3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2016; 13(10):730-740. doi:10.7150/ijms.16132 This issue Cite
Research Paper
Dehydroepiandrosterone Supplementation Combined with Whole-Body Vibration Training Affects Testosterone Level and Body Composition in Mice
1. Center for General Education, Chang Gung University of Science and Technology, Taoyuan 33301, Taiwan;
2. Department of Otorhinolaryngology-Head and Neck Surgery, Sleep Center, Linkou-Chang Gung Memorial Hospital, Taoyuan 33301, Taiwan.
3. Graduate Institute of Sports Science, National Taiwan Sport University, Taoyuan 33301, Taiwan
Emails: 1021302@ntsu.edu.tw (Y.-M.C.)
4. Division of General Surgery, Department of Surgery, Kaohsiung Veterans General Hospital, 813 Kaohsiung, Taiwan.
Received 2016-5-11; Accepted 2016-8-19; Published 2016-9-16
Abstract
Dehydroepiandrosterone (DHEA), the most abundant sex steroid, is primarily secreted by the adrenal gland and a precursor hormone used by athletes for performance enhancement. Whole-body vibration (WBV) is a well-known light-resistance exercise by automatic adaptations to rapid and repeated oscillations from a vibrating platform, which is also a simple and convenient exercise for older adults. However, the potential effects of DHEA supplementation combined with WBV training on to body composition, exercise performance, and hormone regulation are currently unclear. The objective of the study is to investigate the effects of DHEA supplementation combined with WBV training on body composition, exercise performance, and physical fatigue-related biochemical responses and testosterone content in young-adult C57BL/6 mice. In this study, male C57BL/6 mice were divided into four groups (n = 8 per group) for 6-weeks treatment: sedentary controls with vehicle (SC), DHEA supplementation (DHEA, 10.2 mg/kg), WBV training (WBV; 5.6 Hz, 2 mm, 0.13 g), and WBV training with DHEA supplementation (WBV+DHEA; WBV: 5.6 Hz, 2 mm, 0.13 g and DHEA: 10.2 mg/kg). Exercise performance was evaluated by forelimb grip strength and exhaustive swimming time, as well as changes in body composition and anti-fatigue levels of serum lactate, ammonia, glucose, creatine kinase (CK), and blood urea nitrogen (BUN) after a 15-min swimming exercise. In addition, the biochemical parameters and the testosterone content were measured at the end of the experiment. Six-week DHEA supplementation alone significantly increased mice body weight (BW), muscle weight, testosterone level, and glycogen contents (liver and muscle) when compared with SC group. DHEA supplementation alone had no negative impact on all tissue and biochemical profiles, but could not improve exercise performance. However, WBV+DHEA supplementation also significantly decreased BW, testosterone level and glycogen content of liver, as well as serum lactate and ammonia levels after the 15-min swimming exercise when compared with DHEA supplementation alone. Although DHEA supplementation alone had no beneficial effect in the exercise performance of mice, the BW, testosterone level and glycogen content significantly increased. On the other hand, WBV training combined with DHEA decreased the BW gain, testosterone level and glycogen content caused by DHEA supplementation. Therefore, WBV training could inhibit DHEA supplementation to synthesis the testosterone level or may decrease the DHEA supplement absorptive capacity in young-adult mice.
Keywords: dehydroepiandrosterone (DHEA), whole-body vibration (WBV), exercise performance, testosterone, glycogen.
Introduction
Dehydroepiandrosterone (DHEA) is a precursor of sex steroid hormones and serum DHEA levels generally decrease with aging [1]; hormones supplementation is one direct way to increase sex steroid hormone level. In previous study, DHEA levels with aging and obesity had been improved insulin resistance to increasing prevalence of diabetes [2, 3]. DHEA is reversibly converted to dehydroepiandrosterone sulfate (DHEAS) [4]. In addition, sex steroid hormone supplementation and exercise may relate to increase in muscle mass [5, 6], especially in older subjects. Exercise also increased sex steroid hormone levels and increased activation of the glucose metabolism-signaling pathway in skeletal muscle [7].
Whole-body vibration (WBV) is a kind of supplementary training or light-resistance training based on automatic body adaptations to rapid and repeated oscillations of a vibrating platform [8]. Studies have reported that WBV could improve muscle strength and power [9], benefit bone mineral density [10], decrease in abdominal fat [11], and increase hormone content [12]. In addition, WBV training also significantly increased the muscle strength and bone mineral density of older subjects [13, 14], and DHEA supplementation had several benefits in age-advanced subjects [15]. However, to the best of our knowledge, there is no prior report on the effects of a combination of DHEA supplementation and WBV training for body composition, serum biochemical indexes, exercise performance and hormone content. In this study, we combined DHEA supplementation and WBV training for young-adult mice to investigate the beneficial synergistic effects on hormone content, muscle mass, body composition, exercise performance, biochemical profiles, and pathological responses after 6-weeks supplementation.
Materials and methods
Materials, Animals, and Experiment Design
Dehydroepiandrosterone (DHEA) used for supplementation in this study was obtained from General Nutrition Centers, Inc. (GNC, Pittsburgh, PA, USA). Male C57BL/6 mice (6 weeks old) with specific pathogen-free conditions were purchased from National Laboratory Animal Center (NLAC), National Applied Research Laboratories (Taipei, Taiwan). One week of acclimation to the environment and diet was allowed before the experiment began. All animals were provided a standard laboratory diet (No. 5001; PMI Nutrition International, Brentwood, MO, USA) and distilled water ad libitum, and housed at 12-h light/12-h dark cycle at room temperature (24 ± 1 °C) and 50%-60% humidity. The Institutional Animal Care and Use Committee (IACUC) of National Taiwan Sport University inspected all animal experiments in this study, and the study conformed to the guidelines of protocol IACUC-10321 approved by the IACUC ethics committee.
All animals were randomly assigned to 4 groups (8 mice/group) for sedentary control with vehicle (SC), DHEA supplementation (DHEA), whole-body vibration training with vehicle (WBV), and WBV with DHEA supplementation (WBV+DHEA). Food intake and water consumption were recorded daily, and all animals were weighed weekly.
DHEA Supplementation
The oral gavage treated with DHEA once a day for 6-week at 10.2 mg/day. SC group received the same volume of distilled water equivalent to body weight. The DHEA supplementation in WBV+DHEA group was complete WBV training after 30 min. The recommended use of DHEA for humans is about 50 mg per one intake with a normal diet and exercise program. The mouse DHEA dose (10.2 mg/kg) used in this study was converted from a human equivalent dose on the basis of body surface area by the following formula from the US Food and Drug Administration [16]: assuming a human weight of 60 kg, the human equivalent dose of 50 mg/60 kg (0.83 mg/kg) = 0.83 × 12.3 = a mouse dose of 10.2 mg/kg; the conversion coefficient 12.3 was used to account for differences in body surface area between a mouse and a human.
WBV Protocol
Animals in the WBV and WBV+DHEA groups underwent WBV following the training protocol as shown in Figure 1. The frequencies provided by the vibration platform were 5.6 Hz (peak acceleration, 0.13 g). A vibration platform is considered to produce gravitational force < 1 g regardless of frequency. The peak-to-peak amplitude of the vibration was 2 mm. The WBV training was under continuous supervision for 15 min/day, 5 days/week for 6 weeks. Training session was regularly beginning at 9:00 Am.
Forelimb Grip Strength
A low-force testing system (Model-RX-5, Aikoh Engineering, Nagoya, Japan) was used to measure the forelimb grip strength of mice. The amount of tensile force was measured by use of a force transducer equipped with a metal bar (2 mm diameter and 7.5 cm long). The detailed procedures were described in our previous study [17]. Forelimb grip strength was tested after consecutive administration of SC, DHEA, WBV and DHEA + WBV treatment for 6 weeks and 1 h after the last treatment. The maximal force (in grams) recorded by this low-force system was used as the grip strength. The data is a measure of different grip strength (g) adjusted for body weights (g).
Swimming Exercise Performance Test
Mice were pretreated with the SC, DHEA, WBV and WBV+DHEA for 6 weeks, then an exhaustive swimming test was tested after the last treatment. The details of the exhaustive swimming test was described previously [18]. The endurance of each mouse was recorded as the time from the beginning to exhaustion, determined by observing loss of coordinated movements, and failure to return to the surface within 7 s.
Determination of Blood Biochemical Variables
The effect of DHEA, WBV and WBV+DHEA on serum lactate, ammonia, glucose, BUN, and CK levels were evaluated post-exercise. At 1 h after the administration, a 15-min swimming test was performed without weight loading, then blood samples were immediately collected from the submandibular duct of pretreated mice and centrifuged at 1500 ×g and 4 °C for 10 min for serum preparation. Lactate, ammonia, and glucose, CK and BUN level in serum were determined by using an autoanalyzer (Hitachi 7060, Hitachi, Tokyo). In addition, at the end of the experiments, all mice were killed by 95% CO2 asphyxiation, and blood was withdrawn by cardiac puncture. Serum was collected by centrifugation, and levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine (CREA), blood urine nitrogen (BUN), CK, glucose, and uric acid (UA) were assessed by use of an auto-analyzer (Hitachi 7060).
Tissue Glycogen Determination
Liver and muscle tissues were investigated to determine whether DHEA, WBV and WBV+DHEA treatment increased glycogen deposition. About 1 h after the last treatment administration, mice were euthanasia by CO2 inhalation. The liver was excised and weighed. The method of glycogen analysis was described in our previous studies [19,20].
Immunohistochemical staining of gastrocnemius muscles
Gastrocnemius muscles were carefully removed, minced, and fixed in 10% formalin. Tissues were embedded in paraffin and cut into 4-μm thick slices for morphological and pathological evaluations. Immunohistochemical (IHC) staining of tissues involved use of the Leica antibody to myosin heavy chain fast (WB-MHCf) and myosin heavy chain slow (WB-MHCs). By using automated BondMax with double staining, WB-MHCf and WB-MHCs epitope retrieval involved use of ER2 (AR9640) (pH 9) retrieval solution for 30 min once, followed by incubation with WB-MHCf and WB-MHCs antibodies with diluent 100X for 30 min. The detection kit used was the Bond Polymer Refine Detection (DS9800) (incubation with post primary for 8 min, polymer for 8 min and 3'3'-diaminobenzidine for 5 min) and Bond Polymer Refine Red Detection (DS9390) (incubation with post primary for 20 min, polymer for 30 min, Red for 10 min and haematoxylin for 5 min). Finally, results were examined under a light microscope equipped with a CCD camera (BX-51, Olympus, Tokyo) by a veterinary pathologist.
Statistical analysis
All data were expressed as mean ± SEM (n = 8). Differences between groups were analyzed by one-way ANOVA using Duncan's post-hoc test, and p values < 0.05 were considered significant.
Protocol for 6-week whole-body vibration training (WBV).
Results
Effect of DHEA Supplementation Combined with WBV Training on Body Weight (BW), Skeletal Muscle Mass, and Other Metabolism-Related Organ Weights
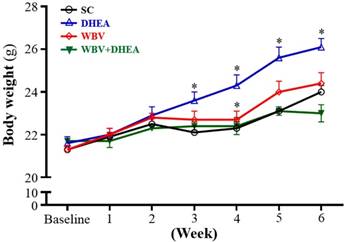
The initial BW of SC, DHEA, WBV, and DHEA + WBV groups was 21.3±0.1, 21.6±0.2, 21.3±0.2, and 21.7±0.1 g, respectively, with no differences among groups (Figure 2). The BW of DHEA group was significantly higher from 3 to 6 weeks than other groups. The BW of SC, WBV, and WBV+DHEA groups did not significantly differ during the 6-week experiment period. At the end of the experiment, the BW in the SC, DHEA, WBV, and WBV+DHEA groups were 24.0±0.3, 26.1±0.4, 24.4±0.5, and 23.0±0.4, respectively. The final BW of the DHEA group was significantly higher by 1.09- fold (p = 0.0008) than the SC group. On the other hand, the final BW of the WBV+DHEA group was significantly lower by 12% (p < 0.0001) compared with the DHEA group. Thus, DHEA supplementation can increase BW. Effect of 6-week DHEA, WBV, and WBV+DHEA on food intake, water intake and tissue changes were shown in Table 1. The food and water intake of all groups did not differ. In addition, there were no significant changes in the liver, epididymal fat pad (EFP), heart, and brown adipose tissue (BAT) weights. The muscle weight of DHEA group was significantly higher, by 1.08-fold (p = 0.0445), compared to the SC group. The lung weight was significantly higher for the WBV than SC group, by 1.14-fold (p = 0.0096). Moreover, relative tissue weight (%) is a measure of different tissue weights adjusted for individual BW. The relative lung weight of the WBV and WBV+DHEA groups was 1.15- (p = 0.0039) and 1.12-fold (p = 0.011) higher than SC group, respectively. Relative liver, EFP, heart, muscle, and BAT weights did not differ among groups. Consistent with the change in BW and tissue weights, the WBV training had no negative impact on appetite, although the tissue weight had slightly changes in kidney and lung of WBV group, the values are still within reasonable limits. In this study, the BW and muscle weights of DHEA-fed mice were significantly increased, but DHEA+WBV group was significantly decreased. Thus, sex steroid hormone levels relate to increase in muscle mass, and WBV training inhibited the DHEA-stimulating BW and muscle weight gain.
Effect of DHEA Supplementation Combined with WBV Training on Forelimb Grip Strength and Endurance Swimming Performance
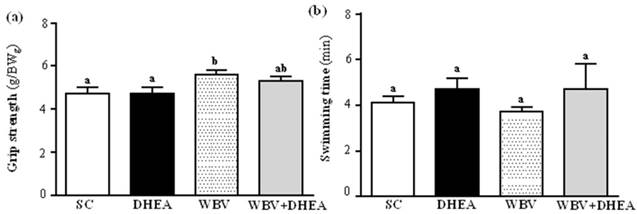
The forelimb grip demonstrates the maximal and explosive force production. The grip strength were 4.7±0.3, 4.7±0.1, 5.6±0.2, and 5.3±0.2 g/BWg for the SC, DHEA, WBV, and WBV+DHEA groups, respectively (Figure 3 a). Compared with the SC group, the grip strength was significantly higher by 1.18-fold (p = 0.0063) in WBV group, and slightly higher by 1.13-fold (p = 0.061) in DHEA+WBV group. Exercise endurance is an important variable in evaluating aerobic capacity. Swimming exhaustive time reflects the endurance exercise capacity. The exercise endurance levels with a swimming test in SC, DHEA, WBV and WBV+DHEA groups were 4.1±0.3, 4.7±0.5, 3.7±0.2, and 4.7±1.1 min, respectively (Figure 3 b). Each groups had no significant effect on the endurance exercise.
Effects of 6-week DHEA, WBV, and WBV+DHEA on body weight, food intake, water intake, and tissue changes.
| Characteristic | SC | DHEA | WBV | WBV+DHEA |
|---|---|---|---|---|
| Food intake (g/day) | 4.7±0.3 | 4.5±0.1 | 4.9±0.2 | 4.9±0.3 |
| Water intake (mL/day) | 4.9±0.3 | 5.1±0.1 | 4.9±0.3 | 4.9±0.2 |
| Liver (g) | 1.11±0.03ab | 1.22±0.05b | 1.11±0.03ab | 1.07±0.04a |
| Kidney (g) | 0.35±0.01b | 0.36±0.01b | 0.32±0.01a | 0.35±0.01b |
| EFP (g) | 0.19±0.01 | 0.20±0.02 | 0.20±0.01 | 0.15±0.02 |
| Heart (g) | 0.13±0.00 | 0.14±0.01 | 0.13±0.01 | 0.13±0.01 |
| Lung (g) | 0.14±0.01a | 0.15±0.01ab | 0.16±0.01b | 0.15±0.01ab |
| Muscle (g) | 0.31±0.01a | 0.33±0.01b | 0.30±0.01a | 0.30±0.01a |
| BAT (g) | 0.05±0.00 | 0.06±0.00 | 0.05±0.00 | 0.05±0.00 |
| Relative (%) | ||||
| liver | 4.62±0.14 | 4.65±0.16 | 4.62±0.08 | 4.63±0.14 |
| Kidney | 1.47±0.05bc | 1.38±0.05ab | 1.34±0.02a | 1.51±0.03c |
| EFP | 0.80±0.04 | 0.77±0.07 | 0.82±0.04 | 0.66±0.10 |
| Heart | 0.55±0.02 | 0.52±0.04 | 0.53±0.02 | 0.54±0.02 |
| Lung | 0.59±0.02a | 0.56±0.02a | 0.68±0.01b | 0.66±0.02b |
| Muscle | 1.27±0.03 | 1.26±0.03 | 1.23±0.04 | 1.28±0.02 |
| BAT | 0.22±0.01 | 0.23±0.02 | 0.21±0.01 | 0.23±0.01 |
Data were mean ± SEM (n = 8). Different letters indicated significant difference at p < 0.05 by one-way ANOVA. Muscle mass includes both gastrocnemius and soleus muscles in the back part of the lower legs. EFP: epididymal fat pad; BAT: brown adipose tissue; sedentary control with vehicle (SC), DHEA supplementation (DHEA), WBV training (WBV), and WBV combined with DHEA supplementation (WBV+DHEA).
Effect of DHEA supplementation combined with WBV training on body weight (BW) for 6 weeks. Data were mean ± SEM (n = 8). * p < 0.05 for DHEA, WBV and WBV+DHEA groups, respectively, compared with SC group by one‐way ANOVA.
Effect of 6-week DHEA, WBV, and WBV+DHEA on forelimb grip strength (a) and swimming exercise performance (b). Male C57BL/6 mice underwent a grip strength test 1 h after the final administered DHEA or WBV training. Swimming performance test were pretreated with DHEA or WBV training and then 1 h later performed an exhaustive swimming exercise with a load equivalent to 5% of the mouse's body weight attached to the tail. Data were mean ± SEM (n = 8). Different letters indicated significant difference at p < 0.05 by one-way ANOVA.
Effect of DHEA Supplementation Combined with WBV Training on the Serum Testosterone Level
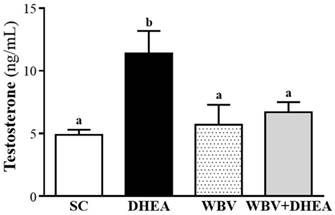
The serum testosterone level of SC, DHEA, WBV, and WBV+DHEA groups were 4.9±0.4, 11.4±1.8, 5.7±1.6, and 6.7±0.8 ng/mL, respectively (Figure 4). The testosterone level was significantly increased by 2.31-fold (p = 0.0013) in DHEA group than SC group, with no significant difference among SC, WBV, and WBV+DHEA groups. According this result, we found that WBV combination with DHEA supplementation could decrease the testosterone level increase by DHEA treatment.
Effect of 6-week DHEA, WBV, and WBV+DHEA on serum testosterone level. Data were mean ± SEM (n = 8). Different letters indicated significant difference at p < 0.05 by one-way ANOVA.
Effect of DHEA Supplementation Combined with WBV Training on Serum Lactate, Ammonia, Glucose, CK, and BUN Levels after Acute Exercise Challenge
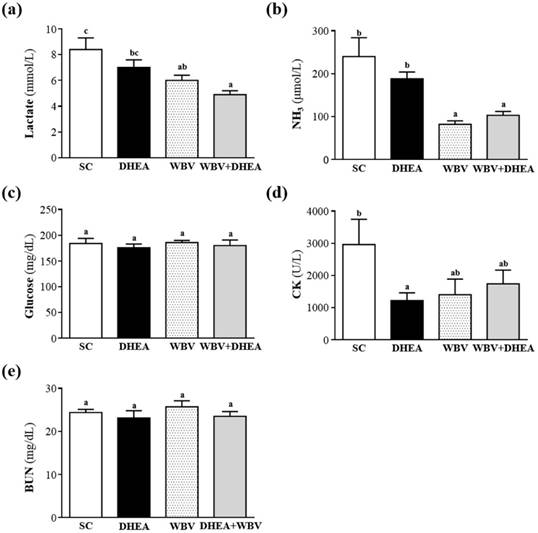
Muscle fatigue after exercise was evaluated by biochemical indicators, including lactate, ammonia, glucose, CK, and BUN [21]. During high-intensity exercise, muscles must obtain sufficient energy from anaerobic glycolysis, and abundant lactate is produced by glycolysis metabolism. Lactate is an oxidizable substrate in skeletal muscle and a precursor to gluconeogenesis in muscles or liver after exercise [22]. In the present study, lactate levels in the SC, DHEA, WBV and DHEA + WBV groups were 8.6±2.7, 7.0±1.6, 6.0±0.4 and 4.9±0.3 mmol/L, respectively; the levels with WBV and WBV+DHEA treatment were significantly lower, by 29% (p = 0.007) and 41% (p = 0.0003), when compared with SC group, respectively (Figure 5a). Therefore, six-week combination of WBV with DHEA supplementation could decrease the serum lactate accumulation after the 15 min acute exercise. Muscle fatigue is associated with deamination of adenine nucleotides, and increased deamination of AMP coincides with decreased phosphocreatine and pH values and failure of the contraction process. Peripheral and central fatigue levels are related to increased ammonia level during exercise [23]. Serum ammonia level in the SC, DHEA, WBV and WBV+DHEA groups was 240±44, 188±16, 92±8 and 103±9 μmol/L, respectively. Serum ammonia levels of WBV and WBV+DHEA groups were significantly lower, by 62% (p = 0.0002) and 57% (p < 0.0004) than the SC group, respectively (Figure 5b). Blood glucose level is an important index for performance maintenance during exercise. Serum glucose level in the SC, DHEA, WBV, and WBV+DHEA groups was 184±10, 176±7, 186±4, and 180±11 mg/dL, respectively; with no significant differences among all groups (Figure 5c). During exercise, carbohydrates are the main substrates for ATP resynthesis in tissues, and glucose mobilization is associated with the metabolic demands of muscles during activity [24]. The maintenance of steady levels of blood glucose during physical exercise involves very precise controls of the hepatic production of glucose, which includes hormonal feedback mechanisms [25]. However, in this study, DHEA and WBV training alone or combination have no beneficial effect on glucose values after the acute exercise. Serum CK is an important clinical biomarker for muscle damage, such as muscular dystrophy, severe muscle breakdown, myocardial infarction, autoimmune myositides, and acute renal failure. Serum CK activity of the SC, DHEA, WBV, and WBV+DHEA groups were 2963±786, 1219±243, 1403±486, and 1737±430 U/L, respectively (Figure 5d), and the CK activity of DHEA group was significantly decreased by 59% (p = 0.0258) than SC group (Figure 4d). Many factors other than renal disease can cause BUN alteration [26]. Urea is formed by the liver and carried with the blood to the kidneys, and urea is an important index correlation with protein breakdown, dehydration, stress, and fatigue [27]. The serum BUN level of the SC, DHEA, WBV, and WBV+DHEA groups were 24.4±0.7, 23.1±1.7, 25.7±1.4, and 23.5±1.1 mg/dL, respectively, with no significant difference among all groups (Figure 4e). DHEA supplementation alone or WBV training alone could reduce serum lactate, ammonia, and CK levels after acute exercise challenge. Thus, DHEA supplementation or WBV training alone may be an ergogenic supplement to recover the fatigue and recovery of muscle damage after acute exercise challenge. The results of WBV training agreed with previous results that WBV training is beneficial to serum lactate, ammonia, and CK levels after exercise [28].
Effect of DHEA Supplementation Combined with WBV Training on Hepatic and Muscle Glycogen Level
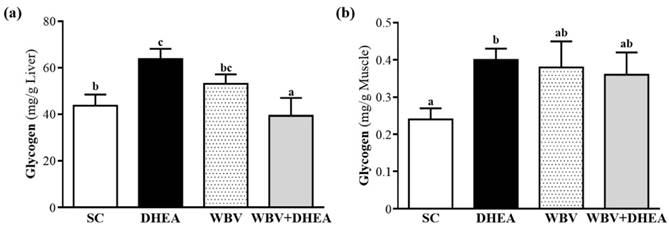
Glycogen is the predominant source of glycolysis [29]. During high-intensity exercise, muscle obtains enough energy from anaerobic glycolysis, and abundant lactate is produced through glycolysis [19]. The glycogen contents of liver and muscle tissues were shown in Figure 6a and 6b. The liver glycogen level of SC, DHEA, WBV, and WBV+DHEA groups was 43.7±4.8, 63.8±4.4, 53.1±4.1, and 39.3±7.7 mg/g liver, respectively, and the liver glycogen content of DHEA group was significantly higher (p = 0.0057) by 1.45-fold than with SC. WBV+DHEA group was significantly lower (p < 0.0001) by 38.37% than DHEA group (Figure 6a). Glycogen content of muscle tissues in SC, DHEA, WBV, and WBV+DHEA groups was 0.24±0.09, 0.40±0.03, 0.38±0.07, and 0.36±0.06 mg/g muscle, respectively. Compared with SC group, muscle glycogen level of DHEA group was significantly increased (p = 0.027) by 1.72-fold, and did not differ among SC, WBV and DHEA+WBV groups (Figure 6b).
Effect of 6-week DHEA, WBV, and WBV+DHEA on serum levels of (a) lactate, (b) ammonia (NH3), (c) glucose, (d) creatine kinase (CK), and (e) blood urea nitrogen (BUN) after an acute exercise challenge. Mice were pretreated with of DHEA, WBV, or WBV+DHEA for six weeks, then 1 h later performed a 15-min swimming test without weight loading. Data were mean ± SEM (n = 8). Different letters indicated significant difference at p < 0.05 by one-way ANOVA.
Effect of 6-week DHEA, WBV, and WBV+DHEA on (a) hepatic glycogen and (b) muscle glycogen levels at rest. Data were mean ± SEM (n = 8). Different letters indicated significant difference at p < 0.05 by one-way ANOVA.
Effect of DHEA Supplementation Combined with WBV Training on Biochemical Analyses at the End of the Experiment
The levels of AST, ALT, BUN, CK, and UA did not differ among groups (Table 2), thus there were no negative effect in WBV+DHEA group in biochemical analyses. In addition, the level of creatinine was significantly lowered 13% (p = 0.0319) in the WBV+DHEA than SC group. The glucose level of WBV+DHEA group was significantly lower 26% (p = 0.005) than DHEA group.
Effect of 6-week DHEA, WBV, and WBV+DHEA on biochemical serum levels at the end of the experiment.
| Variable | SC | DHEA | WBV | WBV+DHEA |
|---|---|---|---|---|
| AST (U/L) | 145 ± 13 | 115 ± 13 | 188 ± 29 | 165 ± 15 |
| ALT (U/L) | 60 ± 4 | 59 ± 10 | 92 ± 10 | 69 ± 12 |
| Creatinine (mg/dL) | 0.39 ± 0.01b | 0.37 ± 0.02ab | 0.38 ± 0.01ab | 0.34 ± 0.02a |
| BUN (mg/dL) | 23 ± 1 | 21 ± 1 | 20 ± 1 | 20 ± 1 |
| CK (U/L) | 926 ± 178 | 654 ± 167 | 1592 ± 428 | 864 ± 530 |
| Glucose (mg/dL) | 164 ± 8ab | 188 ± 10bc | 194 ± 6c | 139 ± 11a |
| UA (mg/dL) | 1.4 ± 0.1 | 1.2 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.2 |
Data were mean ± SEM (n = 8). Different letters indicated significant difference at p < 0.05 by one-way ANOVA. AST, aspartate aminotransferase; ALT, alanine aminotransferase; CK, creatine kinase; BUN, blood urea nitrogen; UA, uric acid.
Histopathological Evaluation and Immunohistochemistry (IHC) of gastrocnemius muscles of DHEA Supplementation Combined with WBV Training at the End of the Experiment
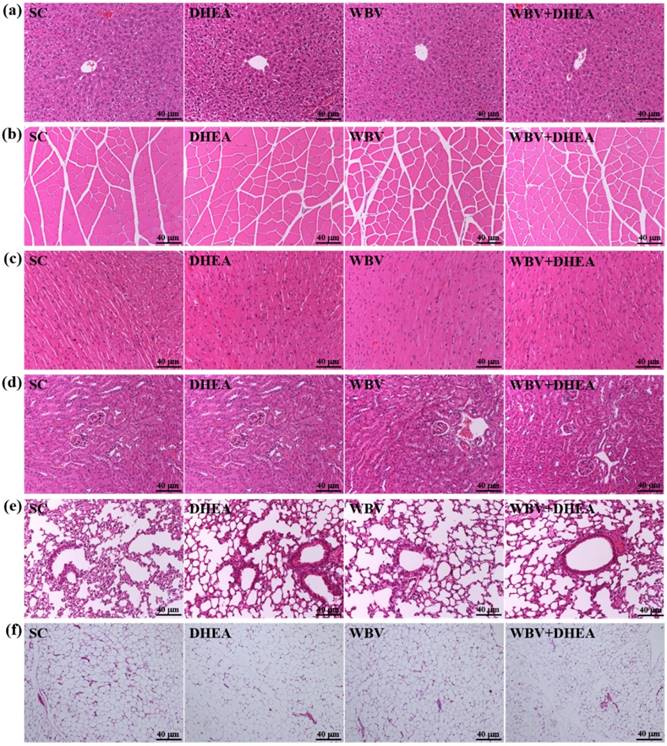
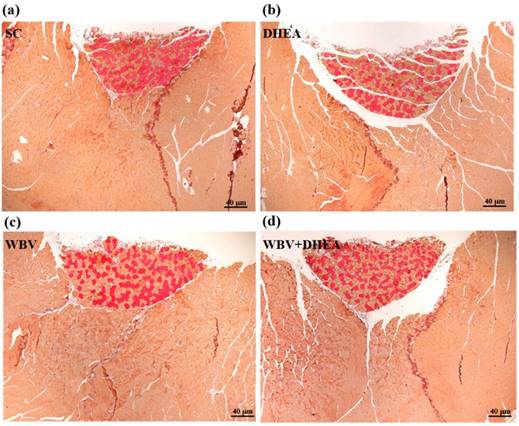
Figure 7 showed that the four groups did not differ according to histological observations of the liver, muscle, heart, kidney, lung, and testis. We also investigated the difference between slow muscle and fast muscle by IHC (Figure 8). Red fibers (slow muscle) and orange fibers (fast muscle) were did not differ among treatments in soleus and gastrocnemius muscle. Thus, DHEA supplementation and WBV training were not transfer the gastrocnemius muscle type in young-adult mice.
Comment
We conducted DHEA treatment combined with WBV training and discovery WBV+DHEA could inhibit the BW gain from DHEA supplementation. In our previous study, after WBV training (5.6 Hz, 2 mm, 0.13 g) for 6-weeks, BW of mice fed with a high fat diet was slightly but not significantly decreased compared with those with a high fat diet only [30]. Several studies have reported WBV training could reduce body weight or arterial stiffness in non-athletes [31-33]. In this study, although BW of WBV group had no differences compared with SC, we speculate that WBV training could inhibit the hormone-stimulating weight increase caused by DHEA.
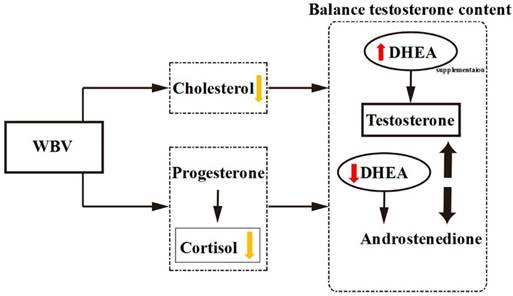
In recent systematic reviews, WBV is as an alternative to conventional training or as supplementary training, and Osawa et al. [34] suggested that the use of WBV training would lead to greater muscle strength and countermovement jump height compared with the identical conditions without WBV training. As indicated previously, combination of DHEA administration with resistance exercise improved muscle mass and strength in older individuals [35, 36]. In our present data, DHEA treatment combined with WBV training could not have synergistic effect on muscle strength and exercise performance in young mice. Thus, the effect of DHEA treatment combined with WBV training on exercise performance has related with age. We also find that combination of DHEA supplementation with WBV could inhibit DHEA-increase testosterone capacity. DHEA, the major adrenal androgens, is the precursor of several metabolites, including sex steroid hormones [37]. According to previous research, both cholesterol and DHEA are substrates for testosterone formation [2], and WBV training has been demonstrated to decrease serum cholesterol content [30]. Therefore, WBV+DHEA treatment may cause cholesterol-lowering to decrease serum DHEA. WBV influences proprioceptive feedback mechanisms and specific neural components to decrease cortisol content which steroid (glucocorticoid) hormone produced by the adrenal gland [38, 39]. WBV and DHEA supplementation may stimulate the hormone variety to open the balancing synthesis of testosterone content via DHEA pathway. Therefore, we suggest that WBV may inhibit DHEA absorption or testosterone synthesis, and influence these biochemical indices in vivo.
In our study, there is no synergistic effect on DHEA supplementation and WBV training, but it demonstrated that DHEA supplementation had effective for muscle protective effects on CK biomarker after acute exercise.
The endogenic effect of WBV training in skeletal muscle can attribute to glycogen synthesis in the muscle, which can continue for more than 5 hours [40]. Previous studies demonstrated DHEA enhanced insulin-stimulated PKCζ/λ activation in muscle and AKT activation in the liver of rats. PKCζ/λ activation may modulate GLUT4 translocation and glucose transport in muscle [41]. AKT and PKC activities induced by insulin are increased in liver and muscle, respectively [42]. According to our results, supplementation with DHEA increased hepatic and muscle glycogen storage and synthesis. However, WBV training affects the concentrations of glucose and several hormones [43] and modulates the glucose metabolism rate of the DHEA supplementation.
The H&E staining of 6-week DHEA, WBV, and WBV+DHEA on the morphology of (a) liver, (b) skeletal muscle, (c) heart, (d) kidney, (e) lung, and (f) epididymal fat pad (EFP) tissues. Specimens were photographed with a light microscope (Olympus BX51). (Magnification: ×200, Scale bar, 40 µm).
The immunohistochemical (IHC) staining of 6-week DHEA, WBV, and WBV+DHEA on type I and type II muscle fibers in gastrocnemius muscle. Red fibers are type I fibers; orange fibers are type II fibers. Specimens were photographed by light microscopy. (Magnification: ×200, Scale bar, 40 µm).
The proposed mechanisms by which DHEA supplementation combined with WBV training acts on the hormone regulation.
In our present study, instead of DHEA increasing the glycogen, WBV+DHEA may consume the glycogen to result in improving energy utilization and a reduction in BW. Our previous study suggested that when carbohydrates are available after exercise, liver glycogen resynthesis is the first priority and muscle glycogen synthesis is secondary [19]. DHEA+WBV may be a safe way to increase energy expenditure and lose weight. On the other hand, one of the main advertising arguments for the use of WBV devices available on the market is that they promote weight loss or decrease fat mass. However, there is a lack of data to support these claims. In our opinion, WBV can influence muscle strength, body weight, or metabolism under specific situations, such as in older [44, 45] and obese subjects [46].
In conclusion, we found that DHEA supplementation increased BW, serum testosterone level and glycogen (liver and muscle) contents, as well as reduced fatigue after acute exercise. However, the combination of WBV training and DHEA supplementation resulted in a significant decrease in testosterone level and glycogen contents when compared with DHEA supplementation alone. WBV and DHEA supplementation may stimulate the hormone variety to open the balancing synthesis of testosterone content via DHEA pathway (Figure 9). This phenomenon shows that WBV could inhibit DHEA absorption or testosterone synthesis and influence these biochemical indices in young-adult mice. Taken together, we suggested that WBV training is not suitable during the supplementation period and DHEA does not affect exercise performance in young-adult mice.
Acknowledgements
This study was supported by the Ministry of Science and Technology of Taiwan (grant no. MOST 104-2410-H-255-003 to Wen-Chyuan Chen). The authors are grateful to Chien-Chao Chiu for pathological examination and Yu-Tuan Chen (Da-Guang construction Inc., Taipei, Taiwan) for technical assistance on plotting the training protocol.
Author Contributions
Wen-Chyuan Chen and Chi-Chang Huang and designed the experiments. Yi-Ming Chen and Chi-Chang Huang carried out the laboratory experiments. Wen-Chyuan Chen, Yi-Ming Chen and Yen-Dun Tzeng analyzed the data, interpreted the results, prepared figures, and wrote the manuscript. Wen-Chyuan Chen, Yi-Ming Chen and Yen-Dun Tzeng revised the manuscript. Wen-Chyuan Chen and Yen-Dun Tzeng contributed DHEA, reagents, materials and analysis platforms.
Conflicts of Interest
The authors declare no conflict of interest.
References
1. Belanger A, Candas B, Dupont A, Cusan L, Diamond P, Gomez JL, Labrie F. Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year old men. J Clin Endocrinol Metab. 2005;79:1086-1090
2. Sato K, Iemitsu M, Aizawa K, Mesaki N, Ajisaka R, Fujita S. DHEA administration and exercise training improves insulin resistance in obese rats. Nutr Metab. 2012;9:1
3. Coza A, Nigg BM, Dunn JF. Effects of vibrations on gastrocnemius medialis tissue oxygenation. Med Sci Sports Exerc. 2011;43:509-515
4. Baulieu EE, Robel P. Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) as neuroactive neurosteroids. Proc Natl Acad Sci U S A. 1998;95:4089-4091
5. Muller M, Grobbee DE, den Tonkelaar I, Lamberts SW, van der Schouw YT. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endcrinol Metab. 2005;90:2618-2623
6. Blackman MR, Sorkin JD, Munzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE. Growth hormone and sex steroid administration in healthy aged women and men. JAMA. 2002;288:2282-2292
7. Frystyk J. Exercise and the growth hormone-insulin-like growth factor axis. Med Sci Sports Exerc. 2010;42:58-66
8. Sato K, Iemitsu M, Aizawa K, Ajisaka R. Testosterone and DHEA activate the glucose metabolism-related signaling pathway in skeletal muscle. Am J Physiol Endocrinol Met. 2008;294:E961-E968
9. Petit PD, Pensini M, Tessaro J, Desnuelle C, Legros P, Colson SS. Optimal whole-body vibration settings for muscle strength and power enhancement in human knee extensors. J Electromyogr Kinesiol. 2010;20:1186-1195
10. Verschueren SM, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a ran- domized controlled pilot study. J Bone Miner Res. 2004;19:352-359
11. Vissers D, Verrijken A, Mertens I. Effect of long- term whole body vibration training on visceral adipose tissue: a preliminary report. Obes Facts. 2010;3:93-100
12. Domingos LL, Giehl PM, Paiva DN, Asad NR, Marin PJ, Bernardo-Filho M. Alterations on the plasma concentration of hormonal and non hormonal biomarkers in human beings submitted to whole body vibration exercises. Sci Res Essays. 2015;10:287-297
13. Bogaerts A, Delecluse C, Claessens AL, Coudyzer W, Boonen S, Verschueren SM. Impact of whole-body vibration training versus fitness training on muscle strength and muscle mass in older men: a 1-year randomized controlled trial. J Gerontol Ser A Biol Sci Med Sci. 2007;62:630-635
14. Lau RW, Liao LR, Yu F, Teo T, Chung RC, Pang MY. The effects of whole body vibration therapy on bone mineral density and leg muscle strength in older adults: a systematic review and meta-analysis. Clin Rehabil. 2011;25:975-988
15. Khorram O, Vu L, Yen SS. Activation of immune function by dehydroepiandrosterone (DHEA) in age-advanced men. J Gerontol Ser A Biol Sci Med Sci. 1997;52:1-7
16. Guidance for Industry on Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Department of Health and Human Services, Food and Drug Administration. https://www.federalregister.gov/articles/2005/07/22/05-14456/guidance-for-industry-on-estimating-the-maximum-safe-starting-dose-in-initial-clinical-trials-for
17. Wang SY, Huang WC, Liu CC, Wang MF, Ho CS, Huang WP, Huang CC. Pumpkin (Cucurbita moschata) fruit extract improves physical fatigue and exercise performance in mice. Molecules. 2012;17:11864-11876
18. Huang WC, Chiu WC, Chuang HL, Tang DW, Lee ZM, Wei L, Huang CC. Effect of curcumin supplementation on physiological fatigue and physical performance in mice. Nutrients. 2015;7:905-921
19. Chen YM, Lin CL, Wei L, Hsu YJ, Chen KN, Huang CC, Kao CH. Sake protein supplementation affects exercise performance and biochemical profiles in power-exercise-trained mice. Nutrients. 2016;8:106
20. Hsu YJ, Chiu CC, Li YP, Huang WC, Huang YT, Huang CC, Chuang HL. Effect of intestinal microbiota on exercise performance in mice. J Strength Cond Res. 2015;29:552-558
21. Chen YM, Tsai YH, Tsai TY, Chiu YS, Wei L, Chen WC, Huang CC. Fucoidan supplementation improves exercise performance and exhibits anti-fatigue action in mice. Nutrients. 2014;7:239-252
22. Brooks GA. Intra-and extra-cellular lactate shuttles. Med Sci Sports Exerc. 2000;32:790-799
23. Carvalho-Peixoto J, Alves RC, Cameron LC. Glutamine and carbohydrate supplements reduce ammonemia increase during endurance field exercise. Appl Physiol Nutr Metab. 2007;32:1186-1190
24. Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998;47:1369-1373
25. Klieverik LP, Janssen SF, van Riel A, Foppen E, Bisschop PH, Serlie MJ, Boelen A, Ackermans MT, Sauerwein HP, Fliers E, Kalsbeek A. Thyroid hormone modulates glucose production via a sympathetic pathway from the hypothalamic paraventricular nucleus to the liver. Proc Natl Acad Sci U S A. 2009;106:5966-5971
26. Langenberg C, Wan L, Egi M, May CN, Bellomo R. Renal blood flow in experimental septic acute renal failure. Kidney Int. 2006;69:1996-2002
27. Wang JJ, Shieh MJ, Kuo SL, Lee CL, Pan TM. Effect of red mold rice on antifatigue and exercise-related changes in lipid peroxidation in endurance exercise. Appl Microbiol Biotechnol. 2006;70:247-253
28. Lin CI, Huang WC, Chen WC, Kan NW, Wei L, Chiu YS, Huang CC. Effect of whole-body vibration training on body composition, exercise performance and biochemical responses in middle-aged mice. Metabolism. 2015;64:1146-1156
29. Greenberg CC, Jurczak MJ, Danos AM, Brady MJ. Glycogen branches out: New perspectives on the role of glycogen metabolism in the integration of metabolic pathways. Am J Physiol Endocrinol Metab. 2006;291:E1-E8
30. Huang CC, Tseng TL, Huang WC, Chung YH, Chuang HL, Wu JH. Whole-body vibration training effect on physical performance and obesity in mice. Int J Med Sci. 2014;11:1218-1227
31. Vissers D, Verrijken A, Mertens I, Van Gils C, Van de Sompel A, Truijen S, Van Gaal L. Effect of long-term whole body vibration training on visceral adipose tissue: a preliminary report. Obes Facts. 2010;3:93-100
32. Behboudi L, Azarbayjani MA, Aghaalinejad H, Salavati M. Effects of aerobic exercise and whole body vibration on glycaemia control in type 2 diabetic males. Asian J Sports Med. 2011;2:83-90
33. Figueroa A, Gil R, Wong A, Hooshmand S, Park SY, Vicil F, Sanchez-Gonzalez MA. Whole-body vibration training reduces arterial stiffness, blood pressure and sympathovagal balance in young overweight/obese women. Hypertens Res. 2012;35:667-672
34. Osawa Y, Oguma Y, Ishii N. The effects of whole-body vibration on muscle strength and power: a meta-analysis. J Musculoskel Neuron. 2013;13:380-390
35. Villareal DT, Holloszy JO. DHEA enhances effects of weight training on muscle mass and strength in elderly women and men. Am J Physiol. 2006;291:1003-1008
36. Kenny AM, Boxer RS, Kleppinger A, Brindisi J, Feinn R, Burleson JA. Dehydroepiandrosterone combined with exercise improves muscle strength and physical function in frail older women. J Am Geriatr Soc. 2006;58:1707-1714
37. Osawa E, Nakajima A, Yoshida S, Omura M, Nagase H, Ueno N, Sekihara H. Chemoprevention of precursors to colon cancer by dehydroepiandrosterone (DHEA). Life Sci. 2002;70:2623-2630
38. Bosco C, Iacovelli M, Tsarpela O, Cardinale M, Bonifazi M, Tihanyi J, Viru A. Hormonal responses to whole-body vibration in men. Eur J Appl Physiol. 2000;81:449-454
39. Roberge C, Carpentier AC, Langlois MF, Baillargeon JP, Ardilouze JL, Maheux P, Gallo-Payet N. Adrenocortical dysregulation as a major player in insulin resistance and onset of obesity. Amer J Physiol Endocrinol Met. 2007;293:E1465-E1478
40. Behboudi L, Azarbayjani MA, Aghaalinejad H, Salavati M. Effects of aerobic exercise and whole body vibration on glycaemia control in type 2 diabetic males. Asian J Sports Med. 2011;2:83-90
41. Farese RV. Insulin-sensitive phospholipid signalling systems and glucose transport. Update II. Proc Soc Exp Biol Med. 2001;226:283-295
42. Campbell CS, Caperuto LC, Hirata AE, Araujo EP, Velloso LA, Saad MJ, Carvalho CR. The phosphatidylinositol/AKT/atypical PKC pathway is involved in the improved insulin sensitivity by DHEA in muscle and liver of rats in vivo. Life Sci. 2004;76:57-70
43. Di Loreto C, Ranchelli A, Lucidi P, Murdolo G, Parlanti N, De Cicco A, Bolli GB. Effects of whole-body vibration exercise on the endocrine system of healthy men. J Endocrinol Invest. 2004;24:323-327
44. Witham MD, Avenell A. Interventions to achieve long-term weight loss in obese older people A systematic review and meta-analysis. Age and Ageing. 2010;39:176-184
45. Mikhael M, Orr R, Amsen F, Greene D, Singh MA. Effect of standing posture during whole body vibration training on muscle morphology and function in older adults: a randomised controlled trial. BMC Geriatrics. 2010;10:1
46. Figueroa A, Gil R, Wong A, Hooshmand S, Park SY, Vicil F, Sanchez-Gonzalez MA. Whole-body vibration training reduces arterial stiffness, blood pressure and sympathovagal balance in young overweight/obese women. Hypertens Res. 2012;35:667-672
Author contact
![]() Corresponding author: Kaohsiung Veterans General Hospital, No.386, Dazhong 1st Rd., Zuoying Dist., Kaohsiung City 81362, Taiwan (Y.-D.T.). Tel.: +886-7-3422121 (ext. 3008) (Y.-D.T.). Electronic addresses: seeoutonycom (Y.-D.T.).
Corresponding author: Kaohsiung Veterans General Hospital, No.386, Dazhong 1st Rd., Zuoying Dist., Kaohsiung City 81362, Taiwan (Y.-D.T.). Tel.: +886-7-3422121 (ext. 3008) (Y.-D.T.). Electronic addresses: seeoutonycom (Y.-D.T.).

 Global reach, higher impact
Global reach, higher impact