3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2016; 13(5):316-324. doi:10.7150/ijms.14552 This issue Cite
Research Paper
Prevalence of the Lyme Disease Spirochete, Borrelia burgdorferi, in Blacklegged Ticks, Ixodes scapularis at Hamilton-Wentworth, Ontario
1. Research Division, Lyme Ontario, Fergus, Ontario, Canada N1M 2L7;
2. Department of Entomology and Center for Vector Ecology and Zoonotic Diseases. The Connecticut Agricultural Experiment Station, New Haven, Connecticut, USA 06511;
3. Department of Biology, Georgia Southern University, Statesboro, Georgia, USA 30458;
4. Environmental Epidemiology Research Laboratory, Department of Public Health, University of North Florida, Jacksonville, Florida, USA 32224.
Received 2015-11-28; Accepted 2016-3-21; Published 2016-4-10
Abstract
Lyme disease has emerged as a major health concern in Canada, where the etiological agent, Borrelia burgdorferi sensu lato (s.l.), a spirochetal bacterium, is typically spread by the bite of certain ticks. This study explores the presence of B. burgdorferi s.l. in blacklegged ticks, Ixodes scapularis, collected at Dundas, Ontario (a locality within the region of Hamilton-Wentworth). Using passive surveillance, veterinarians and pet groomers were asked to collect blacklegged ticks from dogs and cats with no history of travel. Additionally, I. scapularis specimens were submitted from local residents and collected by flagging. Overall, 12 (41%) of 29 blacklegged ticks were infected with B. burgdorferi s.l. Using polymerase chain reaction (PCR) and DNA sequencing, two borrelial amplicons were characterized as B. burgdorferi sensu stricto (s.s.), a genospecies pathogenic to humans and certain domestic animals. Notably, three different vertebrate hosts each had two engorged I. scapularis females removed on the same day and, likewise, one cat had three repeat occurrences of this tick species. These multiple infestations suggest that a population of I. scapularis may be established in this area. The local public health unit has been underreporting the presence of B. burgdorferi s.l.-infected I. scapularis in the area encompassing Dundas. Our findings raise concerns about the need to erect tick warning signs in parkland areas. Veterinarians, medical professionals, public health officials, and the general public must be vigilant that Lyme disease-carrying blacklegged ticks pose a public health risk in the Dundas area and the surrounding Hamilton-Wentworth region.
Keywords: Blacklegged tick, Ixodes scapularis, Lyme disease, Borrelia burgdorferi, prevalence, Dundas, Ontario.
Introduction
The blacklegged tick, Ixodes scapularis (northern populations previously treated as I. dammini), is the primary vector of the Lyme disease bacterium, Borrelia burgdorferi sensu lato (s.l.) east of the Rocky Mountains [1]. Worldwide, the B. burgdorferi s.l. complex consists of at least 23 genospecies or genomospecies. In North America, at least 9 B. burgdorferi s.l. genospecies are present, namely B. americana, B. andersonii, B. bissettii, B. burgdorferi sensu stricto (s.s.), B. californiensis, B. carolinensis, B. garinii, B. kurtenbachii, and B. mayonii [2-9]. Of these 9 genospecies, B. andersonii, B. americana, B. bissettii, B. burgdorferi s.s., B. garinii, and B. mayonii are known to be pathogenic to humans [9-12].
In nature, I. scapularis can harbor and transmit a wide diversity of tick-associated pathogens. These zoonotic pathogens include: Babesia spp. (e.g., B. microti, B. duncani), Bartonella spp. (e.g., B. henselae), Ehrlichia spp. (E. ewingii), Mycoplasma spp. (e.g., M. fermentans), Anaplasma spp. (e.g., A. phagocytophilum), Borrelia miyamotoi (relapsing fever group spirochete), an Ehrlichia muris-like agent, and Deer Tick Virus (Powassan virus group). Of note, several tick-associated pathogens have been reported in I. scapularis collected from avian hosts, such as B. burgdorferi s.l. [13, 14], A. phagocytophilum [15], B. microti [16], and B. miyamotoi [17]. Also, Hersh et al. [18] reported a triple co-infection of B. burgdorferi s.l., B. microti, and A. phagocytophilum in an I. scapularis nymph collected from a Veery, Catharus fuscescens, a ground-frequenting Neotropical songbird.
In southwestern Ontario, several small mammals that are reservoir-competent hosts of B. burgdorferi s.l. include: eastern chipmunks, Tamias striatus [19, 20]; white-footed mice, Peromyscus leucopus [21]; deer mice, Peromyscus maniculatus [22, 23]; northern short-tailed shrews, Blarina brevicauda [24, 25]; and meadow vole, Microtus pennsylvanicus [26]. Although white-tailed deer, Odocoileus virginianus, are hosts of I. scapularis and support I. scapularis reproduction, they are not competent reservoir hosts of B. burgdorferi [27].
Songbirds act as a source of I. scapularis in the Dundas area. Migratory songbirds widely disperse larval and nymphal I. scapularis ticks across central and eastern Canada during spring migration [13,14, 28-30]. Neotropical and southern-temperate passerines transport I. scapularis as far north as northern Alberta [28] and, likewise, carry Lyme disease vector ticks during trans-border migration to as far north as southern Yukon [13]. When passerine migrants make landfall at established populations of I. scapularis, they can be parasitized by questing I. scapularis larvae and nymphs. Peak questing activity for I. scapularis nymphs coincides with peak northward spring migration [31, 32]. Subsequently, northbound migrants could transport I. scapularis immatures from central, southern, northeastern, and mid-Atlantic states and, likewise from established populations in southwestern Ontario, including Long Point, Turkey Point and Wainfleet Marsh, and then release them in the Dundas area. Although there are currently no bird banding stations operating in the Hamilton-Wentworth region, there are stations to the south and to the north that provide substantive data on songbird-transported I. scapularis immatures [30].
Banerjee et al. [33] provided the first record of a B. burgdorferi s.l.-infected I. scapularis in the Hamilton metropolitan area, which encompasses Dundas; it was collected from an untraveled dog in 1997. In addition, a 10-year, tick-host study (1993-2002), which was conducted across Ontario, reported B. burgdorferi s.l.-infected I. scapularis adults in the Hamilton-Wentworth area [34]. These tick researchers found that all I. scapularis, which were submitted from dogs, cats, horses, and people, were adults; no larvae or nymphs of this tick species were presented. However, the same study reported larvae and nymphs of several other tick species on mammalian hosts. Because residents in the Dundas area were being bitten by ticks and contracting Lyme disease, we wanted to determine the prevalence of B. burgdorferi s.l. in I. scapularis ticks in the Dundas area, and ascertain whether these ticks are a public health risk.
Materials and Methods
Study area. A 2-year study was conducted in the vicinity of Dundas, Ontario (43.2643° N, 79.9533° W), and encompassed a well-drained, hilly block of land situated at the west end of Lake Ontario in the Hamilton-Wentworth region, which is situated on the cusp of the Niagara escarpment. This 56 km2 area consists of deciduous forests that are interspersed with residential areas, and bordered by Highway 99, Highway 52, Highway 5, and Rock Chapel Road. The predominate tree species in this locality are: sugar maple, Acer saccharum; red maple, Acer rubrum; red oak, Quercus rubra; white oak, Quercus alba; white ash, Fraxinus americana; and black cherry, Prunus serotina.
The Dundas area supports a wide array of wildlife mammals that are common in deciduous forests in northeastern North America. The principal large mammal in this wooded area is the white-tailed deer. Mid-sized mammals include gray squirrels, Sciurus carolinesis; red squirrels, Tamiasciurus hudsonicus; eastern cottontail rabbits, Sylvilagus floridanus; raccoons, Procyon lotor; and striped skunk, Mephitis mephitis. Small mammals include deer mice; white-footed mice; meadow vole; northern short-tailed shrew; eastern chipmunk; and house mouse, Mus musculus. All of these resident mammals are hosts for I. scapularis ticks [35]. Not only do I. scapularis adults parasitize white-tailed deer, they also parasitize eastern cottontails, raccoons, and striped skunks [35].
Tick collection. We asked local veterinarians and pet groomers to participate in our study because dogs and cats are good sentinels of ixodid ticks. This study was conducted from mid-April to early December. We prepared and distributed a chart showing adults of four species of ticks, which are commonly found in the area, and asked local veterinarians and pet groomers to submit three of the four illustrated species. Each participant was given 'Tick-Host Information' sheets, and asked to complete and submit one with each tick specimen. The background information included: host, location/residency of companion animal, travel history, date collected, and collector. Animals with a history of travel were excluded from the study. We discouraged submissions of American dog ticks, Dermacentor variabilis, which are not a vector of B. burgdorferi s.l. We emphasized that I. scapularis ticks were the focus of our study, but invited other tick species. In addition, a local volunteer flagged 2 ha of forests and ecotones (woods edge); these are areas where I. scapularis are most commonly found. Flagging was timed to correspond with the bimodal questing activity periods at this lattitude of I. scapularis adults in the spring and fall. The flagging cloth was made of flannel-back vinyl (88 × 70 cm), and the length of the pole was 229 cm. Collection permits were not required because ticks are blood-sucking ectoparasites, and there are no proprietary issues with these arthropod pests.
The I. scapularis adults which attached to the flag, were removed with fine-pointed, stainless steel tweezers and placed in round-bottom, 8.5 mL polypropylene tubes (15.7 X 75 mm) with labels consisting of background information. A 7-mm hole was drilled in the polyethylene push caps (15.7 mm diameter) for ventilation and, to prevent ticks from escaping, tulle netting was placed over the mouth of the vial before the push cap was inserted. The capped vials were then placed in self-sealing, double-zipper, plastic bags with a slightly moistened paper towel. Ticks were sent directly by express mail to the tick identification laboratory (JDS) for examination and recording. Ticks were identified morphologically using taxonomic keys [36, 37].
Spirochete detection. The first portion of ticks were sent by courier to the culturing and PCR amplification laboratory (JFA). These ticks were directly tested for B. burgdorferi s.l. using DNA extraction and PCR analysis. The DNA detection methods have been previously described [38-40]. During the second phase of our study, I. scapularis ticks were put in 2 mL micro tubes containing 94% ethyl alcohol, and sent by courier to a separate laboratory (KLC).
DNA extraction. DNA was extracted from the ethanol-preserved ticks using a salting out procedure similar to that described previously [10]. Each tick was cut into several pieces with a sterile scalpel blade within a 2-mL microtube. Then, 500 µl 1x Tissue and Cell Lysis Buffer (MasterPure, Epicentre, Madison, WI) and 200 µg of proteinase K were added. After the sample was heated in a water bath at 65°C for 1 hour, the liquid was transferred to a clean tube, and then chilled at -20°C for 5 min. Then, 200 µl of 7.5M ammonium acetate was added, and the tube was vortexed on high speed for 30 sec. The sample was then chilled at -20°C for 5 min again, centrifuged at 16,000 RCF for 5 min at room temperature in a tabletop centrifuge (Eppendorf model 5424) to pellet protein, and the supernatant was transferred to a clean tube. To enhance DNA precipitation, 3 µl of polyacryl carrier (MRC, Cincinnati, OH) was added, and mixed by vortexing for 15 sec, followed by the addition of 700 µl of 100% isopropanol. Each tube was inverted 50 times to gently mix, and then chilled overnight at -20°C. DNA was pelleted by centrifuging at 16,000 RCF for 30 min at room temperature. Supernatant was discarded, and the pellet was washed twice with 1 mL of 75% ethanol, by inverting and rotating the tube gently 5 times to rinse the pellet, and inside of the tube, and then centrifuging for 5 min at 16,000 RCF for each rinse. The residual ethanol was removed, and the pellet was air dried at room temperature for 10 min. To rehydrate, 100 µl tris-EDTA buffer (pH 8.0) was added, and the tube was heated at 65°C for 5 min. Thereafter, each sample was stored at 0-4°C.
PCR testing. Tick extracts were initially screened for B. burgdorferi s.l. by two nested (hemi-nested) PCR assays designed to amplify separate portions of the 41-kDa chromosomal flagellin (flaB) gene. The assays are hereafter referred to as PCR1 and PCR2. Primary/outer reaction primers for PCR1 were 313F (5'-GCA-GAC-AGA-GGT-TCT-ATA-CAA-ATT-G-3') and 551R (5'-GCT-TCA-TCT-TGG-KTT-GCT-CCA-ACA-T-3'), which amplify a 238-bp fragment; inner reaction primers were 313F and 506R (5'-GCT-TGA-GAY-CCT-GAA-AGT-GAT-GCT-GG), which amplify a 194-bp product. Primers for PCR2 were 481F (5'-CCA-GCA-TCA-CTT-TCA-GGR-TCT-CA-3') and 737R (5'-GCA-TCA-ACT-GTR-GTT-GTA-ACA-TTA-ACA-GG-3', which amplify a 257-bp product, followed by 532F (5'-GGA-GCA-AMC-CAA-GAT-GAA-GCT-ATT-GC-3') and 737R, amplifying a 206-bp product. Positive results with PCR1 and PCR2 were confirmed with additional B. burgdorferi s.l. specific primers for the 5S-23S rRNA intergenic spacer and flaB gene as described previously [41, 42], as well as Borrelia species primers for the 16S-23S rRNA intergenic spacer [43].
First round PCR amplifications contained 2.5 µl of tick DNA extract in a total reaction volume of 50 µl. Each inner/nested reaction used 1 µl of outer reaction product as template. First round amplifications utilized a hot start PCR master mix (HotMasterMix, 5 Prime, Gaithersburg, MD) resulting in a final concentration of 1.0 U of Taq DNA polymerase, 45 mM KCl, 2.5 mM Mg2+, 200 µM of each deoxynucleoside triphosphate, and 0.5 µM of each primer. Second round amplifications used GoTaqGreen® PCR Master Mix (Promega, Madison, WI), which allowed samples to be directly loaded into agarose gels without the addition of a gel loading buffer. All PCRs were carried out in an Applied Biosystems AB2720 thermal cycler (Life Technologies, ThermoFisher Scientific, Waltham, MA). Each primary PCR consisted of initial denaturation at 94°C for 2 min, followed by 35 cycles at 94°C for 30 sec, primer annealing at 5°C below the lowest primer's calculated melting temperature for 30 sec, and extension at 65°C for 1 min, with a final extension at 65°C for 5 min. Nested reactions included initial denaturation at 94°C for 1 min, followed by 35 cycles of amplification with an annealing temperature of 55°C, and extension temperature of 72°C.
PCRs were set up in an area separate from DNA extractions, and within a PCR clean cabinet (CleanSpot Workstation, Coy Laboratory Products, Grass Lake, MI) equipped with a germicidal UV lamp. Other precautions to prevent carryover contamination of amplified DNA included different sets of pipettes dedicated for DNA extraction, PCR setup, and post-amplification activities, the use of aerosol barrier filter pipette tips, and soaking pipettes used for handling DNA samples in 10% bleach solution after setup of each PCR. Each PCR test included negative control samples with nuclease free TE buffer as template. As a further measure to prevent DNA artifact contamination of PCR testing, no positive control samples were used. PCR products were electrophoresed in 2% agarose gels, which were stained with ethidium bromide, and visualized and recorded with a digital gel documentation unit.
DNA sequencing. PCR products from PCR1 and PCR2 positive samples were purified using the Wizard® SV Gel and PCR Clean-Up System (Promega, Madison, WI). DNA templates were sequenced [44] using both the forward and reverse primers used in the nested PCRs. Investigator-derived sequences were compared with those obtained by searching the GenBank database (National Center for Biotechnology Information) using the Basic Local Alignment Search Tool (BLAST) [45], and aligned using Clustal X [46]. Of note, DNA sequencing was not conducted during Phase 1; it was only available in Phase 2.
Nucleotide sequences. In order to confirm the identity of B. burgdorferi s.l. in the Dundas area, we obtained and sequenced borrelial amplicons from two I. scapularis females (14-5A183, 14-5A184) that were collected in Phase 2. DNA sequences for amplicons of flaB gene from PCR1 (base position 313 to 506) for both ticks, as well as the sequence for PCR2 (base position 532 to 737) for tick 14-5A184, were deposited in the GenBank database under the accession numbers KX011445, KT764114, and KT764115.
Results
Based on PCR amplification, 12 (41%) of 29 I. scapularis adults, which were collected in the Dundas area, were positive for B. burgdorferi s.l. (Figure 1). After DNA sequencing two B. burgdorferi s.l. amplicons, which were obtained from two I. scapularis females (14-5A183, 14-5A184), we determined that they both belonged to B. burgdorferi s.s., which is pathogenic to humans and certain domestic animals.
Multiple parasitisms were experienced by 4 hosts (2 cats, 1 dog, 1 human). One particular cat had 3 separate infestations of I. scapularis. During flagging, two I. scapularis adults were collected in the spring, while three I. scapularis adults were collected in the fall.
Map of Dundas study area showing sites where Ixodes scapularis ticks were collected.
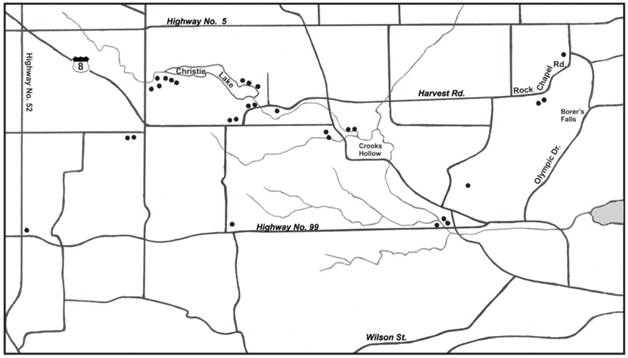
Additionally, we collected 3 other ticks species in the Dundas area: Amblyomma americanum (lone star tick), D. variabilis (American dog tick), and Ixodes cookei (groundhog tick) from dogs, cats, and humans. Although we did not test these ticks for B. burgdorferi s.l., they are all known to carry multiple tick-borne pathogens, which can cause associated pathologies in humans. While flagging, we observed that D. variabilis and I. scapularis are sympatric in the Dundas area.
Discussion
Our study documents a 41% infection prevalence for B. burgdorferi s.l. in I. scapularis adults collected in the Dundas area, Ontario. Small mammals and migratory songbirds play a supportive role in the infection prevalence of B. burgdorferi s.l. Some of these infected ticks are likely introduced by migratory songbirds during northbound spring migration. Importantly, these B. burgdorferi s.l.-infected I. scapularis ticks pose a public health risk to outdoors people and companion animals.
Factors affecting B. burgdorferi prevalence. Recent studies show prevalence rates of B. burgdorferi s.l. in I. scapularis adults collected in the upper Midwest and the northeastern United States ranging from 27-47% [47, 48]. Our results correlate with the prevalence rate of B. burgdorferi s.l. in I. scapularis adults in northeastern North America. Interestingly, it is analogous to the 35% infection prevalence in I. scapularis nymphs collected from migratory songbirds in eastern and central Canada [30]. When these engorged nymphs molt to adults, they retain the same level of B. burgdorferi s.l. infection due to transstadial transmission of spirochetes in I. scapularis. Since no nymphs were collected in our study, we suggest that I. scapularis females are the most likely life stage to bite humans, and present the greatest risk for B. burgdorferi s.l. infection in this bioregion.
Songbirds introduce I. scapularis ticks. Based on earlier bird-tick studies, migratory songbirds transport I. scapularis immatures into southwestern Ontario during spring migration [13, 14, 28-30]. After feeding to repletion, engorged ticks detach from ground-frequenting songbirds at stopovers, and descend into the cool, moist leaf litter. Within 5-8 weeks, these ticks molt to the next life stage, and conduct host-seeking activities. In the Dundas area, I. scapularis adults start questing for hosts in October and, if they are unsuccessful in parasitizing a suitable host, will overwinter and quest for a host in the spring. Because transstadial transmission of B. burgdorferi s.l. is apparent in I. scapularis, adults have an equivalent infection prevalence to that of replete nymphs. Since 35% of songbird-transported I. scapularis nymphs are infected with B. burgdorferi s.l. [30], and 41% of the I. scapularis adults in our study were infected, we suggest that migratory songbirds have a supportive role in supplying I. scapularis immatures to the Dundas area.
In addition, larval and nymphal A. americanum ticks are transported into the Dundas area by migratory songbirds during cross-border spring migration [13]. After these bird-transported ticks have fed to repletion, they detach from their hosts and, likewise, descend into the leaf litter to molt to the next development life stage in 6-8 weeks. By August, unfed lone star ticks are ready to conduct host-seeking activities, and parasitize people and domestic animals, such as dogs, cats, and people.
Long-distance migrants can transport bird-feeding ticks hundreds of kilometres during northward spring migration from as far south as the southern United States, the Caribbean, and Central and South America, and disperse them widely across southern Canada [13, 14, 28-30, 49-54]. Not only do songbirds provide an influx of I. scapularis, they contribute to the establishment of blacklegged tick populations [50, 55], and support the enzootic maintenance of B. burgdorferi s.l. in these populations [11, 12]. Since songbirds widely disperse Lyme disease vector ticks, people do not have to visit an endemic area to contract Lyme disease.
High deer count influence I. scapularis numbers. The high prevalence of deer can greatly influence high numbers of I. scapularis in tick habitats. During a deer count in the Dundas area in 2009, wildlife researchers documented 0.35 deer/ha, which is 4 times the number considered ecologically desirable [56] and, in 2013, they reported 0.3 deer/ha, again, triple the ideal number. In 2009, the highest concentration in urban areas was 1.54 deer/ha. Because deer are hosts of male and female I. scapularis, these cervids facilitate and promote the development and maintenance of I. scapularis populations. In addition, these high deer counts signify the potential to promote all 4 motile life stages of I. scapularis. Such an overpopulation of white-tailed deer exacerbates the abundance of I. scapularis in the Dundas area.
In this study, the highest concentration of I. scapularis occurred in parkland areas, namely Christie Lake Conservation Area, Crooks Hollow, and Borer's Falls (Figure 1). These no-hunt areas provide sanctuary for white-tailed deer, especially during the fall hunting season. These woodland parks have a variety of acorn-bearing oak trees, especially red oak, which are a nutritious source of food for deer. Not only do acorns act as an energy reserve for deer, they act as a nutritious source of food for small rodents (i.e., white-footed mice, deer mice, eastern chipmunks) [57]. A bumper crop of acorns sparks a mouse boom the following year and helps to support and maintain I. scapularis [58]. Mast production provides one of the key components to bring I. scapularis and vertebrate hosts together to promote enzootic Lyme disease transmission. In essence, parkland areas in the Dundas area have natural biotic and climatic attributes that support I. scapularis and reservoir-competent hosts.
When there is a bumper acorn crop, white-tailed deer will move from maple-dominated areas to oak-dominated areas [59]. Acorns are rich in protein and fats, and are a favorite energy source for deer and mice. The acorn-laden canopy area, at the base of the oak trees, becomes a focal hub for deer and mice. With the abundant food supply, mice increase in numbers and, in the following year, act as preferential hosts for questing I. scapularis larvae. Stafford [59] revealed that I. scapularis larvae move up to 2 m from the egg laying site, and Carroll [60] found I. scapularis larvae at a height of 2 m on the trunks of oaks. These findings indicate that gravid female ticks often drop close to the trunks of oaks [59, 60]. Although it would be next to impossible to see or track the drop of replete I. scapularis females, questing larvae are commonly found on well-drained soils either at field-forest ecotones (woods edge) where deer pasture and bed-down or in wooded areas, especially at the base of oak trees [61]. Based on odoriferous vapors (i.e., ammonia from small mammal droppings, composting oak leaves), replete females lay eggs in this nut-producing area that provides an ideal microhabitat [61, 62]. With the combination of deer and other mammalian hosts, this community of hosts maintains the enzootic cycle of Lyme disease spirochetes.
Potential for I. scapularis establishment. Although I. scapularis nymphs are touted as being the vectors of B. burgdorferi s.l., our data revealed that I. scapularis females may be the primary source of spirochetal infection in the Dundas area. We recorded four occurrences where more than one I. scapularis parasitized the same host. In one case, three I. scapularis ticks were individually removed from a cat on two separate occasions; two ticks were collected on one day and one of these ticks was positive for B. burgdorferi s.l. As well, two I. scapularis females were removed from a dog on the same day. Additionally, two I. scapularis females were removed from a human on the same day; one of these ticks was positive for B. burgdorferi s.l. Collectively, these co-infestations of I. scapularis females provide supportive evidence that I. scapularis may be established in the Dundas valley.
Underreporting of B. burgdorferi-positive I. scapularis. This study provides new information regarding Lyme disease vector ticks in the Dundas area. Over a 5-yr period (2009-2013), the local health unit (Hamilton Public Health Services) reported no occurrence of locally-acquired, B. burgdorferi s.l.-infected blacklegged ticks in their service area (1117 km2) [62, 63]. In comparison, we documented 12 B. burgdorferi s.l.-positive I. scapularis during a 2-yr period in the Dundas sample area (56 km2), which is 20 times smaller in size. This sharp contrast reveals a significant difference in the actual prevalence of B. burgdorferi s.l.-infected I. scapularis in the Hamilton-Wentworth area. In essence, our study shows that dogs and cats, which roam outdoors, are better sentinels in representing the occurrence of I. scapularis. Underreporting of I. scapularis can easily occur because people are not expecting ticks in the early spring and late fall when they have bimodal questing activity. Furthermore, I. scapularis anesthetize the skin when they bite, and people are unlikely to be aware of a feeding tick. Moreover, I. scapularis larvae and nymphs are very tiny, and can be easily overlooked as they take a blood meal. Whenever a surveillance team is trying to determine whether I. scapularis ticks are present in an area, dogs and cats can provide a reliable gauge of tick activity.
Tick and Lyme disease warning signs. Deer management strategies need to be implemented to limit the number of deer in the Dundas area. During the hunting season, deer congregate in no-hunt parklands and, naturally, increase the mating potential of I. scapularis males and females and, thus, produce a new generation of ticks. Since B. burgdorferi s.l.-infected I. scapularis were collected at several locations in the Dundas area, signs need to be erected in no-hunt parkland areas to warn the public of questing ticks infected with B. burgdorferi s.l. According to the Ontario Health Protection and Promotion Act, the Medical Officer of Health is legally responsible to erect warning signs where any area contains a reportable disease or communicable disease that poses a public health hazard.
Clinical and Zoonotic Implications. Our findings reveal that, in the Dundas area, I. scapularis harbor B. burgdorferi s.s. which is pathogenic to humans. Anyone bitten by an I. scapularis tick should have it tested for B. burgdorferi s.l. and other tick-transmitted pathogens; currently, this can be done via the local health unit. When a B. burgdorferi s.l.-infected I. scapularis takes a blood meal, it typically transmits spirochetes that migrate outward from the bite site. Sometimes, a homogenous or bull's-eye rash will develop. The outward expansion of the rash signifies that B. burgdorferi s.l. spirochetes are spreading in the body, and are evading the immune system. In vivo, B. burgdorferi s.l. has diverse forms (i.e., spirochetes, blebs, granules, round bodies, atypical forms) [64] and, collectively, form biofilms [65]. Spirochetes are able to evade the immune system, and will attach to, invade, and kill human B and T lymphocytes [66]. As spirochetes disseminate in the body, a myriad of clinical manifestations commonly unfold, including fatigue, headaches, low-grade fever, stiff neck/back, disturbed sleep, memory and sensory loss. Migratory joint ache and pain and tingling are classic symptoms. This febrile illness can affect several body systems: cardiac, endocrine, gastrointestinal, musculoskeletal, neurological, otological, and ophthalmic [67]. Neurological deficits are common in both children and adults. As spirochetes attack nerves and ligamentous tissue, they produce neurotoxins that cause an inflammatory response in the surrounding tissue [68, 69]. As spirochetemia progresses in the central nervous system, demyelination and apoptosis occurs. In addition, as this tick-borne zoonosis advances, it induces inflammatory cytokines, such as interleukin 1, interleukin 6, and TNF-alpha, and produces mitochondrial dysfunction, oxidative stress, physical and hormonal abnormalities and, ultimately, profound fatigue [69, 70]. Left untreated or inadequately treated, B. burgdorferi s.l. will sequester in deep-seated tissues, such as bone [71], brain [72-74], eye [75], muscle [76], collagenous tissues (ligaments, tendons) [77, 78], glial and neuronal cells [79, 80], and fibroblast/scar tissue [81]. In some cases, this persistent infection is fatal [72, 82]. Since B. burgdorferi s.l. can be persistent, Lyme disease spirochetes have been detected in and cultured from tissues and body fluids after conventional, short-term antibiotic treatment in animals, including humans [83-87].
Conclusions
We document that 41% of I. scapularis adults in the Dundas area were infected with Lyme disease spirochetes, and confirm that B. burgdorferi s.s., which pathogenic to humans, is present in the Hamilton-Wentworth region. Advocacy and surveillance programs conducted by the local public health unit fail to reflect and authenticate the presence of B. burgdorferi s.l. in locally-acquired I. scapularis, and give the false impression that there is no Lyme disease in the Hamilton-Wentworth area. The number of white-tailed deer needs to be significantly reduced to minimize the public health risk of Lyme disease. Signs need to be erected to warn hikers that B. burgdorferi s.l.-infected I. scapularis are present in the parkland areas. This high risk area presents a health-care challenge for people bitten by I. scapularis. Veterinarians, clinicians, medical professionals, public health officials, and the populace must be aware that Lyme disease can be contracted in the Dundas area and surrounding Hamilton-Wentworth region.
Acknowledgements
We thank veterinarians and groomers who collected ticks from companion animals. We are grateful to Elizabeth A. Alves for technical assistance. We are indebted to John Ward for computer graphics.
Competing Interests
The authors confirm that they have no conflicts of interest that could bias any aspect of the present paper. They have no financial interests in, relationships with, or have received no funding from any clinical laboratories or test kit companies or reagent manufactures mentioned in the paper.
References
1. Burgdorfer W, Barbour AG, Hayes SF. et al. Lyme disease―a tick-borne spirochetosis? Science. 1982;216:1317-1319 doi:10.1126/science.7043737
2. Baranton G, Postic D, Saint Girons I. et al. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov. and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992;42:378-383 doi: 10.1099/00207713-42-3-378
3. Marconi RT, Liveris D, Schwartz I. Identification of novel insertion elements, restriction fragment length polymorphism patterns, and discontinuous 23S rRNA in Lyme disease spirochetes: phylogenetic analysis of rRNA genes and their intergenic spacers in Borrelia japonica sp. nov. and genomic group (Borrelia andesonii sp. nov.) isolates. J Clin Microbiol. 1995;33:2427-2434
4. Postic D, Ras NM, Lane RS. et al. Expanded diversity among Californian Borrelia isolates and description of Borrelia bissettii sp. nov. (formerly Borrelia group DN127). J Clin Microbiol. 1998;36:3497-3504
5. Smith RP, Muzaffar SB, Lavers J. et al. Borrelia garinii in seabird ticks (Ixodes uriae), Atlantic coast, North America. Emerg Infect Dis. 2006;12:1909-1912 doi:10.3201/eid1212.060448
6. Rudenko N, Golovchenko M, Grubhoffer L. et al. Borrelia carolinensis sp. nov, a new (14th) member of the Borrelia burgdorferi sensu lato complex from southeastern region of the United States. J Clin Microbiol. 2009;47:134-141 doi: 10.1099/ijs.0.021436-0
7. Rudenko N, Golovchenko M, Lin T. et al. Delineation of a new species of the Borrelia burgdorferi sensu lato complex, Borrelia americana sp. nov. J Clin Microbiol. 2009;47:3875-3880 doi: 10.1128/JCM.01050-098
8. Margos G, Hojgaard A, Lane RS. et al. Multilocus sequence analysis of Borrelia bissettii strains from North America reveals a new Borrelia species, Borrelia kurtenbachii. Ticks Tick Borne Dis. 2010;1:151-158 doi: 10.1016/j.ttbdis.2010.09.002
9. Pritt BS, Mead PS, Hoang Johnson DK. et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016
10. Clark KL, Leydet B, Hartman S. Lyme borreliosis in human patients in Florida and Georgia, USA. Int J Med Sci. 2013;10:915-931 doi: 10.7150/ijms.6273
11. Rudenko N, Glovchenko M, Vancova M. et al. Isolation of live Borrelia burgdorferi sensu lato spirochetes from patients with undefined disorders and symptoms not typical for Lyme borreliosis. Clin Microbiol Infect. 2016:22 doi:10.1016/j.cmi.2015.11.009
12. Golovchenko M, Vancová M, Clark K. et al. A divergent spirochete strain isolated from a resident of the southeastern United States was identified by multilocus sequence typing as Borrelia bissettii. Parasit Vectors. 2016;9:68
13. Scott JD, Lee MK, Fernando K. et al. Detection of Lyme disease spirochete, Borrelia burgdorferi sensu lato, including three novel genotypes in ticks (Acari: Ixodidae) collected from songbirds (Passeriformes) across Canada. J Vect Ecol. 2010;35:124-139 doi:10.1111/j.1948.7134.2010.00068.x
14. Scott JD, Anderson JF, Durden LA. Widespread dispersal of Borrelia burgdorferi-infected ticks collected from songbirds across Canada. J Parasitol. 2012;98:49-59
15. Ogden NH, Lindsay RL, Hanincová K. et al. Role of migratory birds in introduction and range expansion of I. scapularis ticks and of Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Appl Environ Microbiol. 2008; 74: 1780-1790. doi:10.1128/AEM.01982-07. Erratum: 2008. Appl Environ Microbiol. 2008;74:3919
16. Hersh MH, Tibbetts M, Strauss M. Reservoir competence of wildlife host species for Babesia microti. Emerg Infect Dis. 2012;18:1951-1957
17. Hamer SA, Hickling GJ, Keith R. et al. Association of passerine birds, rabbits, and ticks with Borrelia miyamotoi and Borrelia andersonii in Michigan, U.S.A. Parasit Vectors. 2012;5:231. doi:10.1186/1756-3305-231
18. Hersh MH, Ostfeld RS, McHenry DJ. et al. Co-infection of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts. PLoS ONE. 2014;9:e99348. doi:10.1371/journal.pone.0099348
19. Anderson JF, Magnarelli LA, Burgdorfer W. et al. Spirochetes in Ixodes dammini and mammals from Connecticut. Am J Trop Med Hyg. 1983;32:818-824
20. McLean RG, Ubico SR, Cooksey. Experimental infection of the eastern chipmunk (Tamias striatus) with the Lyme disease spirochete (Borrelia burgdorferi). J Wildl Dis. 1993;29:527-532
21. Anderson JF, Johnson RC, Magnarelli LA. et al. Identification of endemic foci of Lyme disease: isolation of Borrelia burgdorferi from feral rodents and ticks (Dermacentor variabilis). J Clin Microbiol. 1985;22:36-38
22. Loken KL, Wu C-C, Johnson RC. et al. Isolation of the Lyme disease spirochete from mammals in Minnesota. Exper Biol Med. 1985;3:300-302 doi:10.3181/00379727-179-42100
23. Peavey CA, Lane RS. Transmission of Borrelia burgdorferi by Ixodes pacificus nymphs and reservoir competence of deer mice (Peromyscus maniculatus) infected by tick-bite. J Parasitol. 1995;81:175-178
24. Anderson JF, Magnarelli LA. Avian and mammalian hosts for spirochete-infected ticks and insects in a Lyme disease focus in Connecticut. Yale J Biol Med. 1984;57:627-641
25. Telford SR III, Mather TN, Adler GH. et al. Short-tailed shrews as reservoirs of the agent of Lyme disease and human babesiosis. J Parasitol. 1990;76:681-683
26. Markowski D, Ginsberg HS, Hyland KE. et al. Reservoir competence of the meadow vole (Rodentia: Cricetidae) for the Lyme disease spirochete Borrelia burgdorferi. J Med Entomol. 1998;35:804-808
27. Telford SR, III, Mather TN, Moore SI. et al. Incompetence of deer as reservoirs of the Lyme disease spirochete. Am J Trop Med Hyg. 1988;39:105-109
28. Scott JD, Fernando K, Banerjee SN. et al. Birds disperse ixodid (Acari: Ixodidae) and Borrelia burgdorferi-infected ticks in Canada. J Med Entomol. 2001;38:498-500 doi:10.1603/0022-2585-38.4.493
29. Morshed MG, Scott JD, Fernando K. et al. Migratory songbirds disperse ticks across Canada, and first isolation of the Lyme disease spirochete, Borrelia burgdorferi, from the avian tick, Ixodes auritulus. J Parasitol. 2005;91:780-790 doi:10.1645/GE-3437.1
30. Scott JD, Durden LA. New records of the Lyme disease bacterium in ticks collected from songbirds in central and eastern Canada. Int J Acarol. 2015;41:241-249
31. Jones CJ, Kitron UD. Populations of Ixodes scapularis (Acari: Ixodidae) are modulated by drought at a Lyme disease focus in Illinois. J Med Entomol. 2000;37:408-415
32. Mannelli A, Kitron U, Jones CJ. et al. Influence of season and habitat on Ixodes scapularis infestation on white-footed mice in northwestern Illinois. J Parasitol. 1994:80 1038-1043
33. Banerjee SN, Banerjee M, Fernando K. et al. Presence of spirochete causing Lyme disease, Borrelia burgdorferi, in the blacklegged tick, Ixodes scapularis, in southern Ontario. Can Med Assoc J. 2000;162:1567-1569
34. Morshed MG, Scott JD, Fernando K. et al. Distribution and characterization of Borrelia burgdorferi isolates from Ixodes scapularis and presence in mammalian hosts in Ontario, Canada. J Med Entomol. 2006;43:762-773 doi:10.1093/clinids/19.5.891
35. Anderson JF. Mammalian and avian reservoirs for Borrelia burgdorferi. Ann New York Acad Sci. 1988;539:180-191 doi:10.1111/j.1749-6632.1988.tb31852.x
36. Keirans JE, Clifford CM. The genus Ixodes in the United States: a scanning electron microscope study and key to the adults. J Med Entomol. 1978(Supplement 2):149
37. Keirans JE, Hutcheson HJ, Durden LA. et al. Ixodes (Ixodes) scapularis (Acari: Ixodidae): Redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J Med Entomol. 1996;33:297-318
38. Persing DH, Telford SR III, Spielman A. et al. Detection of Borrelia burgdorferi infection in Ixodes dammini ticks with the polymerase chain reaction. J Clin Microbiol. 1990;28:566-572
39. Persing DH, Telford SR, Rys PN. et al. Detection of Borrelia burgdorferi DNA in museum specimens of Ixodes dammini ticks. Science. 1990;249:1420-1423 doi:10.1126/science2402635
40. Scott JD, Anderson JF, Durden LA. First detection of Lyme disease spirochete Borrelia burgdorferi in ticks collected from a raptor in Canada. J Vet Sci Med Diagn. 2013;2:4. doi:10.4172/2325-9590.1000123
41. Rijpkema SG, Molkenboer MJ, Schouls LM. et al. Simultaneous detection and genotyping of three genomic groups of Borrelia burgdorferi sensu lato in Dutch Ixodes ricinus ticks by characterization of the amplified intergenic spacer region between 5S and 23S rRNA genes. J Clin Microbiol. 1995;33:3091-3095
42. Clark KL, Leydet B, Threlkeld C. Geographical and genospecies distribution of Borrelia burgdorferi sensu lato DNA detected in humans in the USA. J Med Microbiol. 2014;63:674-684 doi: 10.1099/jmm.0.073122-0
43. Bunikis J, Garpmo U Tsao J. et al. Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiol. 2004;150:1741-1755
44. McCombie WR, Heiner C, Kelley JM. et al. Rapid and reliable fluorescent cycle sequencing of double stranded templates. DNA Sequence. 1992;2:289-296 doi:10.3109/10425179209030961
45. Altschul SF, Gish W, Miller W. et al. Basic local alignment search tool. J Mol Biol. 1990;215:403-410
46. Thompson J D, Gibson TJ, Plewniak F. et al. The ClustalX-Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876-4882
47. Turtinen LW, Kruger AN, Hacker MM. Prevalence of Borrelia burgdorferi in adult female ticks (Ixodes scapularis), Wisconsin 2010-2013. J VectEcol. 2015;40:195-197 doi: 10.1111/jvec.12152
48. Hutchinson ML, Strohecker MD, Simmons TW. et al. Prevalence rates of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in host-seeking Ixodes scapularis (Acari: Ixodidae) from Pennsylvania. J Med Entomol. 2015;52:693-698
49. Scott JD. Birds widely disperse pathogen-infected ticks. In: (ed.) Mahala G. Seabirds and Songbirds: habitat preferences conservation and migratory behavior. Birds-evolution, behavior and ecology. Nova Science Publishers, Inc, New York. 2015:1-22
50. Scott JD, Scott CM, Anderson JF. The establishment of a blacklegged tick population by migratory songbirds in Ontario, Canada. J Vet Sci Med. 2014;2:5
51. Scott JD, Durden LA. Songbird-transported tick Ixodes minor (Ixodida: Ixodidae) discovered in Canada. Can Entomol. 2015;147:46-50 doi:10.4039/tce.2014.34
52. Scott JD, Durden LA. Amblyomma dissimile tick (Acari: Ixodidae) parasitizes bird captured in Canada. Syst Appl Acarol. 2015;20:854-860
53. Scott JD, Durden LA. First record of Amblyomma rotundatum tick (Acari: Ixodidae) parasitizing a bird collected in Canada. Syst Appl Acarol. 2015;20:155-161
54. Scott JD, Durden LA, Anderson JF. Infection prevalence of Borrelia burgdorferi in ticks collected from songbirds in far-western Canada. Open J An Sci. 2015;5:232-241 http://dx.doi.org/10.4236/ojas.2015.53027
55. Anderson JF, Magnarelli LA, Stafford KC, III. Bird-feeding ticks transstadially transmit Borrelia burgdorferi that infect Syrian hamsters. J Wildl Dis. 1990;26:1-10 doi:10.7589/0090-3558-26.1.1
56. Yagi A, Timmerman A. Ancaster wintering deer survey 2009―with management recommendations. Ministry of Natural Resources. 2010:1-34
57. McCracken KE, Witham JW, Hunter ML. Relationships between seed fall of three tree species and Peromyscus leucopus and Clethrionomys gapperi during 10 years in an oak-pine forest. J Mammal. 1999;80:1266-1296
58. Ostfeld RS, Canham CD, Oggenfuss K. et al. Climate, deer, rodents, and acorns as determinants of variation in Lyme-disease risk. PLoS Biol. 2006;4:e145. doi:10.1371/journal.pbio.0040145
59. Stafford KC III. Ovipositon and larval dispersal of Ixodes dammini (Acari: Ixodidae). J Med Entomol. 1992; 29: 129-132. http://www.researchgate.net/publication/21602250_ 55. [Carroll JF. Occurrence of larval Ixodes scapularis (Acari: Ixodidae) on tree trunks. J Med Entomol. 1996;33:971-975 ]
60. Carroll JF. Occurrence of larval Ixodes scapularis (Acari: Ixodidae) on tree trunks. J Med Entomol. 1996;33:971-975
61. Carey AB, Krinsky WL, Main AJ. Ixodes dammini (Acari: Ixodidae) and associated ixodid ticks in south-central Connecticut, U.S.A. J Med Entomol. 1980;17:89-99
62. City of Hamilton, Ticks Database. Ontario Ministry of Health and Long-Term Care, integrated Public Health Information System database (iPHIS). Accessed, 18 September. 2015 https://www.publichealthontario.ca/en/ServicesAndTools/SurveillanceServices/Pages/Vector-Borne-Disease-Surveillance-Reports.aspx
63. O'Hara C. Be vigilant when checking for ticks. Flamborough Review. 7 August. 2014 http://www.flamboroughreview.com/news-story/5402269-public-health-waterdown-vet-clinic-offer-tick-identification-services/
64. Meriläinen L, Herranen A, Schwarzbach A. et al. Morphological and biochemical features of Borrelia burgdorferi pleomorphic forms. Microbiol. 2015;161:516-527 doi:10.1099/mic.0.000027
65. Sapi E, Bastian SL, Mpoy CM. et al. Characterization of biofilm formation by Borrelia burgdorferi. PLoS ONE. 2012;7:e48277. doi:10.1371/journal.pone.0048277
66. Dorward DW, Fischer ER, Brooks DM. Invasion and cytopathic killing of human lymphocytes by spirochetes causing Lyme disease. Clin Infect Dis. 1997;25(Suppl 1):52-58
67. Cameron DJ, Johnson LB, Maloney EL. Evidence assessments and guideline recommendations in Lyme disease: the clinical management of known tick bites, erythema migrans rashes and persistent disease. Expert Rev Anti-Infect Ther. 2014;12:1103-1135 doi:10.1586/14787210.2014.940900
68. Zijkowska JM, Hermanowska-Szpakowicz T. New aspects of the pathogenesis of Lyme disease. Przeglad Epidemiologiczny. 2002;56(Suppl 1):57-67
69. Horowitz RI. Why can't I get better? Solving the mystery of Lyme & chronic disease; 1st ed. St Martin's Press, New York. 2013
70. Peacock BN, Gherezghiher TB, Hilario JD. et al. New insights into Lyme disease. Redox Biol. 2015;5:66-70
71. Oksi J, Mertsola J, Reunanen M. et al. Subacute multiple-site osteomyelitis cause by Borrelia burgdorferi. Clin Infect Dis. 1994;19:891-896 doi:10.1093/clinids/19.5.891
72. Oksi J, Kalimo H, Marttila RJ. et al. Inflammatory brain changes in Lyme borreliosis: a report on three patients and review of literature. Brain. 1996;119:2143-2154 doi:10.1093/brain/119.6.2143
73. MacDonald AB. Alzheimer's neuroborreliosis with trans-synaptic spread of infection and neurofibrillary tangles derived from intraneuronal spirochetes. Med Hypotheses. 2007;68:822-825 doi:10.1016/j.mehy.2006.08.043
74. Miklossy J. Alzheimer's disease―a neurospirochetosis. Analysis of the evidence following Koch's and Hill's criteria. J Neuroinflammation. 2011;8:90
75. Preac-Mursic V, Pfister HW, Spiegel H. et al. First isolation of Borrelia burgdorferi from an iris biopsy. J Clin Neuroophthalmology. 1993;13:155-161
76. Frey M, Jaulhac B, Piemont Y. et al. Detection of Borrelia burgdorferi DNA in muscle of patients with chronic myalgia related to Lyme disease. Am J Med. 1998;104:591-594 doi:10.1016/S0002-9343(98)00112-0
77. Häupl T, Hahn G, Rittig M. et al. Persistence of Borrelia burgdorferi in ligamentous tissue from a patient with chronic Lyme borreliosis. Arthritis Rheum. 1993;36:1621-1626 doi:10.1002/(ISSN)1529-0131
78. Müller KE. Damage of collagen and elastic fibres by Borrelia burgdorferi - known and new clinical histopathogical aspects. Open Neurol J. 2012;6(Suppl 1-M11):179-186 doi:10.2174/1874205X01206010179
79. Ramesh G, Borda JT, Dufour J. et al. Interaction of the Lyme disease spirochete Borrelia burgdorferi with brain parenchyma elicits inflammatory mediators from glial cells as well as glial and neuronal apoptosis. Am J Pathol. 2008;173:1415-1427 doi:10.2353/ajpath.2008.080483
80. Ramesh G, Santana-Gould L, Inglis FM. et al. The Lyme disease spirochete Borrelia burgdorferi induces inflammation and apoptosis in cells from dorsal root ganglia. J Neuroinflammation. 2013;10:88
81. Klempner MS, Noring R, Rogers RA. Invasion of human skin fibroblasts by the Lyme disease spirochete, Borrelia burgdorferi. J Infect Dis. 1993;167:1074-1081 doi:10.1093/infdis/167.5.1074
82. Liegner K, Duray P, Agricola M. et al. Lyme disease and the clinical spectrum of antibiotic responsive chronic meningoencephalomyelitides. J Spir Tick-borne Dis. 1997;4:61-73
83. Phillips SE, Mattman LH, Hulínská D. et al. A proposal for the reliable culture of Borrelia burgdorferi from patients with chronic Lyme disease, even from those previously aggressively treated. Infection. 1998;26:364-367
84. Miklossy J, Kasas S, Zurn AD. et al. Persisting atypical and cystic forms of Borrelia burgdorferi and local inflammation in Lyme neuroborreliosis. J Neuroinflammation. 2008;5:40. doi:10.1186/1742-2094-5-40
85. Hodzic E, Imai D, Feng S. Resurgence of persisting non-cultivable Borrelia burgdorferi following antibiotic treatment in mice. PLoS ONE. 2014;9:e86907. doi:10.1371/journal.pone.0086907
86. Stricker RB, Johnson L. Persistent Borrelia burgdorferi infection after treatment with antibiotics and anti-tumor necrosis factor-α. J Infect Dis. 2008;197:1352-1353
87. Embers ME, Barthold SW, Borda JT. et al. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS ONE. 2012;7:e29914. doi:10.1371/journal.pone.0029914
Author contact
![]() Corresponding author: John D. Scott, Research Division, Lyme Ontario, 365 St. David Street South, Fergus, Ontario, Canada N1M 2L7. Tel: 519-843-3646. E-mail: jkscottcom.
Corresponding author: John D. Scott, Research Division, Lyme Ontario, 365 St. David Street South, Fergus, Ontario, Canada N1M 2L7. Tel: 519-843-3646. E-mail: jkscottcom.

 Global reach, higher impact
Global reach, higher impact