Impact Factor
ISSN: 1449-1907
Int J Med Sci 2017; 14(10):977-983. doi:10.7150/ijms.20212 This issue Cite
Research Paper
IL-12 Influence mTOR to Modulate CD8+ T Cells Differentiation through T-bet and Eomesodermin in Response to Invasive Pulmonary Aspergillosis
1. Department of Critical Care Medicine, Peking Union Medical College Hospital, Peking Union Medical College & Chinese Academy of Medical Science, Beijing, China;
2. Department of Critical Care Medicine, 4 th Peoples' Hospital of Shenyang, Liaoning Province, China;
3. Department of Critical Care Medicine, Dalizhou People's Hospital, Yunnan Province, China;
4. Department of Critical Care Medicine, Chifeng City Hospital, Inner Mongolia, China;
5. Department of Pathology, Peking Union Medical College Hospital, Peking Union Medical College & Chinese Academy of Medical Science;
6. Department of Clinical Laboratory, Peking Union Medical College Hospital, Peking Union Medical College & Chinese Academy of Medical Science.
Received 2017-3-22; Accepted 2017-6-18; Published 2017-8-18
Abstract
Objective: To investigate whether mTOR signaling pathway regulate the proliferation and differentiation of CD8+ T cells by transcription factors T-bet and Eomes, and explore the role of IL-12 in this biological procedure.
Methods: Aspergillus fumigatus spore suspension nasal inhalation was used to establish the invasive pulmonary aspergillosis (IPA) mouse model. After inoculation, rapamycin (2mg/kg) each day or IL-12 (5ug/kg) every other day was given for 7 days. The blood samples were obtained before the mice sacrificed and lung specimens were taken. Pathological sections were stained with hematoxylin and eosin (HE). The number of CD8+effective memory T cells (Tem) and the expression of IFN-γ, mTOR, ribosomal protein S6 kinase (S6K), T-bet and EOMES were measured by flow cytometry. The levels of IL-6, IL-10 and Galactomannan (GM) were determined by ELISA.
Results: After IL-12 treatment, the number of CD8+ Tem and the expression of IFN-γ increased significantly; while quite the opposite results were observed when the mTOR pathway was blocked by rapamycin. The expression of mTOR and S6K as well as the level of IFN-γ of the IL-12 treatment group were significantly higher than those in IPA and IPA + rapamycin groups. In addition, IL-12 promoted increasing T-bet and down regulating Eomes to make the Tem transformation. The final immune effector was high level of inflammatory cytokines (IL-6) and low level of anti-inflammatory factors (IL-10) and this strengthened immune response to the Aspergillus infection.
Conclusions: The biological effects of Tem could significantly affect IPA infection host immune regulation, which depended on the activation of mTOR signaling pathway by IL-12.
Keywords: IL-12, Mammalian target of rapamycin (mTOR), CD8+effect memory T cells (Tem), Invasive Pulmonary Aspergillosis (IPA).
Introduction
The incidence of invasive fungal infection increases by year and invasive pulmonary aspergillosis (IPA) has become a leading cause of severe fungal infections in critically ill patients with a high mortality rate [1]. IPA susceptible population is mainly with immune dysfunction, including high-dose glucocorticoid therapy, immunosuppressive therapy, solid organ or hematopoietic stem cell transplantation, AIDS and chronic granulomatous disease. Clinical invasive operation and long-term use of broad-spectrum antibiotics are also easily secondary to the disease [2, 3]. T lymphocyte-mediated acquired immune response is an important way in determining whether the host body can quickly clear the pathogens. CD8+ T cell subsets are decreased significantly in the early onset of IPA and the differentiation phenotype of the immune cells is closely related to prognosis of the patients [4]. The effector-phenotype CD8+ memory T cells (Tem) can rapidly generate an adaptive immune response to specific antigens in the early stages of infection. Focusing on the mechanism of differentiation of CD8+ T cells in the early stage of infection would provide a new sight to effectively control infection in severely fungal infected patients [5, 6].
Tem is a subset the memory T cell (Tm) populations. According to homing and effecting function, the CD45RO+ Tm are classified into two subpopulations: CD45RO+CD44+CD62L++ CCR7+ Tm and CD45RO+CD44+CD62L±/-CCR7-Tm. The CD62L and CCR7 are lymph node homing receptors, so these cells so-called central memory T cells (Tcm) will be selectively colonized in the secondary lymph organs; while the other subpopulation Tm which are distributed in peripheral tissues are effective memory T cells (Tem) [7]. Tem expresses high levels of β1 and β2 integrins which are cell surface receptors that mediate the adhesion of T cells to tissue cells and extracellular matrix and this is required for entry into inflammatory tissues [8]. At the same time, high expression of tissue-specific homing receptors, CD103 and cutaneous lymphocyte antigen (CLA), and chemokine receptor also promote Tem into the non-lymphoid tissue [9] whether in tumor or infectious inflammatory tissue. Tem is dominant subgroup in the local infiltrating CD8+ Tm [9, 10]. Tems will trigger a rapid, potent, specific and effective immune response when meeting the same antigen again, thus playing an important role in protective immunity.
In the early animal experiments, the Mammalian target of rapamycin (mTOR) signal pathway activity was significantly changed in the immune cells proliferation and differentiation in fungal infection rates [11]. mTOR is an evolutionarily conserved serine /threonine protein kinase that exists in mammals. The mTOR signaling pathway regulates important cellular biochemical metabolic pathways and the process. When the mTOR pathway is activated, it will promote cell anabolism and inhibit the catabolism. The mTOR downstream signaling pathway contains two key substrates, the ribosomal S6 kinase (S6K) and the eukaryotic translational initiation factor 4E binding protein 1(4E-BP1). mTOR activated phosphorylates S6K and 4E-BP1, thereby lifting the inhibition of eIF4E and then start the transcription process to synthesize new proteins for the cell proliferation [12]. The mTOR activity level of activated CD8+ T cells was correlated with the expression of T-bet and Eomesodermin (Eomes), suggesting that the mTOR pathway affects the effector CD8+ T cell differentiation by regulating the expression of T-bet and Eomes.
T-bet is a key transcription factor that regulates T-cell differentiation and belongs to the T-box transcription factor family [13]. Eomes, another transcription factor, plays a synergistic role in T-bet function and regulates the differentiation of CD8+ T cells into effector T cells and memory T cells. Overexpression of T-bet or Eomes could induce CD8+ T cells to up-regulate IFN-γ, perforin and granzyme B expression. Knockout of T-bet and Eomes genes results in a lack of CD8+ T cells in mice and a defect in gene expression associated with cytotoxic T cells. Recent evidence suggests that IL-12 can affect memory / effect CD8+ T phenotype differentiation by regulating T-bet and Eomes [14]. IL-12 can enhance the activity of mTOR kinase in naïve CD8+ T cells [15]. Treatment of CD8+ T cells with rapamycin inhibited the activity of mTOR kinase, thereby blocking the expression of T-bet and promoting the expression of Eomes. Increased expression of Eomes in CD8+ T cells may promote the generation of memory T cells; while low expression of T-bet also promotes long-term survival of CD8+ memory T cells [16].
Interleukin (IL)-6 is a multifunctional cytokine may promote or inhibit inflammation. Because of this pleiotropic activity, IL-6 plays a certain role in different pathologic condition. In IPA murine model, IL-6 deficient (IL-6-/-) mice showed more susceptible than wild-type (WT). Susceptibility was associated with decreased antifungal effectors generation and increased inflammatory pathology. The exogenous IL-6 would restore the antifungal effector activity [17].While, as a cytokine down-regulation of Th1 and macrophage response, IL-10 result in the opposite effect to IL-6. When C56Bl/6 IL-10-/- (KO) and WT are infected with Aspergillus fumigatus, KO mice survive longer and with significantly lower fungal burdens in the kidneys and brain compared with WT [18]. These results demonstrate that although beneficial in some other infections, IL-10 is deleterious in fungal infection to the host.
Based on the theories and experimental results mentioned above, we hypothesized that in the IPA individual, IL-12 might enhance the activity of mTOR pathway, and then influenced the expression of transcripts factors of T-bet and Eomes; after that, the differentiation of CD8+Tem cells would be promoted, eventually caused the IL-6 secretion increased and IL-10 decreased, which would be conducive to removal of fungi. In this study, we determined the molecular mechanism in naive CD8+ T cells differentiated to the Tem by regulating mTOR pathway through T-bet and Eomes expression. In addition, we observed the role of inhibition of mTOR activity on the influence on T-bet and Eomes expression as well as the memory generation. Finally, we also explored the role of IL-12 in the regulation of CD8+ T cell proliferation, differentiation and the function of IL secretion. Our study showed that mTOR is a key regulating factor on control of CD8+Tem differentiation and IL-12 influences the procedure positively in the IPA mouse model.
Materials and Methods
Pathogen Preparation
The strain of Aspergillus fumigatus, provided by Department of Clinical Laboratory, Peking Union Medical College Hospital, was obtained from a case of pulmonary aspergillosis. Viable conidia (> 95%) were obtained by growth on Sabourard dextrose agar for 5 days at 35°C. Conidia were harvested with 10ml 0.1% Tween-80/PBS and filtered through five-layer of gauze. Concentration of conidia was adjusted to 1×108 CFU/ml by the method of Turbidity adjustment.
Animal Model Preparation
Healthy BALB/c mice, female, 4 - 5weeks old, weight of 20 ± 5g, were obtained from the Animal Facility Center, PUMCH. All animals were housed in a pathogen-free facility and used according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) of PUMCH. Total 24 mice were randomly divided into the following groups (6 mice each group): (1). Control group: non-treated normal mice. (2). Invasive pulmonary aspergillosis (IPA) group: animals were infected with Aspergillus fumigatus. 0.1ml conidia solution was inhaled by the nose. (3) IPA plus rapamycin treatment group (IPA + RAPA): mice were given rapamycin after infected with Aspergillus fumigatus. The dose of rapamycin was 2mg/kg as the following 7 consecutive days. (4) IPA plus IL-12 treatment group (IPA + IL-12). Mice were given IL-12 after infected with Aspergillus fumigatus. The dose of IL-12 was 5ug/kg for the following 7 days every other day. Blood samples were obtained through mice eyes. Part of lung tissue was minced and used for Aspergillus fumigatus culture. Part of lung tissue was also fixed with 4% formaldehyde and the sections were stained with HE (hematoxylin and eosin), masson, and PASM (periodic acid-silver metheramine) for histology.
CD8+Tem cell counts and IFN-γ, mTOR, S6K, T-bet, and EOMES expressed by the cells
Peripheral blood mono-nuclear cells (PBMC) were isolated from blood samples and counted by flow cytometry. Cells were then labeled with the following fluorescence-conjugated monoclonal antibodies: anti-Rat CD45 PE (12-0451-81, eBioscience, San Diego, CA, USA), anti-Rat CD8a APC (17-0081-81, eBioscience), anti-Rat CD44 PE (25-0441-81, eBioscience), anti-Rat CD62L (104432, Biolegend). CD8+Tem cells were sorted by flow cytometry (EPICS-XL, Beckman-Coulter, USA), then stained for IFN-γ (11-7311-81, eBioscience), mTOR (ab87540, Abcam, Cambridge, MA), S6K (ab32529, Abcam), T-bet (ab91109, Abcam), and Eomes (53-4875-82, eBioscience) expression.
Cytokine Quantification
Cytokines or proteins of transcription factors were quantified using the following ELISA kits as per the manufacture's instruction; IL-6 (cat# ab168538, Abcam), IL-10 (cat#: ab176665, Abcam), and GM (cat#: 85-86051, Affymetrixe Bioscience, San Diego, CA, USA).
Statistical Analysis
Data were analyzed using SPSS 18.0 software (SPSS Inc., Armonk, NY, USA). All the data for the continuous variables in this study were proved to have normal distributions, and are given as means ± standard deviations (SD). Results for continuous variables that were not normally distributed are given as medians (interquartile ranges) and were compared using non-parametric tests. Student's t-test or analysis of variance (ANOVA) followed by Bonferroni's test were used to determine the statistical significance (P) of differences. P values of P < 0.05 were considered as statistically significant.
Results
Tissue Culture and Histology
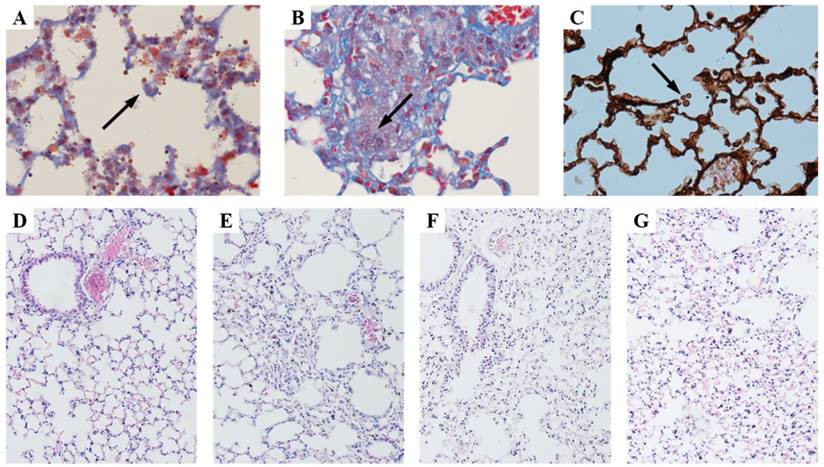
Viability of the conidia was examined by infected lung tissue culture. Viable Aspergillus fumigatus was positively cultured in IPA, IPA+RAPA and IPA + IL-12 groups (Figure 1A-C), while it was negative in control. Histological examination indicated that lung tissue structure was intact in normal control (Figure 1D). In contrast, infiltration of inflammatory cells, blood congestion and interstitial lung tissue injury were found in the mouse lungs of IPA-infected, IPA+RAPA or IPA + IL-12 infection (Figure 1-G). Compared with IPA + IL-12 (Figure 1F), Figure 1 suggested that IPA + RAPA group (Figure 1G) had serious congestion and hemorrhage in the interstitial lung tissue.
Histology of lung tissue stained with HE, masson, and PASM. A, B and C showed the fungal spores of aspergillus fumigatus. D: Control animal. E: Animals infected with invasive pulmonary aspergillosis (IPA). F: IPA plus IL-12 treatment group (IPA+IL-12). G: IPA plus rapamycin treatment group (IPA+RAPA). Magnification: A = masson staining 200×; B = masson staining 400×; C = PASM staining 600×; D-G = HE staining 100×.
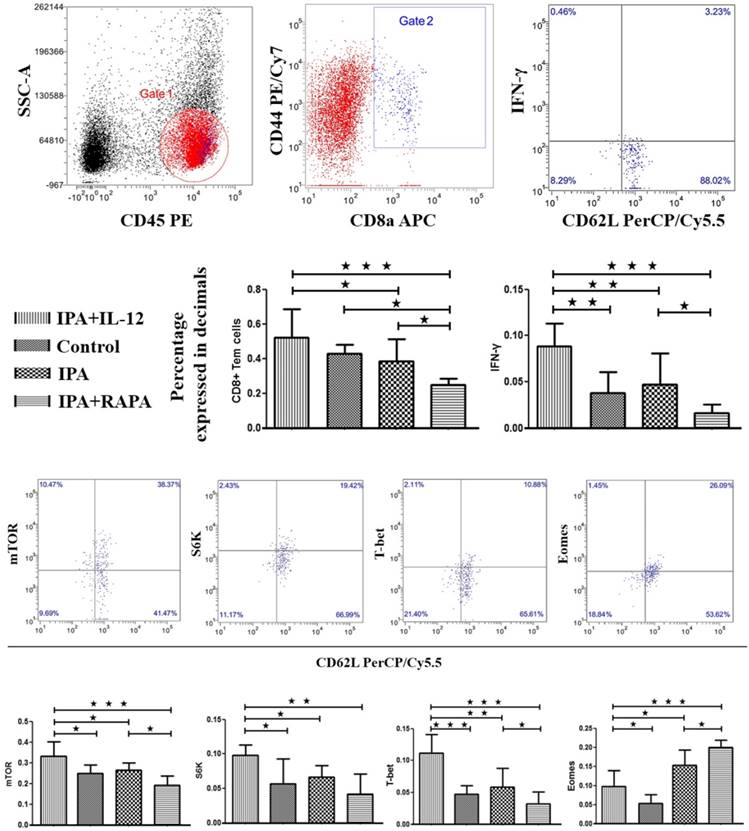
IL-12 enhances T-cell differentiation by activating mTOR pathway to modulate T-bet and Eomes expression
As shown in Figure 2, the proportion of CD8+ Tem cells and IFN-γ production significantly increased in IPA + IL-12 group (0.52 ± 0.16; 0.09 ± 0.02) than in IPA group (0.39 ± 0.13, p = 0.043; 0.05 ± 0.03, p = 0.01). The result means addition of IL-12 could improve CD8+ Tem cells differentiation and resulted in robust IFN-γ production in CD8+ Tem cells during IPA infection. Similarly, compared to control (0.25 ± 0.04) and IPA groups (0.27 ± 0.04), the mTOR activity was increased in IPA + IL-12 group (0.33 ± 0.07), which had statistical significance (p = 0.01; p = 0.03). To verify that the induction of mTOR phosphorylation also led to its kinase activity, we monitored the kinetics of S6K, a direct target of mTOR kinase activity. As anticipated, in correlation with mTOR, the presence of IL-12 could significantly enhance the expression of S6K in CD8+ Tem cells (0.10 ± 0.01) than in control (0.06 ± 0.04, p = 0.011) and IPA groups (0.07 ± 0.02, p = 0.046).
To determine whether sustained mTOR activity was required for CD8+ Tem cells differentiation, we blocked mTOR activity by adding rapamycin during IPA infection. As shown in Figure 2, the expression of mTOR was significantly lower in IPA + RAPA group (0.19 ± 0.04) than in IPA group (0.27 ± 0.04, p = 0.018) and IPA + IL-12 group (0.33 ± 0.07, p < 0.001). The expression of S6K also was significantly lower in IPA + RAPA (0.04 ± 0.03) group than in IPA + IL-12 group (0.10 ± 0.01, p < 0.001). More importantly, we found that, as shown in Figure 2, adding rapamycin could significantly decrease the proportion of CD8+ Tem cells and IFN-γ production (0.25 ± 0.03; 0.02 ± 0.01) than in IPA (0.39 ± 0.13, p = 0.042; 0.05 ± 0.03, p = 0.042) and IPA + IL-12 groups (0.52 ± 0.16, p < 0.001; 0.09 ± 0.02, p < 0.001). These results indicate that IL-12-induced commitment of naïve CD8+ T cells for type Ⅰ effector functions requires mTOR activity.
To determine the effect of IL-12 on T-bet and Eomes expression during the immune response to IPA infection, we examined T-bet and Eomes expression in IL-12-conditioned IPA CD8+ Tem cells. As shown in Figure 2, we found that addition of IL-12 could induce T-bet but inhibit Eomes expression in CD8+ Tem cells (0.11 ± 0.03; 0.10 ± 0.04) than control (0.05 ± 0.01, p < 0.001; 0.05 ± 0.02, p = 0.032) and IPA groups (0.06 ± 0.03, p = 0.001; 0.15 ± 0.04, p = 0.01) to favor effector versus memory generation. Second, we examine the effect of mTOR activity on T-bet and Eomes expression by adding rapamycin to IPA CD8+ Tem cells. The results indicated that compared to IPA and IPA + IL-12 groups, IPA + RAPA group was able to significantly decrease T-bet (0.03 ± 0.02) but increase Eomes expression (0.20 ± 0.03) in CD8+ Tem cells (p < 0.05). Thus, IL-12 augmented mTOR activity is also essential for regulating the expression of transcription factors of T-bet and Eomes in CD8+ Tem cells during immune responses of IPA infection.
Alteration of inflammatory responses and severity of fungal infection
IL-6 reflected the inflammatory response and IL-10 reflected anti-inflammatory response. Figure 3 revealed that IL-12-treated group had the highest IL-6 level (2888.78 ± 1114.04 pg/ml), followed by IPA (1848.45 ± 247.03 pg/ml), IPA + RAPA (1632.75 ± 882.91 pg/ml), and control groups (245.65 ± 85.78 pg/ml). While the concentration of IL-10 showed a reverse tendency. The IL-10 level of IL-12-treated group (252.25 ± 54.62 pg/ml), as same as the control group (267.59 ± 46.10 pg/ml), was significantly lower than the IPA (350.93 ± 43.11pg/ml, p = 0.001) and especially the IPA + RAPA group (467.00 ± 37.70 pg/ml, p < 0.001).
IL-12 can enhance CD8+ Tem cells differentiation through T-bet and Eomes influenced by mTOR activated.The peripheral blood mononuclear cells (PBMC) obtained from IPA model mice, IPA treated with IL-12 or rapamycin and control animals were detected using flow cytometry after 7 days Aspergillus fumigatus inhaled by the nose. The side scatter (SSC) and CD8a were applied to gate CD8+T lymphocytes, CD44+CD45+CD62±/- represented effective memory T cell (Tem). And then the TEMs were stained with IFN-γ, mTOR, S6K, T-BET, EOMES. The data were presented as mean ± S.D. *: p < 0.05; **: p < 0.01; ***: p < 0.001.
IL-12 were significantly increased the IL-6 level, which was significantly decreased by rapamycin treatment. In contrast, the concentration of IL-10 and galactomannan showed the reverse trend. Plasma samples obtained from IPA mice, IPA treated with IL-12 or rapamycin and control animals were detected by ELISA seven days after Aspergillus fumigatus intranasal inoculation. The data are presented as the mean ± S.D. * P < 0.05, ** P < 0.01, *** P < 0.001.
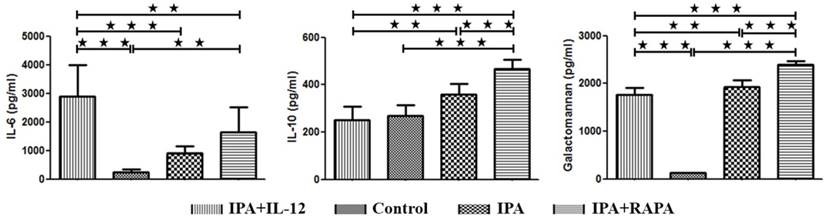
Galactomannan was used to reflect severity of fungal infection in clinical practice. In this study, we found that after Aspergillus fumigates injection, the level of Galactomannan significantly increased in the groups of IPA (1985.98 ± 152.79 pg/ml, p < 0.001), IPA + IL-12 (1720.33 ± 166.86 pg/ml, p < 0.001) and IPA + RAPA (2387.85 ± 87.35 pg/ml, p < 0.001) than in control group (126.82 ± 10.31pg/ml). Compared to the IPA (p = 0.001) and IPA + RAPA (p < 0.001) groups, the level of Galactomannan was significantly lower in IL-12-treated group (Figure 3C).
Discussion
The results of our study showed that IL-12 could increase the number of CD8+ Tem cells and the effector cell response (IFN-γ releasing) during IPA infection, through the mechanism by increasing the expression and activity of mTOR and then enhancing T-bet expression and decreasing Eomes expression. The final effects wereIL-6 level was increased and the IL-10 level was decreased significantly and these really affect the fungal burden of the host. These effects could be blocked by mTOR inhibitor rapamycin.
CD8+ T cells are an important part of the host immune system. After infection, some of the CD8+ T cells will show a memory transformation effect and can survive long-term after infection. CD8+effective memory T cells, the memory T cells with effector cell phenotype will establish rapid immune response when the same pathogen comes into the host [19, 20]. Several studies have shown that different pathogenic bacteria can induce different differentiation phenotype of CD8+ T cells [21]. mTOR signal transduction pathway is the center of the regulation of cellular energy metabolism, proliferation and differentiation. T-cell antigen recognition can be partially achieved by molecular interactions T-bet and Eomes, both belong to the T-box family, and are specific transcription factors that influence T-cell differentiation [13, 22]. T-bet is mainly responsible for Th1-type effector cell differentiation and inflammatory factor synthesis. Eomes expression is increased in late of immune response and promote the Tem transformation.
Many studies have successfully elucidated that cytokine-generated signals during antigen stimulation are instrumental in regulating the transcriptional program of CD8+ T cells for effector and memory functions, but the mechanism by which they are integrated is not entirely clear [23]. Recent evidence suggests that IL-12 induces T-bet but inhibits Eomes expression to favor effector versus memory differentiation during the immune response to Listeria monocytogenes and virus [6, 11], suggesting the importance of understanding cell-intrinsic factors that regulate T-bet and Eomes expression which may enable achieving desirable CD8+ T cell functional outcomes. The results of this in vivo study showed that maintaining the expression and activity of mTOR signaling pathway could increase the number of CD8+Tem proliferation and present the effect of effector T cells (IFN-γ releasing). The expression T-bet was increased and Eomes was decreased in CD8+ Tem and these could be blocked by mTOR inhibitor rapamycin. This suggested that the mTOR signaling pathway may affect the memory / effector CD8+ T cell proliferation and differentiation by regulating the activities of T-bet and Eomes transcription factors. It provided new opportunities for us to modulate CD8+ T cell responses for desirable outcomes during the immune response to fungal infection. The above results exploring the notion that rapamycin exposure may affect effector versus memory functional maturation in IL-12-conditioned IPA CD8+ T cells is provocative because it is likely to reveal the molecular mechanisms by which integration of cytokine-generated signals determine antigen- and co-stimulation induced T cell responses and identify new strategies to generate functionally distinct types of memory CD8+ T cells with heterogeneous efficacy against infectious challenges.
Finally, in this study we found that IL-12 could increase the level of plasma proinflammatory cytokine (IL-6) and reduce the level of anti-inflammatory cytokine (IL-10) in IPA mice compared with the control and IPA groups; as well as reduced the fungal load (Galactomannan, GM) significantly. Galactomannan is a polysaccharide composed of D-galactose and D-mannose units, which is one of the cell wall components of Aspergillus. GM has been used clinically in the diagnosis of IPA. In this study, the level of GM in rats infected with IL-12 was significantly lower than that of IPA, and the level of GM in RAPA-IP group was significantly higher than that in other groups, which indicated that IL-12 had the same level of IPA infection (IL-6) levels, and anti-inflammatory cytokine (IL-10) levels in plasma, which is associated with decreased fungal load in infected hosts.
In summary, the results of this study showed that IL-12 could regulate the CD8+ T cell proliferation and differentiation and the biological function, and this can significantly affect the immune regulation of IPA-infected host. This was a preliminary study, but we can deduce that the regulation mechanism was closely related to mTOR signaling pathway. Through the regulation of CD8+ Tem proliferation and differentiation, increased IL-6 and decreased IL-10 could significantly enhance the immune response of IPA infected host. The immune regulation of the mTOR target of anti-fungal infection need further study to confirm, using mTOR-knock out cell or animal models or more technical immunological methods.
Acknowledgements
The work was supported by the Beijing Municipal Natural Science Foundation (no. 7152119) and Special Project Funds for clinical research of Chinese Medical Association (no. 14030250562).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L. et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-50
2. Maschmeyer G, Haas A, Cornely OA. Invasive aspergillosis: epidemiology, diagnosis and management in immunocompromised patients. Drugs. 2007;67:1567-601
3. Vandewoude KH, Blot SI, Benoit D, Colardyn F, Vogelaers D. Invasive aspergillosis in critically ill patients: attributable mortality and excesses in length of ICU stay and ventilator dependence. J Hosp Infect. 2004;56:269-76
4. Cui N, Wang H, Long Y, Liu D. CD8(+) T-cell counts: an early predictor of risk and mortality in critically ill immunocompromised patients with invasive pulmonary aspergillosis. Crit Care. 2013;17:R157
5. Williams MA. Instant recall: a key role for effector-phenotype CD8(+) memory T cells in immune protection. Immunity. 2013;38:1090-1
6. Plumlee CR, Sheridan BS, Cicek BB, Lefrancois L. Environmental cues dictate the fate of individual CD8+ T cells responding to infection. Immunity. 2013;39:347-56
7. Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204:1625-36
8. Schnoor M, Alcaide P, Voisin MB, van Buul JD. Crossing the Vascular Wall: Common and Unique Mechanisms Exploited by Different Leukocyte Subsets during Extravasation. Mediators Inflamm. 2015;2015:946509
9. Tufail S, Badrealam KF, Sherwani A, Gupta UD, Owais M. Tissue specific heterogeneity in effector immune cell response. Front Immunol. 2013;4:254
10. Gebhardt T, Mackay LK. Local immunity by tissue-resident CD8(+) memory T cells. Front Immunol. 2012;3:340
11. Cui N, Su LX, Wang H, Xiao M, Yang F, Zheng M. et al. mTOR Modulates Lymphocyte Differentiation through T-bet and Eomesodermin in Response to Invasive Pulmonary Aspergillosis in Rats. Chin Med J (Engl). 2016;129:1704-10
12. Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487-502
13. Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR. et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236-44
14. Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67-78
15. Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515-9
16. Keppler SJ, Rosenits K, Koegl T, Vucikuja S, Aichele P. Signal 3 cytokines as modulators of primary immune responses during infections: the interplay of type I IFN and IL-12 in CD8 T cell responses. PLoS One. 2012;7:e40865
17. Cenci E, Mencacci A, Casagrande A, Mosci P, Bistoni F, Romani L. Impaired antifungal effector activity but not inflammatory cell recruitment in interleukin-6-deficient mice with invasive pulmonary aspergillosis. J Infect Dis. 2001;184:610-7
18. Clemons KV, Grunig G, Sobel RA, Mirels LF, Rennick DM, Stevens DA. Role of IL-10 in invasive aspergillosis: increased resistance of IL-10 gene knockout mice to lethal systemic aspergillosis. Clin Exp Immunol. 2000;122:186-91
19. Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A. et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687-91
20. Lefrancois L, Marzo AL. The descent of memory T-cell subsets. Nat Rev Immunol. 2006;6:618-23
21. Duong S, Condotta SA, Rai D, Martin MD, Griffith TS, Badovinac VP. Polymicrobial sepsis alters antigen-dependent and -independent memory CD8 T cell functions. J Immunol. 2014;192:3618-25
22. Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM. et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907-10
23. Lertmemongkolchai G, Cai G, Hunter CA, Bancroft GJ. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J Immunol. 2001;166:1097-105
Author contact
![]() Corresponding authors: Na Cui, Tel: +86 15601212623, Email: cuinacn Dawei Liu, Tel: +86 10 69152300, Email: pumchkycncom Department of Critical Care Medicine, Peking Union Medical College and Chinese Academy of Medical Science, Beijing, China 100730
Corresponding authors: Na Cui, Tel: +86 15601212623, Email: cuinacn Dawei Liu, Tel: +86 10 69152300, Email: pumchkycncom Department of Critical Care Medicine, Peking Union Medical College and Chinese Academy of Medical Science, Beijing, China 100730

 Global reach, higher impact
Global reach, higher impact