survival and suppressive function of Tregs.
Keywords: Tregs, ICOS, Immunosuppression
Introduction
Regulatory T cells (Tregs) are a subset of T cells expressing CD4, CD25, and the Foxp3 transcription factor [1], and play a negative role in anti-tumor immune. Many studies demonstrated that Tregs widely existed in various of human malignancies, such as lung, ovarian, head and neck, gastrointestinal and skin cancer [2], and were closely associated with poor prognosis [3]. In addition, tumor-associated immunosuppression is a major obstacle in the generation of potent anti-tumor immunity [4]. Hence, it is essential to clarify the possible cellular and molecular mechanisms regulating immunosuppressive Tregs.
Inducible costimulator (ICOS) is a costimulatory molecule of the CD28 family [5]. According to the expression of ICOS, Tregs further divided into two subsets, ICOS+ Tregs and ICOS-Tregs [6]. The vitro experiment has confirmed that the two subsets had different capacity of cytokine production [7]. ICOS+Tregs have stronger abilities of suppression and survival than ICOS-Tregs [8]. Several studies indicated that ICOS+Tregs is a main suppressive subset, and both the number of ICOS+ Tregs and the ICOS+Tregs/total Tregs ratio increase in many malignancies, such as melanoma [9], gastric cancer [10] and papillary thyroid cancer [11]. However, the mechanism of Tregs accumulation in tumor microenvironment is not well known. Therefore, clarification of Tregs tolerance mechanism helps us to find some useful antitumor strategies.
ICOS and CD28 downstream signaling events are relate but not identical [12]. Previous studies reported that the CD28 cytoplasmic domain (YMNM motif) is a known binding site for growth factor receptor-bound protein 2 (Grb2), which can activate pathway. However, ICOS's motif is YMFM, which can bind phosphatidylinositol 3-kinase (PI3-K) but not Grb2 [13]. During T-cell activation, the activation of Ras up-regulated protein kinases 1/2 (Erk1/2), resulting in activation of AP1 transcription factor (dimers of cFos and cJun). AP1 transcription factor together with NFAT could promote the related genes transcription of T-cell activation. Foxp3 and AP-1 are two competing transcription factors to occupy NFAT, and the combination of NFAT and Foxp3 enhances suppressive function of Tregs [14]. Therefore, the discrepancy of the cytoplasmic domain between ICOS and CD28 may lead to different Ras-Erk1/2 activation and ultimately activate different transcription factor.
In this study, Tregs were isolated from the murine spleen and stimulated with anti-CD3 activating mAb , anti-CD3 activating mAb + anti-ICOS activating mAb or anti-CD3 activating mAb + anti-CD28 activating mAb. The purpose of this study was to explore the possible mechanism by which ICOS regulates the suppressive functions and survival of Tregs.
Materials and methods
Mice
BALB/c male mice (6-8 weeks) were purchased from the Laboratory Animal Center of Southern Medical University. All experimental procedures were in accordance with the Institution Guidelines for the Care and Use of Laboratory Animals.
Cells sorting
To obtained conventional T cells (Tcons), the spleens were collected, grinded and then isolated by lymphocyte separation liquid (Solarbio). CD4+CD25+ Tregs were isolated from Tcons by CD4+CD25+Regulatory T cell isolation kit according to the manufacturers' instructions (Miltenyi Biotec, Auburn, CA, USA). To observe the purity of Tregs, cells were stained with FITC-labeled CD4 antibody (Miltenyi Biotec), PE-labled anti-CD25 antibody (Miltenyi Biotec) for 30 min at 4-8℃ in the dark and then measured by flow cytometry (Becton Dickinson, CA).
Cells culture and stimulation
Tregs were cultured in RPMI 1640 supplemented with 10% FBS, at 37 °C under 5% CO₂ for 4 days. The cells of group1 were cultured in the presence of 1 μg/mL soluble anti-mouse CD3 and CD28 (1:1) antibody; the cells of group2 were cultured in the presence of 1μg/mL soluble anti-mouse CD3 and ICOS(1:1) activating antibody; the cells of group 3 were only cultured in the presence of 1 μg/mL soluble anti-mouse CD3 antibody.
Apoptosis analysis
Tregs were cultured for 2, 4 or 6 days as previously described, and then washed and resuspended at a concentration of 1×106 cells/ml. To evaluate the apoptosis of Tregs, cells were detected using flow cytometry by an Annexin-V/FITC and propidium iodide (PI) apoptosis detection kit (BD Bioscience). AnnexinV(+)/PI(-) and AnnexinV(+)/PI(+) were defined as early apoptosis and late apoptosis, respectively.
Functional analysis
Tcons were isolated as previously described and labeled with 5μM carboxyfluorescein succinimidy lester (CFSE) dilution. Tregs were activated with anti-mouse CD3 and CD28 (1:1) antibody or anti-mouse CD3 and ICOS(1:1) antibody or anti-mouse CD3 antibody alone. To detect Treg-mediated suppression, Tcons were activated with anti-mouse CD3 and CD28 (1:1) antibody and cultured either alone or in the presence of different activation of Tregs at a 3:1 Tcons: Tregs ratio for 2, 4 or 6 days, the proliferation of Tcons was determined by flow cytometry (Becton Dickinson, San Jose, CA).
Western blotting
Tregs were cultured for 4 days as previously described, cells were harvested and lysed by ice-cold lysis buffer (50 mM Tris-HCl pH7.5, 150 mM NaCl, 1% NP40, 1 mM PMSF, and 10 units/ml aprotinin) supplemented with protease inhibitor cocktail tablets, for 20 min at 4°C, then centrifuged at 12,000 rpm for 10 min at 4°C. Protein samples run on SDS-PAGE gels, transferred to PVDF membranes (Millipore, Bedford, MA, USA) and then blocked. The membranes were then incubated with primary antibodies overnight at 4°C, primary antibodies against the following proteins were used: Erk1/2(CST, #9102), Akt (CST, #4691), phospho-Erk1/2(CST, #9101), phospho-Akt(CST, #4060), Ras(CST, #3339), Bcl2(CST, #2870). The membranes were washed with TBST, and incubated with HRP-conjugated IgG secondary antibody (1:10000; Fdbio science) at room temperature for 1 h. Protein binding was detected using the enhanced chemiluminescence detection kit (Millipore, Billerica, MA, USA).
Co-Immunoprecipitation
After culture for 4 days as previously described, cells were harvested and lysed by ice-cold lysis buffer, then the concentration of extracts were determined by BCA Protein Assay Kit (Beyotime, Jiangsu, China). Sufficient amount of NFAT antibody (CST, #4389) added to 200mg proteins and gently rotated at 4°C overnight. To capture the immunocomplex, 25μl protein A+G agarose beads (Beyotime, Jiangsu, China) were added and gently rotated at 4°C for 2 h, and then centrifuged at 1500×g for 5 min at 4°C. The precipitate was washed for three times with ice-cold RIPA buffer, and resuspended with 5 × sample buffer and boiled for 10 min to dissociate the immunocomplex from the beads and obtained the supernatant. The protein levels of AP1/c-Jun and Foxp3 that combined with NFAT1 were detected using Western blot. The equal amounts of samples mixed with normal IgG was the negative control.
PCR primers and sequences
| Forward primer(5'-3') | Reverse primer(5'-3') | |
|---|---|---|
| β-actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
| IL-2 | GTGCTCCTTGTCAACAGCG | GGGGAGTTTCAGGTTCCTGTA- |
| IL-4 | ATCATCGGCATTTTGAACGAGG | TGCAGCTCCATGAGAACACTA |
| IL-6 | CTGCAAGAGACTTCCATCCAG | AGTGGTATAGACAGGTCTGTTGG |
| IL-10 | AGCCTTATCGGAAATGATCCAGT | GGCCTTGTAGACACCTTGGT |
| TGF-β | CTTCAATACGTCAGACATTCGGG | GTAACGCCAGGAATTGTTGCTA |
RNA extractions and reverse transcription real-time polymerase chain reaction (RT-qPCR)
Tregs were cultured for 3 days as previously described, the total RNA of Tregs of three groups were extracted using RNAiso Plus (Code No. 9108, TAKARA, Dalian, China) according to the manufacturer's protocol. The complementary deoxyribonucleic acid (cDNA) was synthesised using a PrimeScript RT reagent Kit with gDNA Eraser (Takara Bio, Dalian, China). The mRNA levels of cytokines were determined using the 7500 Real Time PCR System. The regulation threshold (expression fold-change) and p-values (a measure of the evidence against the null hypothesis according to the statistical test) were set to 1.2 and 0.05, respectively. The mRNA level was normalized againstβ-actin expression using an optimized comparative Ct (△△Ct) value method.
The PCR primers and their sequences were given in Table 1.
Statistical analysis
The results show the most typical one of three independent experiments. All data are indicate as mean ± SD and analyzed with SPSS version 20.0. Statistical comparisons were performed using one-way ANOVA with statistically significant considered at p < 0.05.
Results
ICOS contributes to the survival of Tregs
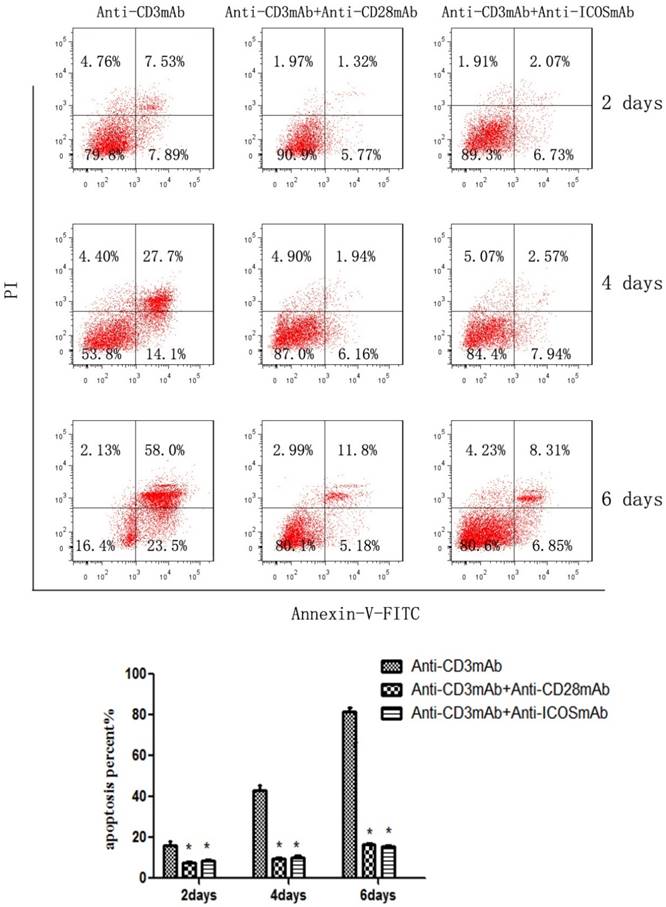
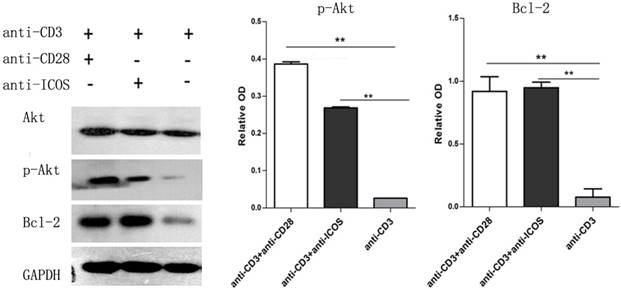
The CD4+CD25+ Tregs were isolated from Tcons by CD4+CD25+Regulatory T cell isolation kit. The results of flow cytometry analysis showed that the sorting rate of Tregs was >95%. Annexin-V/FITC and propidium iodide (PI) apoptosis detection kit was used to evaluate the effect of ICOS on Tregs survival. The results showed that the apoptotic ratio of Tregs was significantly lower in anti-CD3 mAb + anti-ICOS mAb group than that in the anti-CD3mAb group (Fig. 1, p<0.05), but there was no significant difference between anti-CD3 mAb + anti-ICOS mAb group and anti-CD3 mAb + anti-CD28 mAb group. To further exploring the possible mechanism, we examined the levels of apoptosis-related protein (Akt, p-Akt and Bcl2). The results of western blot showed that the levels of Bcl2 and p-Akt were significantly up-regulated in the costimulation-activated Tregs, including CD28 and ICOS (Fig. 2, p<0.05). These results indicated that ICOS contributes to the survival of Tregs.
ICOS enhances the suppressive function of Tregs
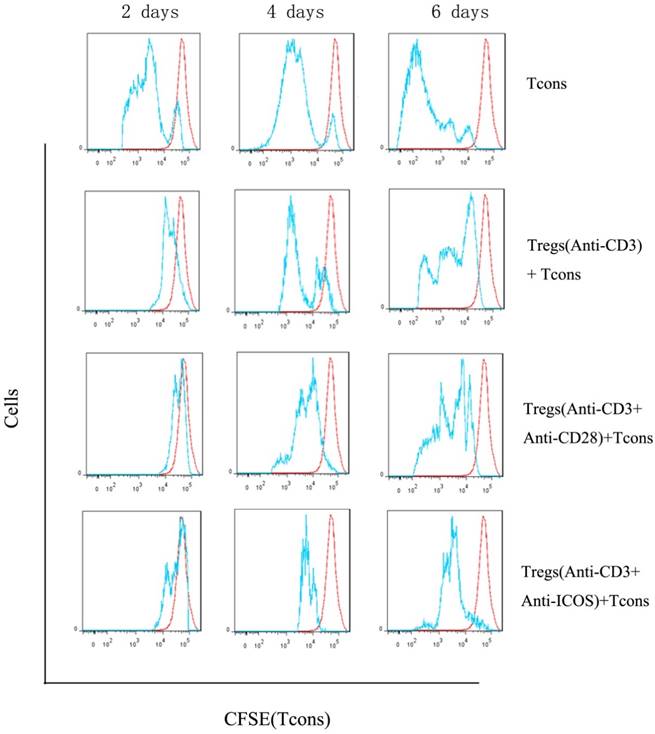
We compared proliferation of Tcons using a CFSE dilution assay. The results indicated that Tregs stimulated with anti-CD3 mAb + anti-ICOS mAb could significantly inhibit the proliferation of Tcons (Fig. 3), but there is no significant difference between anti-CD3 mAb + anti-CD28 mAb group and anti-CD3 mAb group. These results illustrated that ICOS could enhance the suppressive function of Tregs.
ICOS fails to activate Ras-Erk1/2 signal
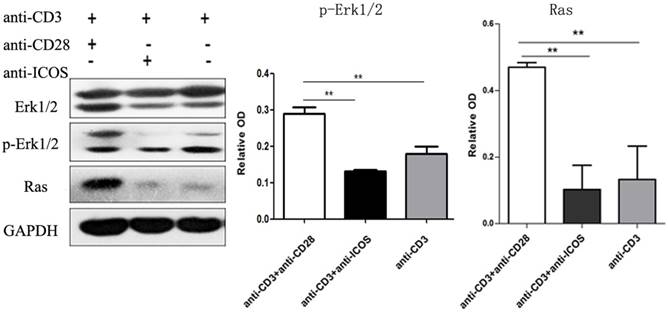
Western blotting showed that anti-CD3mAb + anti-CD28 mAb stimulation resulted in up-regulation of Ras and phosphorylated Erk1/2, but there was no significant difference between anti-CD3mAb + anti-ICOS mAb stimulation and anti-CD3mAb stimulation (Fig. 4, p<0.05). These results indicated that ICOS could not activate Ras-Erk1/2 signal.
ICOS favors NFAT: Foxp3 interaction
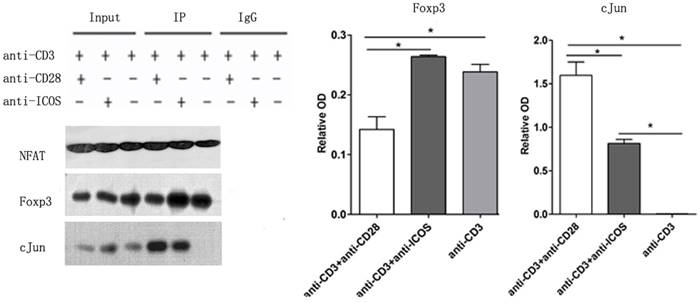
Immune co-precipitation was used to assess the role of ICOS in T-cells related transcription factor. AP1 is a dimer of cFos and cJun, so the detection of cJun stands for AP1 [15]. The results showed that there were more c-Jun in the NFAT precipitates in the Tregs stimulated with anti-CD3 mAb + anti-CD28 mAb. However, Tregs stimulated with anti-CD3 mAb + anti-ICOS mAb contained higher level of Foxp3 in the NFAT precipitates (Fig. 5, p<0.05). These results indicated that CD28 favored the tendency to NFAT: AP1, while ICOS favored the tendency to NFAT: Foxp3 in the balance of NFAT: AP1 and NFAT: Foxp3.
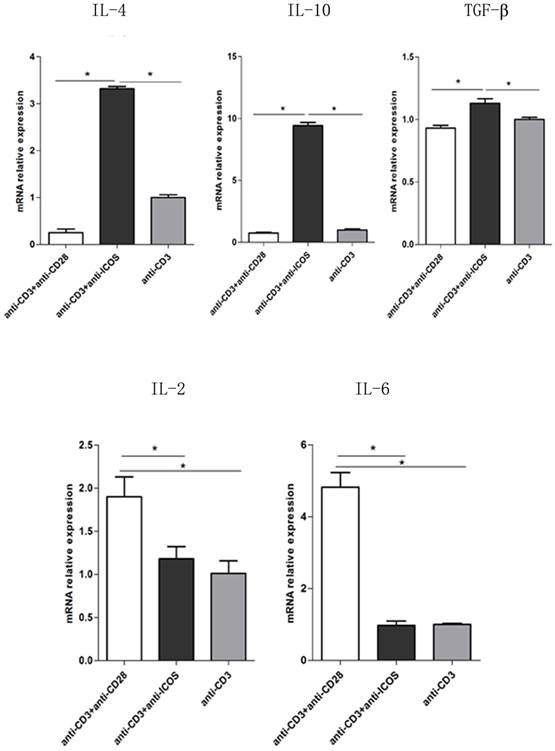
To further exploring the role of ICOS in the balance of NFAT: AP1 and NFAT: Foxp3, the downstream genes of AP1 and Foxp3 were tested. The results of Q-PCR showed that activating ICOS and CD3 could down regulate the AP1 downstream regulatory genes (IL-2 and IL-6) and up regulate the Foxp3 downstream regulatory genes (IL-4, IL-10 and TGF-β) (Fig. 6, p<0.05). The results of Q-PCR are consistent with the results of immune co-precipitation.
ICOS contributes to Tregs survival. The percentage of CD4+CD25+ regulatory T cells in mice after magnetic bead separation was >95%. Tregs were cultured with anti-CD3 antibody, anti-CD3 + anti-CD28 antibody or anti-CD3 + anti-ICOS antibody for 2, 4 or 6 days, and apoptosis was examined by flow cytometry with Annexin-V Apoptosis Detection Kit FITC. All of the experiments were performed in triplicate, *P<0.05.
Expression of Akt, p-Akt and Bcl2 in Tregs. Tregs were cultured with anti-CD3 antibody, anti-CD3 + anti-CD28 antibody or anti-CD3 + anti-ICOS antibody for 4 days. Western blot was used to evaluate the expression of p-Akt and Bcl2. All of the experiments were performed in triplicate, ** P<0.01.
Analysis of suppressive function of Tregs. Tcons were isolated from BALB/c mice and labeled with 5μM CFSE dilution. Tregs were activated with anti-mouse CD3 and CD28 (1:1) antibody or anti-mouse CD3 and ICOS (1:1) antibody or anti-mouse CD3 antibody alone. Tcons were activated with anti-mouse CD3 and CD28 (1:1) antibody and cultured either alone or in the presence of different activation of Tregs at a 3:1 Tcons: Tregs ratio for 2, 4 or 6 days and determined by flow cytometry.
Expression of Erk1/2, p-Erk1/2 and Ras in Tregs. Tregs were cultured with anti-CD3 antibody, anti-CD3 + anti-CD28 antibody or anti-CD3 + anti-ICOS antibody for 4 days. Western blot was used to evaluate the expression of Erk1/2, p-Erk1/2 and Ras. All of the experiments were performed in triplicate, ** P<0.01.
The levels of Foxp3 and cJun protein in co-Immunoprecipitation. Tregs were cultured with anti-CD3 antibody, anti-CD3 + anti-CD28 antibody or anti-CD3 + anti-ICOS antibody for 4 days. Co-Immunoprecipitation was used to evaluate the expression of Foxp3 and c-Jun. All of the experiments were performed in triplicate, *P<0.05.
Discussion
In this study, we demonstrated that both ICOS and CD28 signals could promote the survival of Tregs. However, ICOS but not CD28 improved the suppressive function of Tregs. Mechanistic studies showed that ICOS could promote the transcription activity of Foxp3 by facilitating NFAT: Foxp3 over NFAT: AP-1. Further, ICOS contributed to anti-apoptosis by activating Akt signal. These findings suggested that ICOS signal could facilitate Foxp3 transcription in favor of survival and suppressive function of Tregs.
The present study demonstrated that both ICOS and CD28 signals could promote the survival of Tregs may be by activating the PI3K-Akt pathway. Besides, we verified that activating ICOS could enhance the suppressive function of Tregs by facilitating NFAT: Foxp3 over NFAT: AP-1. Mechanistic research found that activating CD3 and CD28 could activate the Ras-Erk1/2-AP1 pathway and promote the interaction of NFAT: AP-1 for T cell activation. Whereas activating CD3 and ICOS could favor the tendency to the interaction of NFAT: Foxp3 for Tregs suppressive function.
The recognition of antigen peptide-major histocompatibility complex complexes by the T cell receptor (TCR) receptor is not sufficient to activate T cells. It requires costimulatory receptors binding to their ligands on the same antigen-presenting cell (APC) [16]. Our results demonstrated that only activating CD3 is not sufficient to activate T cells, which was consisted with previous studies [17]. The role of CD28 in T cells activation has been well known [18], while limited studies report the role of ICOS in the above function. So we cultured Tregs with anti-CD3 and anti-ICOS activating mAb to assess the activation of ICOS pathway. The results showed that activating ICOS could promote activation and survival of Tregs, which was consistent with activating CD28, but further studies were need to investigate the exact mechanism. Similar to CD28, ICOS is a potent activator of PI3K [5], binding PI3K to its SH2 domain [19-21] to further activate Akt. The results of apoptosis mechanistic study showed that ICOS signal promoted Tregs survival by activating Akt and downstream anti-apoptotic proteins, which was consistent with CD28. It suggested that activating CD3 and ICOS signals have a synergistic effect on the survival of Tregs.
The mRNA levels of IL-2, IL-6, IL-4, IL-10 and TGF-β in Tregs. Tregs were cultured for 3 days in the presence of anti-CD3 antibody, anti-CD3 + anti-CD28 antibody or anti-CD3 + anti-ICOS antibody. The levels of IL-2, IL-6, IL-4, IL-10 and TGF-β were detected by RT-qPCR. All of the experiments were performed in triplicate, *P<0.05.
As we all know, Tregs have obvious effect in inhibiting the proliferation of T cells [22]. The CFSE dilution assay found that activating ICOS could enhance the suppressive function of Tregs, but the exact mechanism is not clear. ICOS and CD28 downstream signaling events were related but distinguished [12] due to the different domains. Previous studies showed that the CD28 cytoplasmic domain (YMNM motif) could bind growth factor receptor-bound protein 2 (Grb2) to activate downstream pathway. However, the corresponding area of ICOS is YMFM, which can bind phosphatidylinositol 3-kinase (PI3-K) but not Grb2 [13]. It is report that cFos and cJun are the dimers of AP1, and the level of cJun is an indicator of AP1 [14]. Activating Ras protein could up-regulate protein kinases 1/2 (Erk1/2) and AP1 transcription factor, which combining with NFAT may induce the activation of T cells. Foxp3 and AP-1 are two competing transcription factors to occupy NFAT [14], and Foxp3 combined with NFAT may enhance the suppressive function of Tregs. The results of immune co-precipitation indicated that activating CD28 favors the tendency to NFAT: AP1 for T cell activation, while activating ICOS favors the tendency to NFAT: Foxp3 for Tregs suppressive function in the balance of NFAT: AP1 and NFAT: Foxp3. These findings suggest that ICOS signal may enhance the suppressive function of Tregs through regulating the balance of the dimer.
In addition, a study reported that Tregs have the ability to produce a huge amount of IL-10 and IL-4 to suppress immune responses [11]. Interestingly, by the interaction of effector T cells with professional APC, ICOS is apparently acting as a driving force and CD28 as an attenuator of IL-10 release [22]. T lymphocytes cultured with anti-CD3 + anti-ICOS mAb mainly secreted IL-4, IL-10 but not IL-2, while that cultured with anti-CD3 + anti-CD28 mAb mainly secreted IL-2 [23]. Our data demonstrated that activating CD3 and CD28 could up-regulate the AP1 downstream regulatory genes (IL-2 and IL-6), and activating CD3 and ICOS could up-regulate the Foxp3 downstream regulatory genes (IL-4, IL-10 and TGF-β). It is suggest that activating ICOS could enhance the suppressive function of Tregs by secreting inhibiting cytokines.
In conclusion, the present study demonstrated that ICOS signal plays a key role in regulating suppressive function and survival of regulatory T cells. In vitro experiments, activating CD3 and CD28 could activate the Ras-Erk1/2-AP1 pathway and promote T cell activation by the interaction of NFAT: AP-1. Whereas activating CD3 and ICOS could favor the tendency to the interaction of NFAT: Foxp3 for Tregs function. In addition, ICOS contributed to the survival of Tregs may through activating the PI3K-Akt pathway. These findings suggested that ICOS plays a significant role in immunological tolerance. Our study provides a clue that the Tregs-related immune escape may be mediated by ICOS signal which facilitates the interaction of NFAT: Foxp3 to favor suppressive function of Tregs. This study may provide insights into finding some useful strategies for cancer immunotherapy.
Acknowledgements
This work was supported by the grants from National Natural Science Foundation of China Grants (No.81373122).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Chen X, Du Y, Lin X, Qian Y, Zhou T, Huang Z. CD4+CD25+ regulatory T cells in tumor immunity. International immunopharmacology. 2016;34:244-9
2. Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P. et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature medicine. 2004;10:942-9
3. Nizar S, Copier J, Meyer B, Bodman-Smith M, Galustian C, Kumar D. et al. T-regulatory cell modulation: the future of cancer immunotherapy? British journal of cancer. 2009;100:1697-703
4. Piccirillo CA. Regulatory T cells in health and disease. Cytokine. 2008;43:395-401
5. Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, Manning S. et al. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13:95-105
6. Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L. et al. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28:870-80
7. Gogali F, Paterakis G, Rassidakis GZ, Kaltsas G, Liakou CI, Gousis P. et al. Phenotypical analysis of lymphocytes with suppressive and regulatory properties (Tregs) and NK cells in the papillary carcinoma of thyroid. The Journal of clinical endocrinology and metabolism. 2012;97:1474-82
8. Kornete M, Sgouroudis E, Piccirillo CA. ICOS-dependent homeostasis and function of Foxp3+ regulatory T cells in islets of nonobese diabetic mice. Journal of immunology. 2012;188:1064-74
9. Strauss L, Bergmann C, Szczepanski MJ, Lang S, Kirkwood JM, Whiteside TL. Expression of ICOS on Human Melanoma-Infiltrating CD4+CD25highFoxp3+ T Regulatory Cells: Implications and Impact on Tumor-Mediated Immune Suppression. The Journal of Immunology. 2008;180:2967-80
10. Huang XM, Liu XS, Lin XK, Yu H, Sun JY, Liu XK. et al. Role of plasmacytoid dendritic cells and inducible costimulator-positive regulatory T cells in the immunosuppression microenvironment of gastric cancer. Cancer science. 2014;105:150-8
11. Yu H, Huang X, Liu X, Jin H, Zhang G, Zhang Q. et al. Regulatory T cells and plasmacytoid dendritic cells contribute to the immune escape of papillary thyroid cancer coexisting with multinodular non-toxic goiter. Endocrine. 2013;44:172-81
12. Riley JL, Mao M, Kobayashi S, Biery M, Burchard J, Cavet G. et al. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11790-5
13. Harada Y, Ohgai D, Watanabe R, Okano K, Koiwai O, Tanabe K. et al. A Single Amino Acid Alteration in Cytoplasmic Domain Determines IL-2 Promoter Activation by Ligation of CD28 but Not Inducible Costimulator (ICOS). The Journal of Experimental Medicine. 2003;197:257-62
14. Rudensky AY, Gavin M, Zheng Y. FOXP3 and NFAT: partners in tolerance. Cell. 2006;126:253-6
15. Bandukwala HS, Wu Y, Feuerer M, Chen Y, Barboza B, Ghosh S. et al. Structure of a domain-swapped FOXP3 dimer on DNA and its function in regulatory T cells. Immunity. 2011;34:479-91
16. Dong C, Nurieva RI, Prasad DV. Immune regulation by novel costimulatory molecules. Immunologic research. 2003;28:39-48
17. Hennecke J, Wiley DC. T cell receptor-MHC interactions up close. Cell. 2001;104:1-4
18. van Berkel ME, Oosterwegel MA. CD28 and ICOS: similar or separate costimulators of T cells? Immunology letters. 2006;105:115-22
19. Arimura Y, Kato H, Dianzani U, Okamoto T, Kamekura S, Buonfiglio D. et al. A co-stimulatory molecule on activated T cells, H4/ICOS, delivers specific signals in T(h) cells and regulates their responses. International immunology. 2002;14:555-66
20. Fos C, Salles A, Lang V, Carrette F, Audebert S, Pastor S. et al. ICOS Ligation Recruits the p50 PI3K Regulatory Subunit to the Immunological Synapse. The Journal of Immunology. 2008;181:1969-77
21. Feito MJ, Vaschetto R, Criado G, Sanchez A, Chiocchetti A, Jimenez-Perianez A. et al. Mechanisms of H4/ICOS costimulation: effects on proximal TCR signals and MAP kinase pathways. European journal of immunology. 2003;33:204-14
22. Witsch EJ, Peiser M, Hutloff A, Buchner K, Dorner BG, Jonuleit H. et al. ICOS and CD28 reversely regulate IL-10 on re-activation of human effector T cells with mature dendritic cells. European journal of immunology. 2002;32:2680-6
23. Vettermann C, Victor HP, Sun Y, Plewa C, Gupta S. A signaling-enhanced chimeric receptor to activate the ICOS pathway in T cells. Journal of immunological methods. 2015;424:14-9
Author contact
![]() Corresponding authors: Zhiming Hu, Ph.D. School of Laboratory Medicine and Biotechnology, Southern Medical University, 1023 Sha Tai Road, Guangzhou, Guangdong 510515, China. Tel: +86-20-62789135; E-mail: hzmedu.cn and Jinlong Li, Ph.D. School of Laboratory Medicine and Biotechnology, Southern Medical University, 1023 Sha Tai Road, Guangzhou, Guangdong 510515, China. Tel: +86-20-62789135; E-mail: lijinlongedu.cn
Corresponding authors: Zhiming Hu, Ph.D. School of Laboratory Medicine and Biotechnology, Southern Medical University, 1023 Sha Tai Road, Guangzhou, Guangdong 510515, China. Tel: +86-20-62789135; E-mail: hzmedu.cn and Jinlong Li, Ph.D. School of Laboratory Medicine and Biotechnology, Southern Medical University, 1023 Sha Tai Road, Guangzhou, Guangdong 510515, China. Tel: +86-20-62789135; E-mail: lijinlongedu.cn

 Global reach, higher impact
Global reach, higher impact