3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2016; 13(3):220-224. doi:10.7150/ijms.13853 This issue Cite
Research Paper
Pathological Analysis of Cell Differentiation in Cholesterol Granulomas Experimentally Induced in Mice
1. Department of Hard Tissue Research, Matsumoto Dental University Graduate School of Oral Medicine, Shiojiri, Japan
2. Department of Life Science, Faculty of Science, Okayama University of Science, Okayama, Japan
3. Department of Oral Health Promotion, Matsumoto Dental University Graduate School of Oral Medicine, Shiojiri, Japan
4. Department of Oral Pathology, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan
Received 2015-9-14; Accepted 2016-2-5; Published 2016-2-19
Abstract
In this study, cholesterin was implanted in the subcutaneous tissue in mice to induce the formation of cholesterol granuloma. Histological examination was carried out to determine the type and source of cells. The tissue surrounding the embedded cholesterin was examined histologically within the period of 6 months. Cell differentiation in cholesterol granulomas was investigated using ddY mice and GFP bone marrow transplanted mice. Cholesterin was embedded in mice subcutaneously and histopathological examination was carried out in a period of 6 months. Results showed that at 2 weeks, cholesterin was replaced partly by granulation tissues. The majority of cells in the granulation tissues were macrophages and foreign body giant cells and the center consists of small amount of fibroblasts, collagen fibers and capillaries. At 3 months, more granulation tissue was observed compared to 2 weeks. Similar cells were observed, however, there were more fibroblasts, collagen bundles and capillaries present compared to 2 weeks. At 6 months, the cholesterin was mostly substituted by fibrous tissues consisting mainly of fibroblasts and collagen fibers with some macrophages and foreign body giant cells. Specifically, the outer part of the tissue consists of fibroblasts, collagen bundles and capillaries and the inner portion is filled with collagen bundles. Immunohistochemistry revealed that macrophages and foreign body giant cells were positive to GFP and CD68 although the fibroblasts and capillaries in the outer portion of cholesterol granulomas were GFP negative. Some spindle shape fibroblasts were also GFP positive. Immunofluorescent double staining revealed that cells lining the blood vessels were both positive to GFP and CD31 indicating that those were endothelial cells and were actually derived from the transplanted bone marrow cells. The results suggest that macrophages, foreign body giant cells as well as fibroblasts and capillary endothelial cells are bone marrow derived mesenchymal cells.
Keywords: Cholesterol granuloma, Green fluorescent protein (GFP), Cell differentiation, Immunohistochemistry
Introduction
Granulation tissue formation is a part of secondary wound healing and tissue repair in vivo. Many studies have shown the formation of foreign body granulomas in vivo. For instance, charcoal (1), silicone (2, 3) and other foreign matter (4-6) induced the formation of foreign body granulomas. Cholesterol granuloma is a term given to foreign body granuloma present in various lesions caused by cholesterin (3, 7). The most common cells found in cholesterol granulomas are macrophages and foreign body giant cells (FBGC). However, the source of these cells as well as other cellular components such as fibroblasts and capillary endothelial cells has not been elucidated. Cholesterin is generally produced in the body. Therefore, cholesterol granuloma was experimentally induced and the tissue reaction was studied histopathologically with emphasis on cell migration and differentiation.
Materials and Methods
Laboratory animals
The animals used in this experiment were 7-week ddY mice purchased from Japan SLC, Inc. (Hamamatsu, Japan), 7-week old, female, C57BL/6 recipient (CAG-EGFP), and 7-week old, female C57BL/6Tg (CAG-EGFP) GFP transgenic mice (Shimizu Laboratory Supplies, Kyoto, Japan). Bone marrow derived cells (BMDCs) were harvested by sacrificing GFP transgenic mice under ether anesthesia. Briefly, femurs were excised followed by soft tissue removal and harvest of BMDCs. The cells were washed with RPMI 1640 and then the medium was replaced with HBBS. After X-ray irradiation of recipient mice with 10 Gray, the cells (1×107) were imjected in 7-week old female cognates from the tail vein. The engrafted mice were used 4 weeks after transplantation (8, 9). The mice were housed in metal cage lined with beddings (Paper clean: Peparlet Co., Ltd, Hamamatsu, Japan) in an air-conditioned controlled environment at 24±1 oC. During breeding, the animals had free access to water and food (Picolab Rodent Diet 20: SLC Japan Inc.).
The mice were placed under Isoflurane inhalation anesthesia and 10 mg of cholesterin was implanted subcutaneously by making an incision on the back of the mouse and then closed with sutures. After 2 weeks, 3 weeks, 3 months and 6 months, the embedded tissue was removed en bloc and fixed in 10 % neutral buffered formalin. The examined animals are shown in Table 1.
Periods and Number of Experimental Animals (ddY and GFP)
| ddY | Experimental | Total | |||||
|---|---|---|---|---|---|---|---|
| Periods | 2 weeks | 3 weeks | 3 months | 6 months | |||
| Number | 3 | 3 | 3 | 3 | 12 | ||
| GFP | Experimental | ||||||
| Periods | 2 weeks | 3 weeks | 3 months | 6 months | |||
| Number | 1 | 1 | 1 | 1 | 4 |
Histopathological examination
The fixed samples were dehydrated in series of alcohol, embedded in paraffin and sectioned into 5μm. Then after, specimens were stained with hematoxylin and eosin and examined under a microscope.
Immunohistochemistry
After deparaffinization, specimens were immersed in citric acid buffer solution with pH 6.0 (LSI Medience Co., Tokyo, Japan) and placed in autoclave at 121oC for 10 min. Blocking was carried out using serum-free protein block (Dako Japan Co., Ltd, Tokyo, Japan) at room temperature for 30 min. Slides were incubated with primary antibodies; anti-CD68 for detection of macrophages (1:100; ab125047, Abcam, Cambridge, UK), anti-CD31 for detection of endothelial cells (1:100; ab28364, Abcam, Cambridge, UK), anti-GFP ChIP Grade (1:2000; ab290, Abcam, Cambridge, UK) at 4oC overnight. This was followed by secondary antibody using anti-rabbit Ig for 30 min. Finally the slides were washed with PBS and developed with DAB.
Immunofluorescent double staining
After deparaffinization, slides were pre-treated in citrate buffer in microwave for 1 min and blocked with 10 % donkey normal serum for 30 min at room temperature. Primary antibodies anti-GFP, anti-CD31, anti-CD68 were each diluted at 1:100 with Can Get Signal (Toyobo Co., Ltd, Osaka, Japan) and allowed to react overnight at 4 oC.
For secondary antibody, Alexa Fluor 568 Labeled Donkey ant-goat Ig G antibodies (Life Technologies, Palo Alto, CA, USA) and Alexa Fluor 488 Labeled Donkey anti-rat IgG Antibodies (Life Technologies, Palo Alto, CA, USA) and Can Get Signal (Toyobo Co., Ltd, Osaka, Japan) were diluted at 1:200 and allowed to react for 60 min at room temperature. Then after, the nuclei were stained with 1 mg/ml of DAPI for 3 min. Slides were then washed with TBS and mounted using Fluorescent Mounting Medium (Dako Japan Co., Ltd, Tokyo, Japan).
The study conformed to the experimental guidelines in animal experiment of Matsumoto Dental University approved by the Animal Laboratory Examination Committees of the University (Approval number # 233-13).
Results
Histopathological evaluation (Fig. 1)
There was no difference between ddY mice and GFP bone marrow transplanted mice histopathologically. At 2 weeks, the growing mass of granulation tissues surrounded the irregularly shaped cholesterin spaces (Fig. 1-a). The cholesterin spaces were actually the areas previously occupied by cholesterin crystals dissolved during tissue preparation. The growth of granulation tissue replaced some of the mass of cholesterin crystals. The granulation tissue primarily consists of macrophages and FBGCs. Within the granulation tissues, large and small blood vessels were observed. FBGCs have large vacuolated cytoplasm. Few fibroblasts and collagen bundles were observed in between macrophages and FBGCs. Some capillaries were also seen especially in the outer portion of granulation tissues where cell proliferation was evident. However, cell proliferation was not observed in the center of granulation tissue where large cholesterin spaces were still present. Moreover, nuclear staining of macrophages and FBGCs in contact with large vessels near residual cholesterin was extremely poor. The outer layer of granulation tissue has relatively thick fibrous tissue formation. There was proliferation of fibroblasts producing collagen fibers as well as capillaries.
At 3 weeks, the granulation tissues became bigger replacing more of the embedded cholesterin crystals. This resulted to a smaller irregular space in the center (Fig. 1-b). Cell morphology was not apparent due to the loss of nucleus in macrophages and FBGCs. The partition between the cell proliferation and remaining cholesterin spaces was still apparent. Many small and large blood vessels were spotted at the periphery of the granulation tissue. Moreover, capillaries were seen in between fibroblasts and collagen fiber in relatively thin peripheral layer of fibrous tissue.
Histopathological views of ddY mice. The growing mass of granulation tissues proliferate the irregularly shaped cholesterin crystals (a). The granulation tissues become bigger replacing more of the embedded cholesterin crystals (b). The granulation tissue had grown and replaced the small dividing mass of cholesterin crystals (c). The outer layer compose of fibroblasts, collagen bundles and capillaries but mostly collagen bundles (d). a: 2 weeks; b: 3 weeks; c: 3 months, d: 6months; scale bars: a, b=100μm, c, d=50μm.
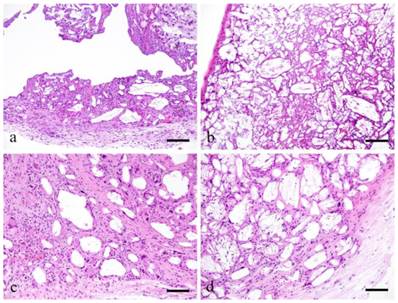
At 3 months, the granulation tissue replaced clumps of embedded cholesterin crystals. Granulation tissue had grown and replaced the small dividing mass of cholesterin crystals. Macrophages and FBGCs surround the cholesterin spaces. Hyperplasia of fibroblasts, collagen bundles and capillaries separating the cholesterin spaces were noted (Fig. 1-c). The outer layer was also filled with fibroblasts, collagen bundles and capillaries.
At 6 months, granulation tissue has replaced the entire cholesterin crystals mainly composed of fibroblasts and collagen bundles. The periphery was replaced with finely organized fibrous tissues. Fibroblasts and collagen bundles also penetrated the center of the granulation tissues. The outer layer was composed of fibroblasts, collagen bundles and capillaries but mostly collagen bundles (Fig. 1-d).
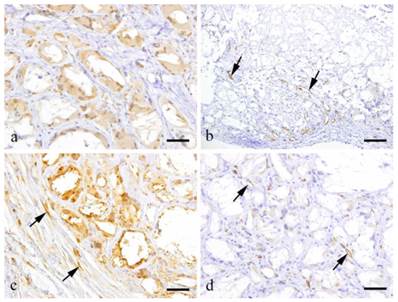
Immunohistochemistry (Fig. 2)
At 2 weeks, the granulation tissue was filled with GFP-positive cells. Macrophages and FBGCs in the granulation tissue were positive to CD68 (Fig. 2-a). However, some of the fibroblasts in the granulation tissues were GFP negative. Relatively thick fibrous tissue was present in the outer layer of the granulation tissues. The granulation tissue was formed within the spaces left by cholesterin crystals. Most CD31 positive cells were localized at the periphery of the granulation tissues but also few cells in the center were positive to CD31 (Fig. 2-b, arrows).
At 6 months, a large amount of fibrous tissue separated the macrophages and FBGCs (Fig. 2-c). Fibroblasts and capillaries distributed in the collagen bundles were mostly GFP negative. The fibroblasts, collagen fibers and capillaries in the outer layer did not express GFP. However, fibroblasts having spindle shaped nuclei were GFP positive (Fig. 2-c, arrows). Capillaries that were present in the granulation tissue were positive to CD31 in the central portion of then granulation tissues (Fig. 2-d, arrows).
Immunohistochemical results showing a: CD68-positive-macrophages and FBGCs (3 weeks, scale bar=50μm); b: CD31-positive capillary endothelial cells (arrows) (2 weeks, scale bar=100 μm); c: GFP-positive fibroblasts (arrows) (6 months, scale bar=50μm); d: CD31-positive capillary endothelial cells (arrows) (6 months, scale bar=50μm).
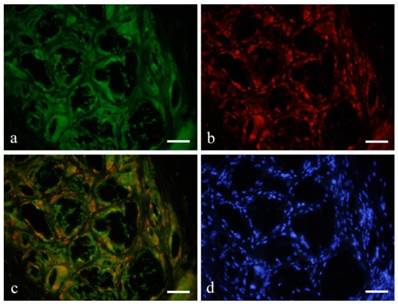
Immunofluorescent double staining (Figs. 3, 4)
Immunofluorescent double staining revealed that macrophages and FBGCs were both GFP (green) and CD68 (red) positive (red) (Fig. 3-a, b). Double staining confirmed that both green and red fluorescence was observed on relatively large and irregular macrophages and FBGCs (Fig. 3-c). However, the spindle shape cells were only positive to GFP.
Immunofluorescent double staining with GFP (green) and CD31 (red) showed GFP expression in the cytoplasm of endothelial cells in distinct blood vessels (Fig. 4-a), and also in the cross-linked-macrophages and FBGCs. Double staining with CD31 and DAPI (blue) showed red fluorescence on vascular endothelial cells indicating CD31 expression. Nuclei were stained blue for DAPI (Fig. 4-b).
Double staining revealed that macrophages and FBGCs were both GFP (green: a) and CD68 (red: b) positive (orange: c). a: GFP; CD68; c: GFP+CD68; d: DAPI; 6months, scale bars=50μm.
Double staining with GFP (green: a) and CD31 (red: b with DAPI) showed GFP expression in the cytoplasm of endothelial cells in distinct blood vessels (a, arrow). Double staining with CD31 and DAPI (blue) showed red fluorescence on vascular endothelial cells indicating CD31 expression. Nuclei were stained blue for DAPI (d). a: GFP; b: CD31+DAPI; c: GFP+CD31; d: DAPI; 6months, scale bars=50μm.
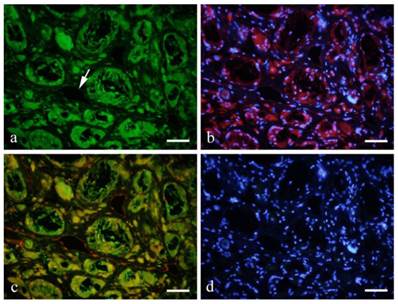
A number of GFP-positive cells were spindle or polygonal in shape and some were lining the lumen of the blood vessels. CD31 expression was observed as red fluorescence in endothelial cells lining the blood vessel. Double staining showed that vascular endothelial cells were both positive to GFP and CD31 (Fig. 4-c).
Discussion
Macrophages and FBGCs mainly gather in reaction to a large external matter producing the so-called foreign body granuloma. Clinical reports showed that charcoal (1) and silicone (2, 3) are some of the foreign materials that can induce foreign body granuloma formation. Previous studies showed the formation of granulation tissue induced by dermal filler (5), subcutaneous injection of acetate in the treatment of prostate cancer (10) and bone wax (11). Moreover, Nakahori et al reported a case of lung cancer from silicone granuloma (3). Kim JS et al (7) and Lsaacson B (12) described the formation of granulation tissue induced by cholesterin. Cholesterol granuloma is a granulation tissue growth on cholesterin crystals. Macrophages mainly proliferate in the granuloma and then coalesce to form multinucleated giant cells having similar characteristics with FBGCs.
In this study, cholesterin was implanted in the subcutaneous tissue in mice to induce the formation of cholesterol granuloma. Histological examination was carried out to determine the type and source of cells. Biological evaluation of several materials have been made. This process conformed to ISO standards (13). The tissue surrounding the embedded cholesterin was examined histologically within the period of 6 months.
The examination model is effective in the examination for determine the origin of the component cells due to the histopathological results. The bone marrow transplantation mouse model was used since it was assumed that the cells would originate from the bone marrow (14-16). Clinical application on the pluripotency of BMDCs had already begun. In fact cell transplantation in the treatment of myocardial and cerebral infarctions has been started with the objective that the cells would differentiate into what they are expected. In the oral region, trials concerning bone regeneration using cultured mesenchymal stem cells from human bone marrow have been conducted. This was for the purpose of regeneration of the jaw with thickness and strength required for oral implant placement. Bone marrow mesenchymal cells of patients were cultured, harvested and then grafted for the purpose that the cells would differentiate into osteoblasts for bone formation. However, studies on regenerative medicine using BMDCs have only been reported in the reconstruction of a very limited area in bone tissue. It is expected that other areas would be explored in the future. Therefore, studies on the migration and cell differentiation of BMDCs for oral tissue regeneration would contribute to the advancement in regenerative medicine. Using GFP transgenic mice, cells that constitute the tissue would express GFP if the transplanted BMDCs differentiate into any cell. Hence, tracking of differentiated cells is possible.
Tsujigiwa et al.'s study on pluripotent BMDCs revealed the migration and differentiation of mesenchymal cells into odontogenic and periodontal tissue cells (17). Transplanted GFP-positive BMDCs migrated to the oral region in a short period of time as early as a month after implantation. The same research group showed GFP positive cells in mice periodontal tissue, dendritic cells, Langerhans cells, osteoclasts etc. However, there is no clear data of differentiation into vascular endothelial cells (18, 19).
The present results showed that some GFP-positive cells that also expressed CD31 are either BMDCs or vascular endothelial cells and their differentiation was evident. This experiment showed that macrophages and FBGCs were derived from transplanted bone marrow mesenchymal cells shown by their GFP expression. Remarkably, GFP positive cells also expressed CD31 indicating that they are endothelial cells. Several studies mentioned that bone marrow derived cells migrate to the site of injury and can differentiate into vascular endothelial cells however this has not been clearly shown (14-16, 20, 21). Another interesting idea is the chronic inflammation and angiogenesis mostly observed in tumors. The continuous angiogenesis seems not only due to local endothelial cell proliferation but also from the movement of mesenchymal cells from the bone marrow (22-24). The formation of granulation tissue over a long period of time was due to the presence of cholesterin. Co-expression of GFP and CD31 was observed in vascular endothelial cells. Fibroblasts and endothelial cells in the granulation tissue expressed GFP suggesting that the cells were derived from the bone marrow. This was further supported by double immunofluorescent staining showing co-expression of GFP and CD31 in vascular endothelial cells seen in distinct blood vessels. In summary, the study presented that macrophages and FBGCs as well as some of the fibroblasts and capillary endothelial cells were bone marrow derived mesenchymal cells.
Acknowledgements
This study was supported in part by Grant-in-Aid for Scientific Research (C) #26463031 from the Japan Society for the Promotion of Science.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Choi JW, Moon W-J, Choi N, Roh HG, Kim MY, Kim NR, Moon SG, Chung HW, Lim SD, Yang J-H. Charcoal-induced granuloma that mimicked a nodal metastasis on ultrasonography and fdg-pet /ct after neck dissection. Korean J Radiol. 2015;16:196-200
2. Seeger JB, Ahmed GA, Basad E, Rickert, Ishaque BA. Gluteal silicone injections and total hip arthroplasty: a case report. J Med Case Rep. 2014;8:140
3. Nakahori R, Takahashi R, Akashi M, Tsutsui K, Harada S, Matsubayashi RN, Nakagawa S, Momosaki S, Akagi Y. Breast carcinoma originating from a silicone granuloma: A case report. World J Surg Oncol. 2015;13:72
4. Luo J, Mao Y, Cai S, Shen X, Chen S, Xie L. Post-nephrectomy foreign-body granuloma in the retroperitoneum mimicking lymph node metastasis of renal cell cancer. Oncol Targets Ther. 2014;7:2137-2141
5. Lee JM, Kim YJ. Foreign body granulomas after the use of dermal fillers: Pathophysiology, clinical appearance, histologic features, treatment. Arch Plast Surg. 2015;42:232-239
6. Mak S, Lui YH, Li KKW. Synthetic fiber granuloma of the conjunctive. Hong Kong Med J. 2015;21:77-79
7. Kong JS, Kim M-S, Lee K-Y. A case of a cholesterol granuloma occluding the external auditory canal in a 12-year-old-girl. Korean J Audiol. 2014;18:89-92
8. Wall Da, Hamberg SD, Reynoldy DS, Burakoff SJ, Abbas AK, Ferrara JL. Immunodeficiency in graft-versus-host disease. I. Mechanism of immune suppression. J Immunol. 1988;140:2970-2976
9. Zijlmans JM, Visser JW, Laterveer L, Kleiverda K, Heemskerk DP, Kluin PM, Willemze R, Fibbe WE. The early phase of engraftment after murine blood cell transplantation is mediated by hematopoietic stem cells. Proc Natl Acad Sci USA. 1998;95:725-729
10. Kawai M, Ikoma N, Yamada A, Ota T, Manabe Y, Kato M, Mabuchi T, Ozawa A, Higure T, Terachi T. A case of foreign body granuloma induced by subcutaneous injection ofleuprorelin acetate -Clinical analysis for 335 cases in our hospital-. Tokai J Exp Clin Med. 2014;39:106-110
11. Allen-Wilson N, Beatty R, Sharpe J. Severe bone wax foreign-body reaction causing peroneal tendon destruction. J Am Podiatr Med Assoc 105: 74-79. 2015
12. Lsaacson B. Cholesterol granuloma and other petrous apex lesions. Otolaryngol Clin North Am. 2015;48:361-373
13. International Organization for Standardization (ISO). Biological Evaluation of Medical Devices -Part 1: Evaluation and testing, ISO 10993-1: 2003 (E). Geneva, Switzerland.
14. Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221-228
15. Crosby JR, Kaminski WE, Schatteman G, Martin PJ, Raines EW, Seifert RA, Bowen-Pope DF. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formstion. Circ Res. 2000;87:728-730
16. Zampetaki A, Kirton JP, Xu Q. Vasclar repair by endothelial progenitor cells. Cardiovasc Res. 2008;78:413-421
17. Tsujigiwa H, Katase N, Sathi GA, Buer RR, Hirata Y, Kubota M, Nakano K, Kawakami T, Nagatsuka H. Transplanted bone marrow derived cells differentiated to tooth, bone and connective tissues in mice. J Hard Tissue Biol. 2011;20:147-152
18. Tomida M, Tsujigiwa H, Nakano K, Muraoka R, Nakamura T, Okafuji N, Nagatsuka H, Kawakami T. Promotion of transplanted bone marrow-derived cell migration into the periodontal tissues due to orthodontic mechanical stress. Int J Med Sci. 2013;10:1321-1326
19. Muraoka R, Tsujigiwa H, Nakano K, Katase N, Tamamura R, Tomida M, Okafuji N, Nagatsuka H, Kawakami T. Transplanted Bone Marrow-derived Cell Migration into Periodontal Tissues and Cell Differentiation. J Hard Tissue Biol. 2011;20:301-306
20. Schier R, El-Zein R, Cortes A, Liu M, Collins M, Rafat N, Teschendorf P, Wu H-K, Heymach J, Mehran R, Riedel B. Endothelial progenitor cell mobilization by preoperative with postoperative outcome. Br J Anaesth. 2014;113:652-660
21. Crosby JR, Kaminski WE, Schatteman G, Martin PJ, Raines EW, Seifert RA, Bowen-Pope DF. Endothelial cell of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res. 2000;87:728-730
22. Melero-Martin JM, Dudley AC. Vasclar Stem Cells and Tumor Angiogenesis. Stem Cells. 2011;29:163-168
23. Carneiro GD, Godoy JA, Werneck CC, Vicente CP. Differentiation of C57/BL6 mice bone marrow mononuclear cells into early endothelial progenitors cells in different culture conditions. Cell Biol Int. 2015 doi: 10.1002/cbin. 10487
24. Yan Z, Zeng L, Li Z, Zhang H, Chen W, Jia L, Chen C, Cheng H, Cao J, Xu K. Bone marrow-derived endothelial progenitor cells promote hematopoietic reconstitution after hematopoietic stem cell transplantation. Transplant Proc. 2013;45:427-433
Author contact
![]() Corresponding author: kawakamimdu.ac.jp
Corresponding author: kawakamimdu.ac.jp

 Global reach, higher impact
Global reach, higher impact