Impact Factor
ISSN: 1449-1907
Int J Med Sci 2015; 12(10):773-779. doi:10.7150/ijms.11610 This issue Cite
Research Paper
Progesterone Inhibits Leptin-Induced Invasiveness of BeWo Cells
1. Department of Obstetrics and Gynecology, College of Medicine, St. Vincent's Hospital, The Catholic University of Korea, Suwon, Republic of Korea
2. Department of Obstetrics and Gynecology, College of Medicine, Bucheon St. Mary's Hospital, The Catholic University of Korea, Bucheon, Republic of Korea
Received 2015-1-16; Accepted 2015-9-2; Published 2015-9-19
Abstract
Background: This study investigated the roles of progesterone and leptin in placenta invasion, which is closely related to pregnancy prognosis. We examined the effects of leptin and progesterone on the invasion of BeWo cells, a human trophoblastic cell line, and the effect of concurrent treatment.
Methods: Cells were treated with leptin (0, 5, 50, or 500 ng/mL) or progesterone (0, 2, 20, or 200 µM) and cultured in an invasion assay. Cells treated with 500 ng/mL leptin were also treated with progesterone (0, 2, 20, or 200 µM) in the invasion assay for 48 h. The number of cells that invaded the lower surface was counted in five randomly chosen fields using a light microscope with a 200× objective. The mRNA expression levels of MMP-9, TIMP1, TIMP2, and E-cadherin were detected by semi-quantitative PCR.
Results: Invasion of BeWo cells was promoted by leptin and influenced by both leptin concentration and treatment duration. Invasion was most effective at 500 ng/mL leptin and 48 h culture. Leptin-induced invasiveness was suppressed by progesterone in a dose-dependent manner. Leptin significantly decreased the expression levels of TIMP1 and E-cadherin, whereas progesterone significantly decreased expression of MMP-9 and significantly increased levels of TIMP1, TIMP2, and E-cadherin.
Conclusions: Leptin promotes invasion of BeWo cells, and progesterone suppresses leptin-induced invasion by regulating the expressions of MMP-9, TIMP1, TIMP2, and E-cadherin. The balance between leptin and progesterone may play an important role in human placenta formation during early pregnancy.
Keywords: Leptin, Progesterone, Placenta, Trophoblast, Tissue Inhibitor of Metalloproteinases, Cadherins, Matrix Metalloproteinase
Introduction
The process of placental invasion into the uterus during the early stage of pregnancy is essential for a successful pregnancy. Invasion actively occurs during weeks 8-16 of pregnancy, and invasion of extravillous trophoblasts into the maternal decidual spiral artery lowers the resistance of uterine blood flow, increasing blood flow into the uterus up to 40-fold. If invasion is insufficient to decrease resistance, growth retardation in utero or preeclampsia may occur because the blood flow increase to the uterus is disrupted and placental function deteriorates [1, 2]. This theory is supported by the observation of shallow intrauterine invasion and almost no permeation into the maternal decidual spiral artery in the placenta of a pregnant woman affected by growth retardation in utero and preeclampsia. Excessive placental invasion into the myometrium is also problematic, as it causes placenta accreta. Accreta is a condition that prevents separation of the placenta from the uterus after delivery, which causes a large amount of bleeding and can lead to transfusion, hysterectomy, or maternal death. Therefore, the invasion process must be balanced between acceleration and suppression. However, the control mechanisms for placenta invasion are not clearly understood. Defining the mode of placenta invasion will ultimately contribute to understanding the causes of these diseases related to dysregulation of invasion. Previous studies suggest that leptin is expressed 50-fold higher in the placenta during early pregnancy relative to late pregnancy and that leptin receptor is found in extratrophoblasts, which promotes placenta invasion during early pregnancy [3, 4].
Progesterone is an important hormone that is found at high concentrations in the placenta during early pregnancy. However, few studies have investigated the role of progesterone in placenta generation. One study found increased progesterone during early pregnancy in mothers with preeclampsia, and another showed that it suppressed MMP-2 and MMP-9, two genes related to placenta invasion, in vitro [3, 5]. Together, these results suggest that progesterone may suppress placenta invasion.
In this study, we examined the effect of leptin on invasion of BeWo cells, a human trophoblastic cell line, and the effect of concurrent treatment with both leptin and progesterone.
Methods
1) Invasion assay
We conducted an invasion assay using a 24-well transwell support (BD, MA, USA). A PET (polyethylene terephthalate) membrane (8-µm pores) was coated on the outside with 0.1% gelatin and on the inside with 4:1 diluted Matrigel culture medium (BD, MA, USA) without fetal bovine serum (FBS). The lower chamber was filled with 10% FBS medium containing 0, 5, 50, or 500 ng/mL leptin (Peprotech, NJ, USA) and 0, 2, 20, or 200 µM progesterone P-7556 (Sigma, MO, USA) as a chemoattractant. BeWo cells (5×104/200 µL) were seeded into the upper chamber wells. After incubation for 24 or 48 h at 37 °C, the filter was fixed with 100% methanol (1 min) and stained with hematoxylin-eosin. Similarly, invasion was monitored for 48 h by adding 500 ng/mL leptin to each concentration of progesterone (0, 2, 20, or 200 µM). The cells in five randomly chosen fields were counted under a light microscope with a 200× objective.
2) BeWo cell culture
BeWo human choriocarcinoma trophoblast cells (American Type Culture Collection, Manassas, VA, USA) were cultured in F12K medium (Invitrogen, Carlsbad, CA) supplemented with 10% (v/v) FBS, 100 U/mL penicillin, and 100 mg/mL streptomycin (Invitrogen, Carlsbad, CA). Cells were maintained in monolayer cultures at 37 °C in 5% CO2, 95% air and 100% humidity. BeWo cells were cultivated in 0, 5, 50, or 500 ng/mL leptin or in 500 ng/mL leptin with varying concentrations of progesterone (0, 2, 20, or 200 µM) for up to 48 h.
3) Semi-quantitative PCR
Total RNA was extracted from 100 mg placenta tissues using an RNA Extraction Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. RNA concentration and purity were assessed using a NanoDrop. cDNA was processed using RT Master Premix (Elpis, Daejeon, Korea) in 20 µL total volume. The following primer sequences were used (forward and reverse, respectively): MMP-9, 5′-TCTATGGTCCTCGCCCTGAA-3′ and 5′-CATCGTCCACCGGACTCAAA-3′; TIMP1, 5′-TCTGCAATTCCGACCTCGTC-3′ and 5′-CTGTTCCAGGGAGCCACAAA-3′; TIMP2, 5′-AGCTTTGCTTTATCCGGGCT-3′ and 5′-ATGCTTAGCTGGCGTCACAT-3′; E-cadherin, 5′-TCATGAGTGTCCCCCGGTAT-3′ and 5′-TCTTGAAGCGATTGCCCCAT-3′; β-actin, 5′-GTGGGGCGCCCCAGGCACCAGGGC-3′ and 5′-CTCCTTAATGTCACGCACGATTTC-3′. Each primer was added at a final concentration of 0.5 μM to a 10 μL PCR Master mixture (Promega, WI, USA). An initial denaturation step was conducted for 5 min at 95 °C, and 35 cycles of denaturation at 94 °C for 30 s, annealing at 53-62 °C for 30 s (MMP-9: 62 °C, TIMP1: 55.4 °C, TIMP2: 60 °C, E-cadherin: 53 °C, β-actin: 62 °C), and extension at 72 °C for 30 s were followed by a final extension of 10 min at 72 °C. Ethidium bromide-stained bands were visualized by UV transillumination, and the fluorescence intensity was quantified using Quantity One (Bio-Rad, CA, USA). RT-PCR data were collected from at least five independent experiments (Table 1).
4) Statistical analysis
All experiments were repeated at least three times, and data are presented as the mean ± SD unless noted otherwise. All statistical analysis was performed by SAS version 8 (SAS Institute Inc., Cary, USA). Differences between data groups were evaluated for significance using ANOVA and sample t-test. P-values less than 0.05 indicate statistical significance.
Results
1) Invasion assay
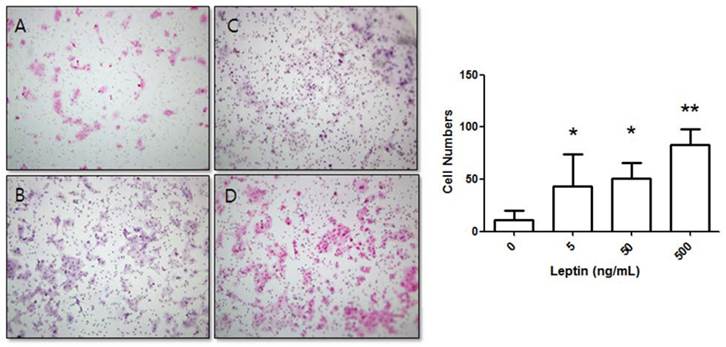
BeWo cells were treated with leptin (0, 5, 50, or 500 ng/mL) and cultured for 24 h. The number of cells that invaded the lower surface was counted in five regions at 200× magnification. The control group (0 ng/mL leptin) showed 11 ± 9.04 invasive cells. In comparison, groups treated with 5, 50, and 500 ng/mL leptin showed significant increases with 43.22 ± 30.7, 50.7 ± 14.93, and 83.1 ± 15.05 invasive cells, respectively (P = 0.025, 0.011, and 0.001, respectively; Fig. 1).
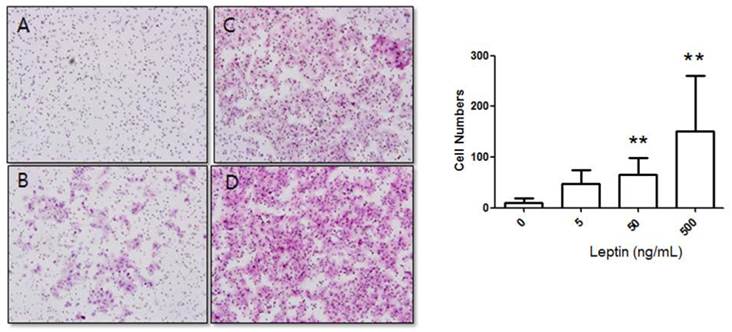
Cells were cultured for 48 h in 0, 5, 50, or 500 ng/mL leptin, and the number of cells that invaded the lower surface was counted in five regions at 200× magnification. The number of invasive cells in the control group (0 ng/mL leptin) was 8.17 ± 9.08, whereas those of the 5, 50, and 500 ng/mL leptin groups were 55.42 ± 27.84, 80.48 ± 38.89, and 109.37 ± 34.58, respectively (Fig. 2). Although the increase for the 5 ng/mL leptin group was insignificant, both the 50 and 500 ng/mL leptin groups showed statistically significant increases in the number of invasive cells relative to the control group (P = 0.008 and 0.001, respectively). Furthermore, the 500 ng/mL leptin group showed a significantly greater number of invasive cells in the 48 h culture relative to the 24 h culture (P = 0.001).
BeWo cells were treated with progesterone (0, 2, 20, or 200 µM) and cultured for 48 h. The number of invasive cells in the control group (0 µM progesterone) was 56.59 ± 61.97, whereas those of the 2, 20, and 200 µM progesterone groups were 80.00 ± 57.89, 49.56 ± 24.85, and 31.44 ± 21.30, respectively. The differences between the groups were not statistically significant (P = 0.165).
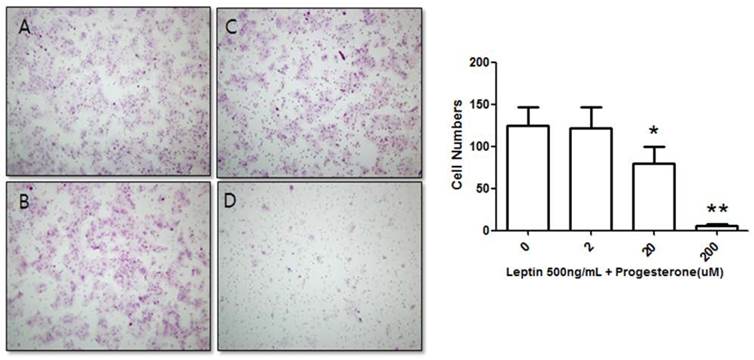
We further tested the effect of progesterone on BeWo cells treated with 500 ng/mL leptin. The number of invasive cells was counted after treatment with 0, 2, 20, or 200 µM progesterone and 500 ng/mL leptin for 48 h. The number of invasive cells in the control group (500 ng/mL leptin and 0 µM progesterone) was 124.83 ± 21.43, while those of cells treated with 500 ng/mL leptin and 2, 20, or 200 µM progesterone were 121.75 ± 24.49, 79.75 ± 20.16, and 5.67 ± 1.93, respectively (Fig. 3). The number of invasive cells decreased with increased progesterone concentration. Compared to the 500 ng/mL leptin control group, the number of invasive cells did not significantly decrease on addition of 2 µM progesterone; however, addition of 20 and 200 µM progesterone significantly decreased the number of invasive cells (P = 0.02 and 0.001, respectively).
Primer pairs for RT-PCR
| Gene | Accession # | Sequence (5′à3′) | Annealing Temp. (°C) | Size (bp) |
|---|---|---|---|---|
| MMP-9 | NM_004994 | TCT ATG GTC CTC GCC CTG AA | 62 | 219 |
| CAT CGT CCA CCG GAC TCA AA | ||||
| TIMP1 | NM_003254 | TCT GCA ATT CCG ACC TCG TC | 55.4 | 286 |
| CTG TTC CAG GGA GCC ACA AA | ||||
| TIMP2 | NM_003255 | AGC TTT GCT TTA TCC GGG CT | 60 | 175 |
| ATG CTT AGC TGG CGT CAC AT | ||||
| E-cadherin | NM_004360 | TCA TGA GTG TCC CCC GGT AT | 53 | 240 |
| TCT TGA AGC GAT TGC CCC AT | ||||
| β-actin | NM_001101 | GTG GGG CGC CCC AGG CAC CAG GGC | 62 | 540 |
| CTC CTT AAT GTC ACG CAC GAT TTC |
Stimulation of BeWo cell invasion potential with leptin. BeWo cells cultured in the absence or presence of leptin (5, 50, or 500 ng/mL) were subjected to the invasion assay. After 24 h, cells were stained with hematoxylin-eosin (A, 0 ng/mL; B, 5 ng/mL; C, 50 ng/mL; D, 500 ng/mL), and the number of invasive cells was counted in five fields per sample (×200). Significance versus vehicle-treated cells: *P < 0.05, **P < 0.01.
Stimulation of BeWo cell invasion potential with leptin. BeWo cells cultured in the absence or presence of leptin (5, 50, or 500 ng/mL) were subjected to the invasion assay. After 48 h, cells were stained with hematoxylin-eosin (A, 0 ng/mL; B, 5 ng/mL; C, 50 ng/mL; D, 500 ng/mL), and the number of invasive cells was counted in five fields per sample (×200). Significance versus vehicle-treated cells: **P < 0.01.
Suppression of BeWo cell invasion potential by treatment with 500 ng/mL leptin and progesterone. Cells were cultured with different progesterone concentrations (2, 20, and 200 µM) and subjected to the invasion assay. After 48 h, cells were stained with hematoxylin-eosin (A, 0 µM; B, 2 µM; C, 20 µM; D, 200 µM), and the number of invasive cells was counted in five fields per sample (×200). Significance versus the leptin-only treatment: *P < 0.05, **P < 0.01.
2) Quantitative RT-PCR
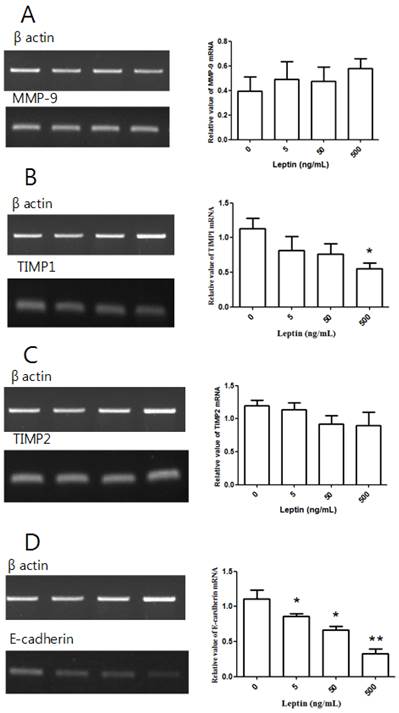
After treating BeWo cells with different leptin concentrations for 48 h, we conducted RT-PCR. Relative to the control group with no leptin, no group showed a significant difference in MMP-9 expression (Fig. 4A). Although there was no significant difference in TIMP1 expression at 5 or 50 ng/mL leptin relative to the control group, the expression significantly decreased with 500 ng/mL leptin (P = 0.036; Fig. 4B). There was no significant difference in TIMP2 expression between the control group and groups treated with leptin (Fig. 4C). However, E-cadherin expression was significantly decreased by treatment with 5, 50, and 500 ng/mL leptin (P = 0.03, 0.012, and 0.004, respectively; Fig. 4D). Furthermore, E-cadherin expression significantly decreased in the groups treated with 50 and 500 ng/mL leptin compared to the group treated with 5 ng/mL and in the 500 ng/mL group relative to the 50 ng/mL group, suggesting that E-cadherin expression is dependent on the leptin dose.
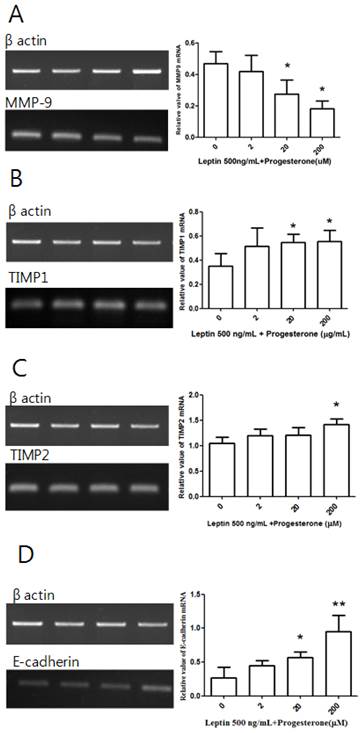
Cells treated with 500 ng/mL leptin alone or with 2, 20, or 200 µM progesterone were cultured for 48 h before conducting RT-PCR. Although there was no significant difference in MMP-9 expression between the leptin-only control group and the 2 µM progesterone group, there were significant decreases in the 20 and 200 µM groups (P = 0.039 and 0.01, respectively; Fig. 5A). A similar dose-dependent effect was observed for TIMP1 expression, which showed significant increases in the 20 and 200 µM progesterone groups (P = 0.027 and 0.02, respectively; Fig. 5B). Significantly increased TIMP2 expression relative to the control group was only observed in the 200 µM progesterone group (P = 0.034; Fig. 5C). There was no difference in E-cadherin expression between the control group and the 2 µM progesterone group, but significant increases were observed in the 20 and 200 µM groups (P = 0.026 and 0.008, respectively; Fig. 5D).
Discussion
Leptin is highly expressed during early pregnancy and is thought to participate in invasion [6-8]. In this study, we demonstrated that invasion is promoted by leptin in BeWo cells, with the greatest effect at high (500 ng/mL) leptin concentration. In a previous test using a human extravillous trophoblast cell line, leptin was found to most effectively promote invasion at 500 ng/mL [4]. This suggests that leptin-induced invasion is influenced by its concentration. We further varied the culture time from 24 to 48 h for each leptin concentration and found that the number of invading cells increased in the 48 h culture. Therefore, we conclude that invasion is influenced by both leptin concentration and culture time.
MMP-9, TIMP1, TIMP2, and E-cadherin mRNA expression with leptin. Total RNA was extracted from cultured BeWo cells treated with different leptin concentrations (0, 5, 50, and 500 ng/mL) and analyzed by RT-PCR. MMP-9 (A), TIMP1 (B), TIMP2 (C), and E-cadherin (D) levels were analyzed by agarose gel electrophoresis (left column), and their relative expression levels were quantified (right column). Significance versus vehicle-treated cells: *P < 0.05, **P < 0.01.
MMP-9, TIMP1, TIMP2, and E-cadherin mRNA expression with leptin and progesterone. Total RNA was extracted from cultured BeWo cells treated with 500 ng/mL leptin and different concentrations of progesterone (2, 20, and 200 µM) and analyzed by RT-PCR. MMP-9 (A), TIMP1 (B), TIMP2 (C), and E-cadherin (D) levels were analyzed by agarose gel electrophoresis (left column), and their relative expression levels were quantified (right column). Significance versus vehicle-treated cells: *P < 0.05, **P < 0.01.
In this study, leptin suppressed TIMP1 mRNA expression. TIMP1 mainly suppresses MMP-9 activity. MMPs decompose type IV collagen, which is a major component of basal membranes, thereby causing invasion. MMP-9 is particularly crucial for placental invasion [9, 10]. A recent study found that leptin increases MMP-9 activity [11]. Our results suggest that this effect on MMP-9 arises from TIMP1 suppression. Although the effect of leptin on TIMP1 in trophoblasts has not been studied, leptin was previously shown to reduce expression of TIMP1 mRNA in cardiomyocytes [12].
We also found that leptin suppressed E-cadherin mRNA expression. E-cadherin is involved in cell-cell adhesion. E-cadherin dysfunction in tumors is related to tumor invasiveness, and E-cadherin expression levels are correlated to malignant alterations in tumors [13]. Reduced E-cadherin expression in extravillous trophoblasts and choriocarcinoma increases invasiveness [14, 15]. Although the effect of leptin on E-cadherin in trophoblasts is unknown, adiponectin was reported to reduce E-cadherin expression in BeWo cells [16]. Herein, we found that leptin reduced E-cadherin expression in BeWo cells and promoted invasiveness. Progesterone is an important pregnancy hormone that is secreted from the ovaries during early pregnancy and later from the placenta. Little is known about the role of progesterone in placental invasion, but some studies have shown its involvement. Progesterone suppresses MMP-9 in trophoblasts [9, 17]. However, to our knowledge, no previous study has examined how progesterone directly affects invasion. In this study, we found that leptin-induced invasiveness is suppressed by progesterone. Leptin activity was also inhibited by progesterone in intrauterine granulosa cells [18], and reduction of progesterone in trophoblasts increases expression of leptin and leptin receptor and, subsequently, invasion [19]. Therefore, we propose that the balance between progesterone and leptin influences successful trophoblast invasion. We found that progesterone-mediated invasion suppression is correlated to decreased MMP-9 expression and increased TIMP1, TIMP2, and E-cadherin expressions. MMP-9 suppression was observed in several previous studies, and progesterone was predicted to negatively regulate invasion [5]. The balance between MMPs and TIMPs is important in determining invasiveness. Progesterone suppresses invasion by reducing the level of MMP-9 and increasing those of its inhibitors, TIMP1 and TIMP2. E-cadherin has a negative effect on invasion. Herein, we found that progesterone increases E-cadherin levels, which led to invasion suppression. Similarly, in human endometrium and pregnant canine uterus, progesterone increased E-cadherin levels [20, 21].
In this study, we demonstrated with a cell invasion assay that leptin promotes invasion of BeWo choriocarcinoma cells and that progesterone suppresses this leptin-induced invasion. This suggests that the balance between leptin and progesterone may play an important role in formation of the human placenta during early pregnancy. Furthermore, our RT-PCR results suggest that leptin promotes invasion by reducing TIMP1 and E-cadherin levels and that progesterone regulates MMP-9, TIMP1, TIMP2, and E-cadherin expression. Future research on the human placenta will be necessary to investigate this possibility and develop clinical applications for the results.
Acknowledgements
The authors acknowledge the financial support of the St. Vincent's Hospital, Research Institute of Medical Science (SVHS-2012-11).
Authors' contributions
YSJ and SJK conceived the study, performed the statistical analysis, and drafted the manuscript.
GSRL and SYN performed some of the experiments. All authors have read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol. 2002;187:1416-23
2. Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69:1-7
3. Chen JZ, Wong MH, Brennecke SP, Keogh RJ. The effects of human chorionic gonadotrophin, progesterone and oestradiol on trophoblast function. Mol Cell Endocrinol. 2011;342:73-80
4. Liu H, Wu Y, Qiao F, Gong X. Effect of leptin on cytotrophoblast proliferation and invasion. J Huazhong Univ Sci Technolog Med Sci. 2009;29:631-6
5. Shimonovitz S, Hurwitz A, Hochner-Celnikier D, Dushnik M, Anteby E, Yagel S. Expression of gelatinase B by trophoblast cells: down-regulation by progesterone. Am J Obstet Gynecol. 1998;178:457-61
6. Kaufmann P, Castellucci M. Extravillous trophoblast in the human placenta. Trophoblast Res. 1997;10:21-65
7. Gonzalez RR, Devoto L, Campana A, Bischof P. Effects of leptin, interleukin-1alpha, interleukin-6, and transforming growth factor-beta on markers of trophoblast invasive phenotype: integrins and metalloproteinases. Endocrine. 2001;15:157-64
8. Smolinska N, Kaminski T, Siawrys G, Przala J. Leptin gene and protein expression in the ovary during the oestrous cycle and early pregnancy in pigs. Reprod Domest Anim. 2010;45:e174-83
9. Staun-Ram E, Shalev E. Human trophoblast function during the implantation process. Reprod Biol Endocrinol. 2005;3:56
10. Staun-Ram E, Goldman S, Gabarin D, Shalev E. Expression and importance of matrix metalloproteinase 2 and 9 (MMP-2 and -9) in human trophoblast invasion. Reprod Biol Endocrinol. 2004;2:59
11. Castellucci M, De Matteis R, Meisser A. et al. Leptin modulates extracellular matrix molecules and metalloproteinases: possible implications for trophoblast invasion. Mol Hum Reprod. 2000;6:951-8
12. Schram K, De Girolamo S, Madani S, Munoz D, Thong F, Sweeney G. Leptin regulates MMP-2, TIMP-1 and collagen synthesis via p38 MAPK in HL-1 murine cardiomyocytes. Cell Mol Biol Lett. 2010;15:551-63
13. Shao ZM, Wu J, Shen ZZ, Barsky SH. Genistein exerts multiple suppressive effects on human breast carcinoma cells. Cancer Res. 1998;58:4851-7
14. Hohn HP, Grummer R, Bosserhoff S. et al. The role of matrix contact and of cell-cell interactions in choriocarcinoma cell differentiation. Eur J Cell Biol. 1996;69:76-85
15. Zhao HB, Wang C, Li RX. et al. E-cadherin, as a negative regulator of invasive behavior of human trophoblast cells, is down-regulated by cyclosporin A via epidermal growth factor/extracellular signal-regulated protein kinase signaling pathway. Biol Reprod. 2010;83:370-6
16. Benaitreau D, Dos Santos E, Leneveu MC, De Mazancourt P, Pecquery R, Dieudonné MN. Adiponectin promotes syncytialisation of BeWo cell line and primary trophoblast cells. Reprod Biol Endocrinol. 2010;8:128
17. Bischof P, Meisser A, Campana A. Involvement of trophoblast in embryo implantation: regulation by paracrine factors. J Reprod Immunol. 1998;39:167-77
18. Koshiba H, Kitawaki J, Ishihara H. et al. Progesterone inhibition of functional leptin receptor mRNA expression in human endometrium. Mol Hum Reprod. 2001;7:567-72
19. Miko E, Halasz M, Jericevic-Mulac B. et al. Progesterone-induced blocking factor (PIBF) and trophoblast invasiveness. J Reprod Immunol. 2011;90:50-7
20. Fujimoto J, Sakaguchi H, Hirose R, Tamaya T. Significance of sex steroids in roles of cadherin subfamily and its related proteins in the uterine endometrium and placenta. Horm Res. 1998;50(Suppl 2):30-6
21. Guo B, Han BC, Tian Z. et al. Expression and hormonal regulation of E-cadherin in canine uterus during early pregnancy. Reprod Domest Anim. 2010;45:e255-9
Author Biography
Yun Sung Jo is currently a Ph. D. student under the supervision of Prof. Sa Jin Kim. She has coauthored over 10 publications. Her current research focuses on pentraxin 3 in complicated pregnancy cases.
Dr. Sa Jin Kim is a professor in the Department of Obstetrics and Gynecology, College of Medicine, The Catholic University of Korea. He obtained his doctor's degree from the College of Medicine, The Catholic University of Korea in 1996. He has coauthored over 40 publications. His research is centered on perinatal outcomes of complicated pregnancies.
![]() Corresponding author: Sa Jin Kim, M.D. Department of Obstetrics and Gynecology, College of Medicine, Bucheon St. Mary's Hospital, The Catholic University of Korea, Sosa-ro 327, Wonmi-gu, Bucheon-si, Gyeonggi-do 420-717, Republic of Korea. Tel: +82. 32-340-2060, Fax: +82. 32-340-2663, E-mail: ksajinac.kr
Corresponding author: Sa Jin Kim, M.D. Department of Obstetrics and Gynecology, College of Medicine, Bucheon St. Mary's Hospital, The Catholic University of Korea, Sosa-ro 327, Wonmi-gu, Bucheon-si, Gyeonggi-do 420-717, Republic of Korea. Tel: +82. 32-340-2060, Fax: +82. 32-340-2663, E-mail: ksajinac.kr

 Global reach, higher impact
Global reach, higher impact