3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2016; 13(1):68-76. doi:10.7150/ijms.13016 This issue Cite
Research Paper
Effects of Acidification and Alkalinization on the Lipid Emulsion-Mediated Reversal of Toxic Dose Levobupivacaine-Induced Vasodilation in the Isolated Rat Aorta
1. Department of Anesthesiology and Pain Medicine, Gyeongsang National University School of Medicine and Gyeongsang National University Hospital, Jinju-si, 52727, Republic of Korea;
2. Department of Anesthesiology and Pain Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea;
3. Department of Anesthesiology and Pain Medicine, Gyeongsang National University School of Medicine, Jinju-si, 52727, Republic of Korea;
4. Department of Anesthesiology and Pain Medicine, Gyeongsang National University Hospital, Jinju-si, 52727, Republic of Korea;
5. Department of Oral and Maxillofacial Surgery, Gyeongsang National University Hospital, Jinju-si, 52727, Republic of Korea;
6. Department of Information Statistics and RINS, Gyeongsang National University, Jinju, 52828, Korea;
7. Department of Anatomy and Cell Biology and Mitochondria Hub Regulation Center, Dong-A University College of Medicine, Busan, South Korea;
8. Institute of Health Sciences, Gyeongsang National University, Jinju, Republic of Korea.
*These two authors contributed equally to this study as co-first authors.
Received 2015-6-23; Accepted 2016-1-6; Published 2016-1-25
Abstract
The goal of this in vitro study was to examine the effects of pre-acidification and pre-akalinization on the lipid emulsion-mediated reversal of toxic dose levobupivacaine-induced vasodilation in isolated rat aorta. Isolated aortic rings with and without the nitric oxide synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME) were exposed to four types of Krebs solution (pH 7.0, 7.2, 7.4, and 7.6), followed by the addition of 60 mM potassium chloride. When the toxic dose of levobupivacaine (3 × 10-4 M) produced a stable and sustained vasodilation in the isolated aortic rings that were precontracted with 60 mM potassium chloride, increasing lipid emulsion concentrations (SMOFlipid®: 0.24, 0.48, 0.95 and 1.39%) were added to generate concentration-response curves. The effects of mild pre-acidification alone and mild pre-acidification in combination with a lipid emulsion on endothelial nitric oxide synthase (eNOS) phosphorylation in human umbilical vein endothelial cells were investigated by Western blotting. Mild pre-acidification caused by the pH 7.2 Krebs solution enhanced the lipid emulsion-mediated reversal of levobupivacaine-induced vasodilation in isolated endothelium-intact aortic rings, whereas mild pre-acidification caused by the pH 7.2 Krebs solution did not significantly alter the lipid emulsion-mediated reversal of the levobupivacaine-induced vasodilation in isolated endothelium-denuded aortic rings or endothelium-intact aortic rings with L-NAME. A lipid emulsion attenuated the increased eNOS phosphorylation induced by the pH 7.2 Krebs solution. Taken together, these results suggest that mild pre-acidification enhances the lipid emulsion-mediated reversal of toxic dose levobupivacaine-induced vasodilation in the endothelium-intact aorta via the inhibition of nitric oxide.
Keywords: Lipid emulsion, Pre-acidification, Levobupivacaine, Nitric oxide, Aorta.
Introduction
Lipid emulsions have been used to treat cardiovascular collapse due to systemic toxicity induced by local anesthetics, including bupivacaine, levobupivacaine, ropivacaine and mepivacaine, as well as other drugs [1,2]. Lipid emulsions, including SMOFlipid®, Intralipid® and Lipofundin® MCT/LCT, reverse severe vasodilation caused by the inhibition of voltage-operated calcium channels induced by toxic doses of levobupivacaine and bupivacaine [3-5]. Cardiovascular collapse due to local anesthetic systemic toxicity is followed by respiratory and metabolic acidosis. Acidosis decreases the serum protein binding of bupivacaine and leads to an increased portion of free bupivacaine, which may contribute to the exaggeration of local anesthetic systemic toxicity [6,7]. On the other hand, acidosis increases the ionized form (cationic form) of local anesthetics and decreases the non-ionized form (base form) of local anesthetics, which leads to a lower amount of the non-ionized form of local anesthetic that is available for penetration of the nerve membrane and suggests a lower potency of the local anesthesia [8, 9]. However, hypercarbia caused by local anesthetic systemic toxicity causes the diffusion of carbon dioxide in the axoplasm of the neuron through the nerve membrane, enhancing the conversion of the local anesthetic into the ionized form, which inhibits sodium channels and exaggerates the local anesthetic toxicity [7,9]. In addition, acidosis produces nitric oxide-induced relaxation, whereas a lipid emulsion attenuates this relaxation [10,11]. However, the effect of acidosis on the lipid emulsion-mediated sequestration of bupivacaine is controversial. For example, Ruan et al. reported that acidosis has no significant effect on lipid emulsion-mediated sequestration of bupivacaine in human serum, whereas Mazoit et al. reported that acidosis decreases the affinity of lipid emulsions for bupivacaine in buffer solutions [12, 13]. Therefore, the goal of this in vitro study was to examine the effect of pre-acidification and pre-alkalinization on the lipid emulsion (SMOFlipid®)-mediated reversal of toxic dose levobupivacaine-induced vasodilation and to investigate the associated cellular mechanism in isolated rat aorta. Given the findings of prior reports, we tested the hypothesis that pre-acidification would evoke the lipid emulsion-mediated reversal of toxic dose levobupivacaine-induced vasodilation, partly via the inhibition of pre-acidification-induced nitric oxide release [10, 11].
Materials and Methods
All experimental procedures and protocols were approved by the Institutional Animal Care and Use Committee of Gyeongsang National University and performed in accordance with the Guide for the Care and Use of Laboratory Animals.
Preparation of aortic rings for tension measurements
The aortic rings were prepared for tension measurements as described previously [5, 14, 15]. Male Sprague-Dawley rats weighing 250-300 g were anesthetized with intramuscular injections of Zoletil 50 (15 mg/kg, Virbac Laboratories, Carros, France). The descending thoracic aorta was dissected free. The surrounding connective tissue and fat were removed under a microscope, and the vessel was bathed in Krebs solution with the following composition (mM): 118 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 2.4 CaCl2, 25 NaHCO3, and 11 glucose. The aorta was then cut into 2.5-mm rings, which were suspended on Grass isometric transducers (FT-03, Grass Instrument, Quincy, MA, USA) under 3.0-g resting tension in a 10-mL Krebs bath at 37°C and continuously aerated with 95% O2 and 5% CO2 to maintain pH values within 7.35-7.45. The rings were equilibrated at a resting tension of 3.0 g for 120 min, during which time the bathing solution was changed every 40 min. Care was taken not to damage the endothelium. In some aortic rings, the endothelium was intentionally removed by inserting a 25-gauge needle tip into the lumen of the ring and gently rolling the ring for a few seconds. Once the phenylephrine (10-7 M)-induced contraction had stabilized, acetylcholine (10-5 M) was added to assess the endothelial integrity. The endothelial integrity was confirmed by the observation of >70% relaxation induced by acetylcholine. The rings were then rinsed with fresh Krebs solution to restore the resting tension. Each ring was used for each concentration-response curve induced by SMOFlipid®. In experimental protocols involving only endothelium-denuded aortae, the Krebs solution contained the nitric oxide synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME: 10-4 M) to prevent the production of endogenous nitric oxide from any residual endothelium, as levobupivacaine induces nitric oxide release in isolated rat aortae with intact endothelium [16].
Experimental protocol
The pH 7.2 Krebs solution was made by both increasing NaCl to 131 mM and decreasing NaHCO3 to 13 mM, as described in a previous study [17]. The pH 7.6 Krebs solution was made by both decreasing NaCl to 101 mM and increasing NaHCO3 to 43 mM [17]. The pH 7.0 Krebs solution was made by both increasing NaCl to 141 mM and decreasing NaHCO3 to 3 mM. The pH was carefully monitored during the experiment using a GEM Premier-3000 (Instrumentation Laboratory Co., Lexington, Massachusetts, USA). Isolated endothelium-intact aortic rings pretreated with or without L-NAME (10-4 M) and endothelium-denuded aortic rings were exposed to four Krebs solutions (pH 7.0, 7.2, 7.4 and 7.6) for 20 min before the addition of 60 mM KCl. After 60 mM KCl had produced a sustained and stable contraction in the endothelium-intact and endothelium-denuded rat aortic rings, a toxic dose (3 × 10-4 M) of levobupivacaine was added. Then, incremental concentrations of SMOFlipid® (0.24, 0.48, 0.95 and 1.39%) were added to generate lipid emulsion concentration-response curves. The higher concentration of SMOFlipid® was added to the organ bath after a previous lower concentration had produced a sustained and stable response.
Cell culture
Human umbilical vein endothelial cells (HUVECs; EA.hy926 cells, American Type Culture Collection, Manassas, VA, USA) were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mmol/L L-glutamine, 100 IU/mL penicillin, and 10 μg/mL streptomycin as described previously [18]. Cells were cultured in 100-mm dishes and grown in a humidified 5% CO2 incubator. HUVECs were plated at a density of 107 cells per 100-mm dish. Cells were used between passage number 6 and passage number 12.
Western blot analysis
Western blot analysis was performed as previously described [18]. Briefly, cells were lysed in PRO-PREP protein extract solution to isolate total cell extracts. After extracts were centrifuged at 13,000 rpm for 20 min at 4°C, protein concentrations were determined by the Bradford method. Samples containing 30 µg of protein were subjected to 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. The separated proteins were then transferred to polyvinylidene difluoride membranes using the SD Semi-dry Transfer Cell® system (Bio-Rad, Hercules, CA, USA). These membranes were incubated with primary antibodies (anti-endothelial nitric oxide synthase [eNOS] and anti-phospho-eNOS [at Ser1177] antibodies; Cell Signaling Technology, Beverly, MA, USA) at a 1:500 dilution (4 μg/mL) in 5% skim milk in TBST overnight at 4°C, and bound antibody was detected by horseradish peroxidase-conjugated anti-rabbit IgG. The membranes were washed and then developed using the Luminol Reagent system (Animal Genetics, Suwon, Korea). Beta-actin was used as the loading control.
Drugs
Acetylcholine, phenylephrine and L-NAME were obtained from Sigma-Aldrich (St. Louis, Missouri, USA). Levobupivacaine was obtained from Abbott Korea (Seoul, Korea). SMOFlipid® was obtained from Fresenius Kabi Korea (Seoul, Korea). DMEM, FBS, penicillin, streptomycin, and glutamine were supplied by Gibco BRL (Rockville, MD, USA).
Data analysis
The cumulative responses to levobupivacaine and lipid emulsion are expressed as percentages of the maximal contraction induced by 60 mM KCl in isolated rat aorta, and the data are presented as the mean ± SD. Areas under the lipid emulsion dose-response curves calculated from vasodilation (baseline) induced by 3 × 10-4 M levobupivacaine were used to evaluate the overall extent of the lipid emulsion-mediated reversal of vasodilation induced by 3 × 10-4 M levobupivacaine [19]. The areas under the lipid emulsion dose-response curves were calculated using GraphPad (GraphPad prism version 5.0 for Windows, GraphPad Software, San Diego, CA, USA). Values related to areas under curves or levobupivacaine-induced vasodilations are presented as medians and interquartile ranges. The effects of pre-acidification and pre-alkalinization on the areas under the lipid emulsion dose-response curves of levobupivacaine-induced vasodilation and on the levobupivacaine (3 × 10-4 M)-induced vasodilation were analyzed using the Kruskal-Wallis test followed by Dunn's test or the Mann-Whitney U test. The effects of pre-acidification alone and pre-acidification in combination with lipid emulsion on eNOS phosphorylation in HUVECs were assessed using one-way analysis of variance followed by Bonferroni's post-test. The effect of pre-acidification on 60 mM KCl-induced contraction was analyzed using the Mann-Whitney U test. When effect size and previous similar studies are not available, the resource equation method can be used as a crude method to calculate sample size [20, 21]. According to the resource equation method, the sample size ranged from 6 to 11 in each group for comparisons between two groups [20, 21]. Thus, we used 6, 7, 8 and 9 samples in each group to compare two groups in the current study. Band intensities, including beta-actin, from Western blot analyses were assessed by scanning densitometry using Image Master VSD (Pharmacia Biotech, San Francisco, CA, USA). Quantitative analysis (phosphorylated eNOS/ total eNOS) of eNOS phosphorylation was normalized to beta-actin. P values less than 0.05 were considered significant.
Results
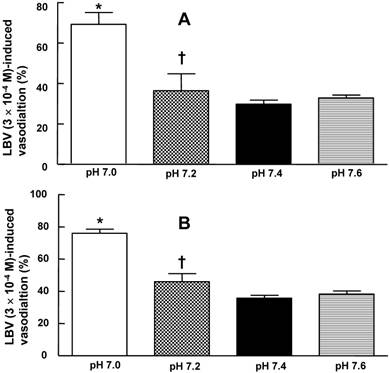
In the isolated rat aortae, pretreatment with pH 7.0 or 7.6 Krebs solution had no effect on the resting tension (data not shown). In isolated rat aortae precontracted with 60 mM KCl, levobupivacaine (3 × 10-4 M)-induced vasodilation was not significantly different between the pH 7.4 Krebs solution and the pH 7.6 Krebs solution (Fig. 1A and B). However, in isolated rat aortae precontracted with 60 mM KCl, levobupivacaine (3 × 10-4 M)-induced vasodilation was attenuated in the pH 7.0 and pH 7.2 Krebs solutions compared with the pH 7.4 Krebs solution (P < 0.01; Fig. 1A and B). In isolated L-NAME-pretreated endothelium-intact rat aortae precontracted with 60 mM KCl, levobupivacaine (3 × 10-4 M)-induced vasodilation was attenuated in the pH 7.2 Krebs solution compared with the pH 7.4 Krebs solution (P < 0.01). Mild pre-alkalinization (pH 7.6) had no effect on the 60 mM KCl-induced contraction (data not shown). Severe pre-acidification (pH 7.0) did not significantly alter the 60 mM KCl-induced contraction (Fig. S1). Precontraction induced by 60 mM KCl was not significantly different between the pH 7.4 Krebs solution and the Krebs solutions at different pH values (data not shown).
Effect of pre-acidification and pre-alkalinization on levobupivacaine (LBV)-induced vasodilation in isolated endothelium-intact (A) and endothelium-denuded (B) rat aortae precontracted with 60 mM KCl. Data are presented as the median and interquartile range. LBV (3 × 10-4 M)-induced vasodilation (%) is expressed as the percentage of 60 mM KCl-induced contraction (endothelium-intact: pH 7.0, N = 6; pH 7.2, N = 8; pH 7.4, N = 21; and pH 7.6, N = 7; endothelium-denuded: pH 7.0, N = 6; pH 7.2, N = 8; pH 7.4, N = 20; and pH 7.6, N = 6). N indicates the number of rats from which descending thoracic aortae were obtained. *P < 0.001 and †P < 0.01 compared with pH 7.4.
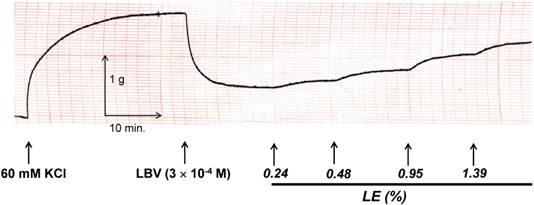
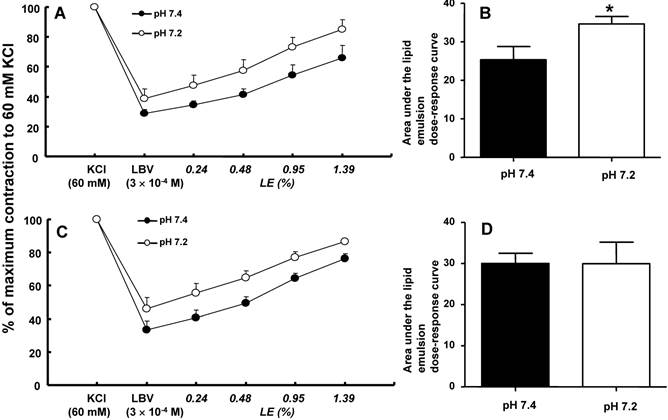
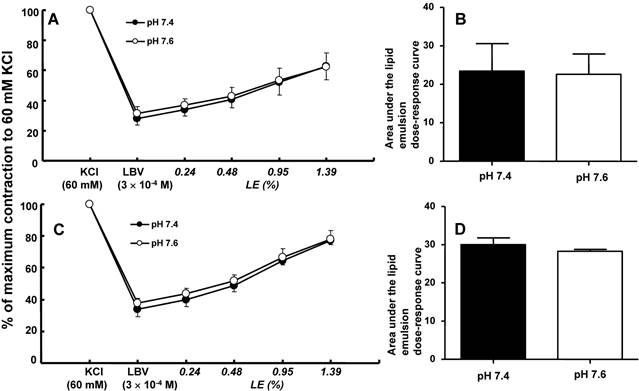
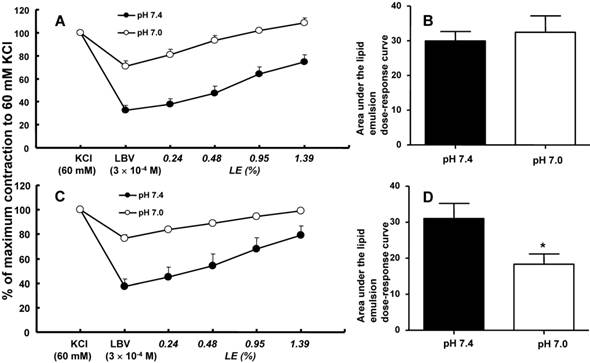
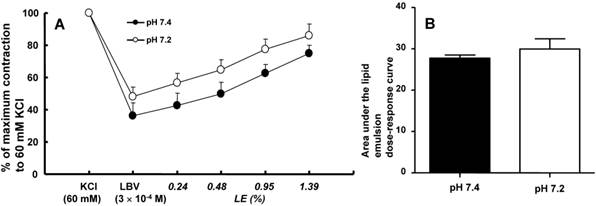
In isolated rat aortae precontracted with 60 mM KCl, levobupivacaine (3 × 10-4 M) produced vasodilation, and a lipid emulsion reversed this levobupivacaine-induced vasodilation (Fig. 2). During the levobupivacaine (3 × 10-4 M)-induced vasodilation of isolated aortae precontracted with 60 mM KCl, the pH 7.2 Krebs solution increased the areas under the lipid emulsion dose-response curves from levobupivacaine (3 × 10-4 M)-induced vasodilation (baseline) in the endothelium-intact aortae (P < 0.001 versus pH 7.4; Fig. 3A and B). However, the pH 7.2 Krebs solution did not significantly alter the areas under the lipid emulsion dose-response curves in the endothelium-denuded aortae (Fig. 3C and D). During the levobupivacaine (3 × 10-4 M)-induced vasodilation of isolated endothelium-intact and endothelium-denuded aortae precontracted with 60 mM KCl, the pH 7.6 Krebs solution did not significantly alter the areas under the lipid emulsion dose-response curves of levobupivacaine (3 × 10-4 M)-induced vasodilation (Fig. 4A, B, C and D). During the levobupivacaine (3 × 10-4 M)-induced vasodilation of isolated aortae precontracted with 60 mM KCl, the pH 7.0 Krebs solution did not significantly alter the areas under the lipid emulsion dose-response curves in the endothelium-intact aortae (Fig. 5A and B), whereas the pH 7.0 Krebs solution decreased the areas under the lipid emulsion dose-response curves of levobupivacaine (3 × 10-4 M)-induced vasodilation in the endothelium-denuded aortae (P < 0.01 compared with pH 7.4; Fig. 5C and D). During the levobupivacaine (3 × 10-4 M)-induced vasodilation of L-NAME pretreated isolated endothelium-intact aortae precontracted with 60 mM KCl, the pH 7.2 Krebs solution did not significantly alter the areas under the lipid emulsion dose-response curves from levobupivacaine (3 × 10-4 M)-induced vasodilation compared with the pH 7.4 Krebs solution (Fig. 6A and B).
Trace showing the change in tension induced by 60 mM KCl, levobupivacaine (LBV) and SMOFlipid® emulsion (LE) in endothelium-intact aortae in Krebs solution at pH 7.4.
A and C: The effects of mild pre-acidification (pH 7.2, N = 8) on the SMOFlipid® emulsion (LE) concentration-response curves during levobupivacaine (LBV, 3 × 10-4 M)-induced vasodilation at a toxic dose in isolated endothelium-intact (A) and endothelium-denuded (C) aortae precontracted with 60 mM KCl. Data are shown as the mean ± SD and are expressed as the percentage of contraction induced by 60 mM KCl. N indicates the number of rats from which descending thoracic aortae were obtained. B and D: The effect of mild pre-acidification (pH 7.2, N = 8) on the areas under the lipid emulsion dose-response curves from LBV (3 × 10-4 M)-induced vasodilation in isolated endothelium-intact (B) and endothelium-denuded (D) aortae precontracted with 60 mM KCl. The areas under the lipid emulsion dose-response curves were calculated from the baseline LBV (3 × 10-4 M)-induced vasodilation. Data are presented as medians and interquartile ranges. *P < 0.001 compared with pH 7.4.
A and C: The effects of mild pre-alkalinization (pH 7.6) on the SMOFlipid® emulsion (LE) concentration-response curves during levobupivacaine (LBV, 3 × 10-4 M)-induced vasodilation at a toxic dose in isolated endothelium-intact (A, N = 7) and endothelium-denuded (C, N = 6) aortae precontracted with 60 mM KCl. Data are shown as the mean ± SD and are expressed as the percentage of contraction induced by 60 mM KCl. N indicates the number of rats from which descending thoracic aortae were obtained. B and D: The effect of mild pre-alkalinization (pH 7.6) on the areas under the lipid emulsion dose-response curves from LBV (3 × 10-4 M)-induced vasodilation in isolated endothelium-intact (B, N = 7) and endothelium-denuded (D, N = 6) aortae precontracted with 60 mM KCl. The areas under the lipid emulsion dose-response curves were calculated from the baseline LBV (3 × 10-4 M)-induced vasodilation. Data are presented as medians and interquartile ranges.
A and C: The effects of severe pre-acidification (pH 7.0, N = 6) on the SMOFlipid® emulsion (LE) concentration-response curves during levobupivacaine (LBV, 3 × 10-4 M)-induced vasodilation at a toxic dose in isolated endothelium-intact (A) and endothelium-denuded (C) aortae precontracted with 60 mM KCl. Data are shown as the mean ± SD and are expressed as the percentage of contraction induced by 60 mM KCl. N indicates the number of rats from which descending thoracic aortae were obtained. B and D: The effect of severe pre-acidification (pH 7.0, N = 6) on the areas under the lipid emulsion dose-response curves from LBV (3 × 10-4 M)-induced vasodilation in isolated endothelium-intact (B) and endothelium-denuded (D) aortae precontracted with 60 mM KCl. The areas under the lipid emulsion dose-response curves were calculated from the baseline LBV (3 × 10-4 M)-induced vasodilation. Data are presented as medians and interquartile ranges. *P < 0.01 versus pH 7.4.
The effects of mild pre-acidification (pH 7.2, N = 9) on the SMOFlipid® emulsion (LE) concentration-response curves (A) and areas under the lipid emulsion dose-response curves (B) during levobupivacaine (LBV, 3 × 10-4 M)-induced vasodilation in Nω-nitro-L-arginine methyl ester (10-4 M)-pretreated endothelium-intact aortae precontracted with 60 mM KCl. N indicates the number of thoracic aortae. A: Data are shown as the mean ± SD and are expressed as the percentage of contraction induced by 60 mM KCl. B: The areas under the lipid emulsion dose-response curves were calculated from the baseline LBV (3 × 10-4 M)-induced vasodilation. Data are presented as medians and interquartile ranges.
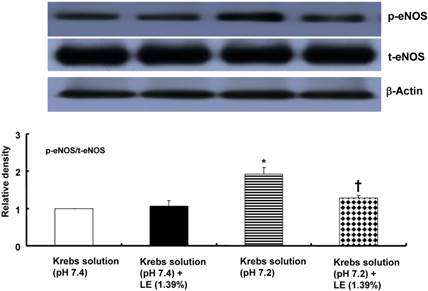
Relative to treatment with the pH 7.4 Krebs solution, treatment with the pH 7.2 Krebs solution increased eNOS phosphorylation at Ser1177 in HUVECs (P < 0.001; Fig. 7). However, combined treatment with the pH 7.2 Krebs solution and a lipid emulsion (1.39%) attenuated eNOS phosphorylation at Ser1177 relative to treatment with the pH 7.2 Krebs solution alone (P < 0.001; Fig. 7). Combined treatment with the pH 7.4 Krebs solution and a lipid emulsion (1.39%) did not significantly alter eNOS phosphorylation at Ser1177 relative to treatment with the pH 7.4 Krebs solution alone (Fig. 7).
Discussion
This study is the first to suggest that mild pre-acidification increases the lipid emulsion-mediated reversal of toxic dose levobupivacaine-induced vasodilation of isolated endothelium-intact rat aortae precontracted with 60 mM KCl. This in vitro study produced the following major findings: 1) mild pre-acidification (at pH 7.2) enhanced the lipid emulsion-mediated reversal of toxic dose (3 × 10-4 M) levobupivacaine-induced vasodilation in endothelium-intact aortae, whereas mild pre-acidification at this pH did not significantly alter the lipid emulsion-mediated reversal of levobupivacaine-induced vasodilation in endothelium-denuded aortae or endothelium-intact aortae with L-NAME; and 2) a lipid emulsion attenuated the mild pre-acidification-induced eNOS phosphorylation in HUVECs at pH 7.2.
Lipid emulsions are widely used to treat intractable cardiovascular collapse induced by toxic doses of local anesthetics and other drugs without specific antidotes [1,2]. One of the proposed underlying mechanisms of lipid emulsion treatment is the lipid sink theory, which states that toxic doses of lipid-soluble local anesthetics can be extracted from tissue using lipid emulsions [2]. Other potential mechanisms include fatty acid oxidation, cardiotonic effect, Akt activation, drug redistribution, and attenuation of local anesthetic-induced sodium channel blockade [2, 22-24]. The toxic dose of levobupivacaine (3 × 10-4 M) in the current study, which exceeds the serum concentration of levobupivacaine (2.55 × 10-5 M) that produces hypotension as a sign of levobupivacaine systemic toxicity in a previous study, caused vasodilation in isolated rat aortae precontracted with 60 mM KCl [25]. Consistent with previous reports, lipid emulsions reversed the type of severe levobupivacaine-induced vasodilation of the isolated rat aortae (Fig. 2) that appears to be involved in cardiovascular collapse induced by toxic doses of local anesthetics [3,4]. Acidosis does not significantly change the tension induced by KCl in rat and adult rabbit aortae [26,27]. Consistent with previous reports, severe pre-acidification (pH 7.0) did not significantly alter 60 mM KCl-induced contraction [26, 27].
The effects of Krebs solution (pH 7.4 or pH 7.2) alone and Krebs solution (pH 7.4 or pH 7.2) in combination with SMOFlipid® emulsion (LE, 1.39%) on the activation of endothelial nitric oxide synthase (eNOS) at Ser1177 in human umbilical vein endothelial cells (HUVECs). HUVECs were treated for 10 min with either Krebs solution (pH 7.4 or pH 7.2) alone or Krebs solution (pH 7.4 or pH 7.2) in combination with SMOFlipid® emulsion (LE, 1.39%). The phosphorylation of eNOS at Ser1177 was investigated as described in the Methods section. Data are presented as the mean ± SD of five independent experiments. *P < 0.001 compared with the pH 7.4 Krebs solution. †P < 0.001 compared with the pH 7.2 Krebs solution alone. t-eNOS: total eNOS; p-eNOS: phosphorylated eNOS.
Reducing the pH of the buffer solution from 7.4 to 7.0 decreases the affinity of the lipid emulsion for bupivacaine and ropivacaine by a factor of 1.68, whereas decreasing the pH of human serum from 7.4 to 6.9 has no effect on the sequestration of bupivacaine by lipid emulsions [12, 13]. In the current study, mild pre-acidification (pH 7.2) caused by Krebs solution enhanced the areas under the lipid emulsion dose-response curves, indicating the enhanced overall extent of lipid emulsion-mediated reversal from levobupivacaine (3 × 10-4 M)-induced vasodilation in endothelium-intact aortae (Fig. 3B). In contrast, mild pre-acidification (pH 7.2) did not significantly alter the overall extent of the lipid emulsion-mediated reversal of levobupivacaine (3 × 10-4 M)-induced vasodilation in endothelium-denuded aortae (Fig. 3D), suggesting that the mild pre-acidification-induced enhancement of the lipid emulsion-mediated reversal appears to be endothelium-dependent. In addition, pretreatment with L-NAME (10-4 M) did not enable the mild pre-acidification-induced enhancement of the lipid emulsion-mediated overall reversal of toxic dose levobupivacaine-induced vasodilation in endothelium-intact aortae (Fig. 6B). Acidosis induces nitric oxide release, and lipid emulsions (including triglycerides) inhibit endothelial nitric oxide release [10, 28, 29]. Triglyceride inhibits nitric oxide-induced relaxation in isolated vessels [11]. Taken together, the mild pre-acidification (pH 7.2)-induced enhancement of the lipid emulsion-mediated overall reversal of toxic dose levobupivacaine-induced vasodilation appears to be associated with the lipid emulsion-mediated inhibition of enhanced nitric oxide release induced by mild acidosis [10,11,28,29]. There were numerous reasons for using area under the curve analysis to ascertain the lipid emulsion-mediated reversal of toxic dose levobupivacaine-induced vasodilation in this study. First, even a slight difference in levobupivacaine (3 × 10-4 M)-induced vasodilation in isolated aortae precontracted with 60 mM KCl between the pH 7.4 Krebs solution and Krebs solutions at different pH values (7.0, 7.2 and 7.6) can affect the magnitude of the subsequent lipid emulsion-mediated reversal of levobupivacaine-induced vasodilation; therefore, we used the area under the lipid emulsion dose-response curve calculated from the baseline toxic dose levobupivacaine (3 × 10-4 M)-induced vasodilation to evaluate the overall extent of the lipid emulsion-mediated reversal [19]. Second, in contrast to the 50% of maximum response, the area under the curve is the integral of the curve generated by plotting the lipid emulsion concentration against a certain response, such as vasoconstriction or vascular tone recovery, and this parameter reflects the overall effect of lipid emulsion-mediated vascular tone recovery [30]. Furthermore, severe pre-acidification (pH 7.0) in the endothelium-denuded aortae attenuated the areas under the lipid emulsion dose-response curves from levobupivacaine (3 × 10-4 M)-induced vasodilation (Fig. 5D) compared with the pH 7.4 Krebs solution, whereas severe pre-acidification (pH 7.0) in endothelium-intact aortae did not significantly alter the areas under the lipid emulsion dose-response curves (Fig. 5B). This severe pre-acidification (pH 7.0)-induced attenuation of the lipid emulsion-mediated reversal in endothelium-denuded aortae appears to be associated with decreased levobupivacaine (3 × 10-4 M)-induced vasodilation compared with the pH 7.4 Krebs solution. Taken together, similar to mild pre-acidification, the difference in the overall extent of lipid emulsion-mediated reversal between endothelium-intact and endothelium-denuded aortae at pH 7.0 may be associated with the lipid emulsion-mediated inhibition of the enhanced endothelial nitric oxide release induced by severe pre-acidification [10, 11, 28, 29]. The pKa (8.1) of levobupivacaine indicates the pH at which 50% of levobupivacaine is in the lipid-soluble non-ionized form that is required for the penetration of nerve membranes, including the perineurium, and 50% of levobupivacaine is in the ionized form that is required to block sodium channels in the axoplasm within the epineurium [9]. Each form (ionized and non-ionized) of levobupivacaine is determined by the pKa and pH of the tissue [9]. As acidification reduces the amount of the lipid-soluble non-ionized form of levobupivacaine that can penetrate the cell membrane, the attenuated levobupivacaine-induced vasodilation at pH 7.2 and 7.0 observed in the current study seems to be associated with a relatively decreased level of intracellular levobupivacaine, which is caused by the fact that only a small amount of non-ionized levobupivacaine can penetrate the cell membrane. Thus, the potency of a local anesthetic upon acidosis appears lower compared with at pH 7.4 [9]. However, hypoventilation and respiratory acidosis due to local anesthetic toxicity in an in vivo state enhance cerebral blood flow, leading to the delivery of more local anesthetic to the brain [31]. The diffusion of carbon dioxide into neuronal cells reduces the intracellular pH, leading to an increased proportion of ionized local anesthetics (ion trapping of local anesthetics) and enhanced toxicity [7, 9, 31]. In addition, nanoemulsions extract more bupivacaine than macroemulsions, suggesting that small lipid emulsion particles are more effective at removing bupivacaine [32]. Considering the effect of pH on both local anesthetics and lipid emulsions, acidosis relatively increases the positively charged portion of levobupivacaine, whereas acidosis induces a less negative zeta potential of a lipid emulsion that leads to flocculation of the lipid emulsion through decreased electrostatic repulsion, leading to decreased efficacy at removing levobupivacaine [8, 33]. Further studies regarding the effects of pH on the ionized and non-ionized forms of local anesthetics, the zeta potential of lipid emulsions, and the intracellular concentration of ionized local anesthetics in rat aortae are needed to elucidate detailed mechanisms.
Consistent with previous reports and our current results for isometric tension measurements, a lipid emulsion attenuated the mild pre-acidification-induced enhancement of eNOS phosphorylation at pH 7.2 [10, 11, 28, 29]. However, because this attenuation was observed using HUVECs instead of the rat aorta endothelium used for the isometric tension measurements, our findings regarding the lipid emulsion-mediated inhibition of pre-acidification-induced eNOS phosphorylation should be interpreted cautiously [34].
The clinical relevance of the mild pre-acidification-induced enhancement of the lipid emulsion-mediated reversal of toxic dose levobupivacaine-induced vasodilation in endothelium-intact aortae should be carefully extrapolated. There are several limitations of this study. First, in a clinical situation, local anesthetic toxicity is followed by acidosis, whereas this experiment investigated the effect of pre-acidification on the lipid emulsion-mediated reversal of toxic dose levobupivacaine-induced vasodilation. Second, we used the aorta, which is regarded as a conduit vessel, whereas small resistance arterioles are the main contributors to organ blood flow and blood pressure [35]. Third, as the body buffer system (including bicarbonate) in the in vivo state attempts to minimize the change in blood pH to acidosis, the mild pre-acidification-induced enhancement of the reversal of the vasodilation induced by a toxic dose of levobupivacaine observed in the current in vitro study may be different in an in vivo state that includes a buffer system [36]. On the other hand, even mild acidosis might have a devastating effect on cardiovascular collapse, including severe hypotension induced by a toxic dose of levobupivacaine in an in vivo state. Thus, further studies regarding the effect of lipid emulsions on cardiovascular collapse followed by acidosis due to levobupivacaine toxicity in an in vivo state are necessary to confirm the effects observed in the current in vitro study. Even with these limitations, when we encounter a clinical situation that comprises pre-existing mild acidosis and severe vascular collapse due to a toxic dose of levobupivacaine, the mild pre-acidification-induced enhancement of the lipid emulsion-mediated reversal of the vascular collapse may provide a beneficial effect in terms of vascular tone recovery.
In conclusion, mild pre-acidification caused by a pH 7.2 Krebs solution enhanced the lipid emulsion-mediated reversal of toxic dose levobupivacaine-induced vasodilation in isolated endothelium-intact rat aortae precontracted with 60 mM KCl. This mild pre-acidification-induced enhancement of the lipid emulsion-mediated reversal appears to be associated with the lipid emulsion-mediated inhibition of nitric oxide evoked by pre-acidification.
Supplementary Material
Fig.S1.
Abbreviations
L-NAME: Nω-nitro-L-arginine methyl ester; eNOS: endothelial nitric oxide synthase; HUVECs: human umbilical vein endothelial cells.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013R1A1A2057459).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ozcan MS, Weinberg G. Update on the use of lipid emulsions in local anesthetic systemic toxicity: a focus on differential efficacy and lipid emulsion as part of advanced cardiac life support. Int Anesthesiol Clin. 2012;49:91-103
2. Weinberg GL. Lipid emulsion infusion: resuscitation for local anesthetic and other drug overdose. Anesthesiology. 2012;117:180-7
3. Ok SH, Sohn JT, Baik JS, Kim JG, Park SS, Sung HJ. et al. Lipid emulsion reverses Levobupivacaine-induced responses in isolated rat aortic vessels. Anesthesiology. 2011;114:293-301
4. Ok SH, Park CS, Kim HJ, Lee SH, Choi BH, Eun SY. et al. Effect of two lipid emulsions on reversing high-dose levobupivacaine-induced reduced vasoconstriction in the rat aortas. Cardiovasc Toxicol. 2013;13:370-80
5. Ok SH, Han JY, Lee SH, Shin IW, Lee HK, Chung YK. et al. Lipid emulsion-mediated reversal of toxic-dose aminoamide local anesthetic-induced vasodilation in isolated rat aorta. Korean J Anesthesiol. 2013;64:353-9
6. Coyle DE, Denson DD, Thompson GA, Myers JA, Arthur GR, Bridenbaugh PO. The influence of lactic acid on the serum protein binding of bupivacaine: species differences. Anesthesiology. 1984;61:127-33
7. Burney RG, DiFazio CA, Foster JA. Effects of pH on protein binding of lidocaine. Anesth Analg. 1978;57:478-80
8. Butterworth JF, Mackey DC, Wasnick JD. Morgan & Mikhail's Clinical anesthesiology. New York, USA: McGraw Hll. 2013
9. Becker DE, Reed KL. Essentials of local anesthetic pharmacology. Anesth Prog. 2006;53:98-108
10. Celotto AC, Restini CB, Capellini VK, Bendhack LM, Evora PR. Acidosis induces relaxation mediated by nitric oxide and potassium channels in rat thoracic aorta. Eur J Pharmacol. 2011;656:88-93
11. Lundman P, Tornvall P, Nilsson L, Pernow J. A triglyceride-rich fat emulsion and free fatty acids but not very low density lipoproteins impair endothelium-dependent vasorelaxation. Atherosclerosis. 2001;159:35-41
12. Ruan W, French D, Wong A, Drasner K, Wu AH. A mixed (long- and medium-chain) triglyceride lipid emulsion extracts local anesthetic from human serum in vitro more effectively than a long-chain emulsion. Anesthesiology. 2012;116:334-9
13. Mazoit JX, Le Guen R, Beloeil H, Benhamou D. Binding of long-lasting local anesthetics to lipid emulsions. Anesthesiology. 2009;110:380-6
14. Sung HJ, Choi MJ, Ok SH, Lee SH, Hwang IJ, Kim HS. et al. Mepivacaine-induced contraction is attenuated by endothelial nitric oxide release in isolated rat aorta. Can J Physiol Pharmacol. 2012;90:863-72
15. Baik J, Ok SH, Cho H, Yu J, Kim W, Nam IK. et al. Dexmedetomidine-induced contraction involves phosphorylation of caldesmon by JNK in endothelium-denuded rat aortas. Int J Biol Sci. 2014;10:1108-15
16. Baik JS, Sohn JT, Ok SH, Kim JG, Sung HJ, Park SS. et al. Levobupivacaine-induced contraction of isolated rat aorta is calcium dependent. Can J Physiol Pharmacol. 2011;89:467-76
17. Kinoshita H, Iranami H, Kimoto Y, Dojo M, Hatano Y. Mild alkalinization and acidification differentially modify the effects of lidocaine or mexiletine on vasorelaxation mediated by ATP-sensitive K+ channels. Anesthesiology. 2011;95:200-6
18. Sung HJ, Choi MJ, Ok SH, Lee SH, Hwang IJ, Kim HS. et al. Mepivacaine-induced contraction is attenuated by endothelial nitric oxide release in isolated rat aorta. Can J Physiol Pharmacol. 2012;90:863-72
19. Huang S, Pang L. Comparing statistical methods for quantifying drug sensitivity based on in vitro dose-response assays. Assay Drug Dev Technol. 2012;10:88-96
20. Mead R. The design of experiments: statistical principles for practical applications. New York, USA: Cambridge University Press. 1994
21. Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4:303-6
22. Partownavid P, Umar S, Li J, Rahman S, Eghbali M. Fatty-acid oxidation and calcium homeostasis are involved in the rescue of bupivacaine-induced cardiotoxicity by lipid emulsion in rats. Crit Care Med. 2012;40:2431-7
23. Fettiplace MR, Ripper R, Lis K, Lin B, Lang J, Zider B. et al. Rapid cardiotonic effects of lipid emulsion infusion. Crit Care Med. 2013;41:e156-62
24. Fettiplace MR, Lis K, Ripper R, Kowal K, Pichurko A, Vitello D. et al. Multi-modal contributions to detoxification of acute pharmacotoxicity by a triglyceride micro-emulsion. J Control Release. 2015;198:62-70
25. Santos AC, DeArmas PL. Systemic toxicity of levobupivacaine, bupivacaine and ropivacaine during continuous intravenous infusion to nonpregnant and pregnant ewes. Anesthesiology. 2001;95:1256-64
26. Nakanishi T, Gu H, Momma K. Effect of acidosis on contraction, intracellular pH, and calcium in the newborn and adult rabbit aorta. Heart Vessels. 1997;12:207-15
27. Loutzenhiser R, Matsumoto Y, Okawa W, Epstein M. H(+)-induced vasodilation of rat aorta is mediated by alterations in intracellular calcium sequestration. Circ Res. 1990;67:426-39
28. Minami M, Yokokawa K, Kohno M, Yasunari K, Yoshikawa J. Suppression of endothelin-3-induced nitric oxide synthesis by triglyceride in human endothelial cells. J Cardiovasc Pharmacol. 1998;31:S467-9
29. Osanai H, Okumura K, Hayakawa M, Harda M, Numaguchi Y, Mokuno S. et al. Ascorbic acid improves postischemic vasodilation impaired by infusion of soybean oil into canine iliac artery. J Cardiovasc Pharmacol. 2000;36:687-92
30. Basu A, Bodycombe NE, Cheah JH, Price EV, Liu K, Schaefer GI. et al. An interactive resource to identify cancer genetic and lineage dependencies targeted by small molecules. Cell. 2013;154:1151-61
31. Berde CB, Strichartz GR. Local anesthetics. In: (ed.) Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Young WL. Miller's Anesthesia, 7th ed. Philadelphia: Churchill Linvingstone Elsvier. 2010:932-4
32. Morey TE, Varshney M, Flint JA, Rajasekaran S, Shah DO, Dennis DM. Treatment of local anesthetic-induced cardiotoxicity using drug scavenging nanoparticles. Nano Lett. 2004;4:757-9
33. Kadam AN, Najlah M, Wan KW, Ahmed W, Crean SJ, Phoenix DA. et al. Stability of parenteral nanoemulsions loaded with paclitaxel: the influence of lipid phase composition, drug concentration and storage temperature. Pharm Dev Technol. 2014;19:999-1004
34. Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP. et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527-61
35. Christensen KL, Mulvany MJ. Location of resistance arteries. J Vasc Res. 2001;38:1-12
36. Malley WJ. Clinical blood gas assessment and interpretation. St. Louis, Missouri, USA: Elsevier Saunder. 2005
Author contact
![]() Corresponding author: Ju-Tae Sohn, MD. Department of Anesthesiology and Pain Medicine, Gyeongsang National University Hospital, 79 Gangnam-ro, Jinju-si, 52727, Korea. E-mail: jtsohngsnu.ac.kr; Tel.: +82-55-750-8586; FAX: +82-55-750-8142.
Corresponding author: Ju-Tae Sohn, MD. Department of Anesthesiology and Pain Medicine, Gyeongsang National University Hospital, 79 Gangnam-ro, Jinju-si, 52727, Korea. E-mail: jtsohngsnu.ac.kr; Tel.: +82-55-750-8586; FAX: +82-55-750-8142.

 Global reach, higher impact
Global reach, higher impact