Impact Factor
ISSN: 1449-1907
Int J Med Sci 2015; 12(9):727-736. doi:10.7150/ijms.11952 This issue Cite
Research Paper
Dexmedetomidine-Induced Contraction in the Isolated Endothelium-Denuded Rat Aorta Involves PKC-δ-mediated JNK Phosphorylation
1. Department of Anesthesiology and Pain Medicine, Gyeongsang National University School of Medicine and Gyeongsang National University Hospital, Jinju-si, 52727, Republic of Korea
2. Department of Anesthesiology and Pain Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea
3. Department of Anesthesiology and Pain Medicine, Gyeongsang National University Hospital, Jinju, Republic of Korea
4. Department of Oral and Maxillofacial Surgery, Gyeongsang National University Hospital, Jinju, Republic of Korea
5. Department of Physiology, Institute for Clinical and Translational Research, Catholic Kwandong University College of Medicine, Gangneung, 25601, Korea
6. Institute of Health Sciences, Gyeongsang National University, Jinju, Republic of Korea
* These authors contributed equally to this study as co-first authors.
Received 2015-2-21; Accepted 2015-8-17; Published 2015-9-4
Abstract
Vasoconstriction mediated by the highly selective alpha-2 adrenoceptor agonist dexmedetomidine leads to transiently increased blood pressure and severe hypertension. The dexmedetomidine-induced contraction involves the protein kinase C (PKC)-mediated pathway. However, the main PKC isoform involved in the dexmedetomidine-induced contraction remains unknown. The goal of this in vitro study was to examine the specific PKC isoform that contributes to the dexmedetomidine-induced contraction in the isolated rat aorta. The endothelium-denuded rat aorta was suspended for isometric tension recording. Dexmedetomidine dose-response curves were generated in the presence or absence of the following inhibitors: the pan-PKC inhibitor, chelerythrine; the PKC-α and -β inhibitor, Go6976; the PKC-α inhibitor, safingol; the PKC-β inhibitor, ruboxistaurin; the PKC-δ inhibitor, rottlerin; the c-Jun NH2-terminal kinase (JNK) inhibitor, SP600125; and the myosin light chain kinase inhibitor, ML-7 hydrochloride. Western blot analysis was used to examine the effect of rottlerin on dexmedetomidine-induced PKC-δ expression and JNK phosphorylation in rat aortic vascular smooth muscle cells (VSMCs) and to investigate the effect of dexmedetomidine on PKC-δ expression in VSMCs transfected with PKC-δ small interfering RNA (siRNA) or control siRNA. Chelerythrine as well as SP600125 and ML-7 hydrochloride attenuated the dexmedetomidine-induced contraction. Go6976, safingol, and ruboxistaurin had no effect on the dexmedetomidine-induced contraction, whereas rottlerin inhibited the dexmedetomidine-induced contraction. Dexmedetomidine induced PKC-δ expression, whereas rottlerin and PKC-δ siRNA transfection inhibited dexmedetomidine-induced PKC-δ expression. Dexmedetomidine also induced JNK phosphorylation, which was inhibited by rottlerin. Taken together, these results suggest that the dexmedetomidine-induced contraction involves PKC-δ-dependent JNK phosphorylation in the isolated rat aorta.
Keywords: dexmedetomidine, protein kinase C, aorta, protein kinase C-δ, vasoconstriction, c-Jun NH2-terminal kinase
Introduction
The highly selective alpha-2 adrenoceptor agonist, dexmedetomidine (DMT) produces vasoconstriction mediated by alpha-2 adrenoceptor stimulation in the vascular smooth muscle [1,2]. The intravenous administration of DMT produces transiently increased blood pressure via this alpha-2 adrenoceptor-mediated vasoconstriction [3-6]. Severe hypertension in humans due to high doses of DMT appears to be associated with DMT-induced alpha-2B adrenoceptor stimulation, which is responsible for vascular smooth muscle contractions [7-10]. DMT-induced contractions involve pathways mediated by protein kinase C (PKC) and Rho kinase, both of which seem to be associated with the increased calcium sensitization that contributes to vascular smooth muscle contractions [11]. In addition, DMT produces vasoconstriction mainly via the involvement of the c-Jun-NH2-terminal kinase (JNK) that belongs to the mitogen-activated protein kinase family [12]. The PKC isoforms that are involved in vascular smooth muscle contractions include PKC-α, -β, -δ, -ε and -ζ [13]. The PKC isoforms that are involved in vascular smooth muscle contraction may be agonist-, vascular bed-, location-, and species-dependent [13]. PKC-α, -β, and -δ are all expressed in the rat aortic vascular smooth muscle [14]. However, the main PKC isoform that is involved in the DMT-induced contraction remains unknown. Therefore, the goal of this in vitro study was to investigate the specific PKC isoform that is involved in the DMT-induced contraction in an isolated endothelium-denuded rat aorta and determine the associated cellular mechanism.
Materials and Methods
Animal preparation
All experimental procedures and protocols were approved by the Institutional Animal Care and Use Committee at Gyeongsang National University. All experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the Institute for Laboratory Animal Research.
Preparation of aortic rings for tension measurements
The aortic rings were prepared for tension measurements as previously described [15,16]. Male Sprague-Dawley rats weighing 250-300 g each were anesthetized with an intramuscular injection of Zoletil 50 (15 mg/kg; Virbac Laboratories, Carros, France). The descending thoracic aorta was removed and dissected from its surrounding connective tissue and fat under microscopic guidance while the aorta was bathed in a Krebs solution of 118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 2.4 mM CaCl2, 25 mM NaHCO3, and 11 mM glucose. The aorta then was cut into 2.5-mm rings, which were suspended on Grass isometric transducers (FT-03, Grass Instrument, Quincy, MA, USA) under a 3.0-g resting tension in a 10-mL Krebs bath at 37°C and continuously aerated with 95% O2 and 5% CO2 to maintain pH values within 7.35-7.45. The endothelium was removed from each aortic ring by inserting a 25-gauge needle into the lumen of the ring and gently rubbing the ring for a few seconds. The rings were equilibrated at a 3.0-g resting tension for 120 min, and the bath solution was changed every 30 min. When the contraction in response to 10-8 M phenylephrine had stabilized, the removal of the endothelium was confirmed by an observation of less than 15% relaxation in response to 10-5 M acetylcholine. The contractile response induced by isotonic 60 mM KCl was measured for all aortic rings and used as a reference value. The isotonic 60 mM KCl solution was prepared by replacing the NaCl in the Krebs solution with an equimolar amount of KCl. After washing out the KCl from the organ bath and allowing the isometric tension to return to baseline, concentration-response curves induced by DMT were obtained as described in the experimental protocols. A single ring was used for each DMT-induced concentration-response curve. As the DMT-induced contraction is attenuated by endothelial nitric oxide release, the nitric oxide synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME, 10-4 M) and the cyclooxygenase inhibitor indomethacin (10-5 M) were included in the Krebs solution to prevent the release of endogenous nitric oxide and prostacyclin, respectively, from any residual endothelial tissue [17,18].
Experimental protocol
The first series of experiments investigated the effect of the pan-PKC inhibitor chelerythrine (10-5 and 3×10-5 M) on the DMT-induced concentration-response curves (10-9 to 10-6 M) in the endothelium-denuded rat aorta [15,19]. Chelerythrine was added to the organ bath for 20 min before the addition of DMT. The DMT-induced concentration-response curves were generated in the presence or absence of chelerythrine.
The second series of experiments was designed to determine the main PKC isoform that is involved in the DMT-induced contraction. The effect of the PKC isoform inhibitors (i.e., PKC-α and -β inhibitor: 10-6 M Go6976, PKC-α inhibitor: 2.5×10-5 M safingol, PKC-β inhibitor: 10-7 M ruboxistaurin, and PKC-δ inhibitor 3×10-6 and 2×10-5 M rottlerin) on the DMT-induced concentration-response curves was assessed by comparing the DMT-induced concentration-response curves in the presence or absence of each PKC isoform inhibitor. Each isoform inhibitor was incubated for 20 min before the addition of DMT to produce the DMT-induced concentration-response curves. The concentrations of the PKC isoform inhibitors used in this experiment were determined based on the concentrations used in previous similar experiments [20-23].
Finally, the effect of the JNK inhibitor SP600125 on the DMT-induced contraction was assessed in the endothelium-denuded rat aorta [12]. After the DMT (10-6 M)-induced contraction had reached a plateau, one of two concentrations of SP600125 (3×10-6 or 10-5 M) was added to the organ bath, and the DMT-induced contraction was measured continuously in the presence or absence of SP600125 for 60 min. In addition, the effect of the myosin light chain kinase inhibitor ML-7 hydrochloride (10-6, 3×10-6, and 10-5 M) on the DMT-induced concentration-response curves was assessed by comparing the DMT-induced contraction in the endothelium-denuded aorta in the presence or absence of ML-7 hydrochloride. ML-7 hydrochloride was added to the organ bath for 20 min before the addition of DMT.
Cell culture
Vascular smooth muscle cells were cultured as described by Ok et al. [24]. Vascular smooth muscle cells were isolated from the thoracic aortas of male rats by enzymatic dissociation and grown in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. The cells were subcultured twice per week by harvesting with trypsin/ethylenediaminetetraacetic acid and seeding in flasks at 7.5 × 105 cells/mm2. For the experiments, cells between passages 2 and 10 were seeded at a density of 107 cells/100-mm dish, fed every other day, and used at 70-80% confluence. The cells were serum-starved overnight prior to treatment.
Western blot analysis
Western blot analysis was performed as described by Ok et al. [24]. In brief, the cells were lysed in PRO-PREP protein extract solution (iNtRON Biotechnology, Houston, TX, USA) for total cell lysates, and the lysates were centrifuged at 100,000 × g for 20 min at 4°C. The protein concentration was determined using the Bradford method. For the preparation of the sample loading, equal volumes of 2× sodium dodecyl sulfate (SDS) sample buffer (0.1 mol/L Tris-HCI, 20% glycerol, 4% SDS, and 0.01% bromophenol blue) and supernatant fractions from the lysates were mixed. Sixty micrograms of protein were separated with 10% SDS-polyacrylamide gel electrophoresis for 90 min at 110 V. The separated proteins were transferred to polyvinylidene difluoride membranes for 2 h at 20 mA using SD Semi-dry Transfer Cells (Bio-Rad Laboratories, Hercules, CA, USA). After blocking the membranes in 5% nonfat milk in Tris-buffed saline (pH 7.0), the membranes were incubated overnight at 4°C with specific antibodies at a dilution of 1:500 in 5% skim milk in Tris-buffed saline containing Tween-20. Bound antibody was detected with horseradish peroxidase-conjugated anti-goat or anti-rabbit IgG. Membranes were washed and developed using a western blotting luminol reagent system (iNtRON Biotechnology) and autoradiography.
Gene silencing with small interfering RNA (siRNA)
Gene silencing experiments were performed using PKC-δ siRNAs, as described by Eun et al. [25]. The vascular smooth muscle cells were transfected with 10-7 M PKC-δ siRNA or control siRNA (Bioneer, Daejeon, Korea) using Lipofectamine™ RNAiMAX (Thermo Fisher Scientific, Waltham, MA, USA) in a medium containing 10% serum. The effect of gene silencing was assessed using Western blot analysis.
Reagents
All drugs were of the highest purity available commercially. L-NAME, indomethacin, chelerythrine, Go6976, safingol, ruboxistaurin, and SP600125 were obtained from Sigma-Aldrich (St. Louis, MO, USA). Rottlerin and ML-7 hydrochloride were purchased from Calbiochem (San Diego, CA, USA). DMT was donated by Orion Pharma (Turku, Finland). Anti-phospho-stress activated protein kinase/JNK (Thr183/Tyr185) and anti-JNK antibodies were obtained from Cell Signaling Technology (Beverly, MA, USA). Anti-PKC-δ antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Indomethacin, Go6976, safingol, rottlerin, ruboxistaurin, and SP600125 were dissolved in dimethyl sulfoxide (final organ bath concentration: 0.1%). All other drugs were dissolved and diluted in distilled water unless otherwise stated.
Data analysis
The values are expressed as the mean ± standard error of mean (SEM) of the numbers of rats (n) from which the descending thoracic aortic rings were obtained. The contractile responses to DMT are expressed as a percentage of the maximum contraction to isotonic 60 mM KCl or DMT (10-6 M). Statistical analyses regarding the contractile response to each concentration of DMT were performed using repeated measures analysis of variance (ANOVA) followed by Bonferroni's post-hoc test (Prism 5.0; GraphPad Software, San Diego, CA, USA). The effects of inhibitors on the DMT-induced contraction were analyzed with two-way repeated measures ANOVA followed by Bonferroni's post-hoc tests. The effect of rottlerin on the DMT-induced PKC-δ expression and JNK phosphorylation and that of DMT on PKC-δ expression in vascular smooth muscle cells transfected with PKC-δ siRNA or control siRNA were analyzed with a one-way ANOVA followed by Tukey's multiple comparison test. The logarithm of the DMT concentration eliciting 50% of the maximum contractile response (ED50) was calculated using the nonlinear regression analysis by fitting the concentration-response relationship for DMT to a sigmoidal curve using commercially available software (Prism version 5.00). Statistical analyses to compare maximal contraction and ED50 between control and inhibitor-treated groups were performed using one-way ANOVA followed by Tukey's multiple comparison test or unpaired Student's t-test. Scanning densitometry was performed using the Image Master VSD® (Pharmacia Biotech Inc., San Francisco, CA, USA). A p-value less than 0.05 was considered statistically significant.
Results
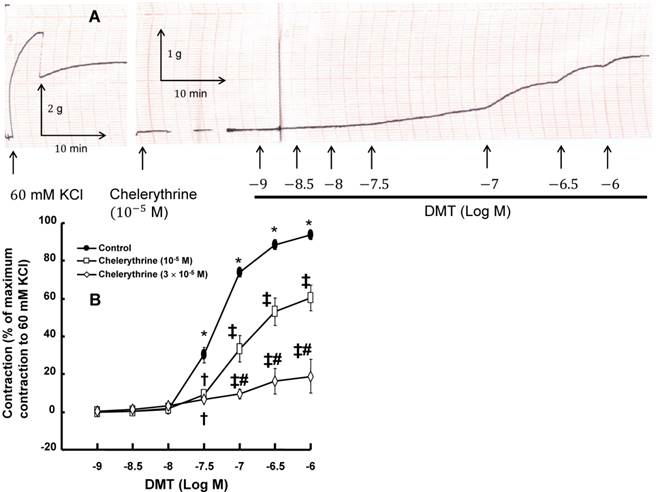
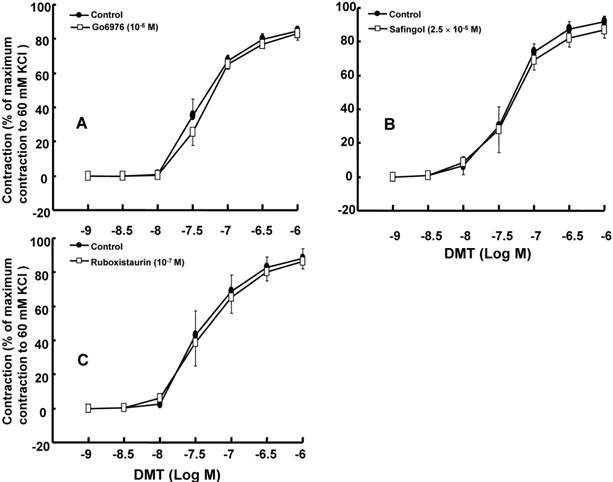
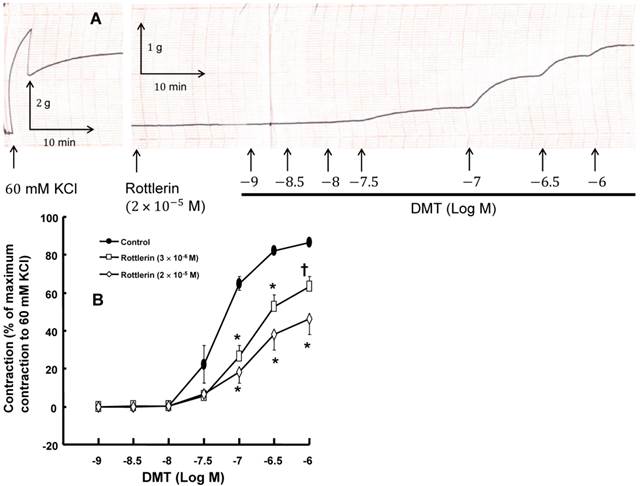
DMT (3×10-8 M) produced vasoconstriction in the isolated endothelium-denuded rat aorta (p < 0.001) (Fig. 1 and 2B). The pan-PKC inhibitor chelerythrine (10-5 and 3×10-5 M) attenuated the DMT-induced contraction in a concentration-dependent manner (p < 0.01 versus control, Fig. 2; Table S1), which suggests that the DMT-induced contraction is mediated by PKC. The PKC-α and -β inhibitor Go6976 (10-6 M), the PKC-α inhibitor safingol (2.5×10-5 M), and the PKC-β inhibitor ruboxistaurin (10-7 M) had no effect on the DMT-induced contraction (Fig. 3A, B, and C, respectively, Table S1), whereas the PKC-δ inhibitor rottlerin (3×10-6 and 2×10-5 M) attenuated the DMT-induced contraction (p < 0.01 versus control, Fig. 4A and B, Table S1). The JNK inhibitor SP600125 (3×10-6 and 10-5 M) attenuated the DMT-induced contraction in a concentration-dependent manner (p < 0.001 versus control at 20 to 60 min; Fig. 5A and B). The myosin light chain kinase inhibitor ML-7 hydrochloride (3×10-6 and 10-5 M) also attenuated the DMT-induced contraction (p < 0.05 versus control; Fig. 6A and B, Table S1).
DMT (10-6 M) induced PKC-δ expression (p < 0.001 versus control, Fig. 7A), whereas the PKC-δ inhibitor rottlerin (3×10-6 M) attenuated the DMT-induced PKC-δ expression (p < 0.01 versus 10-6 M DMT alone, Fig. 7A). DMT (10-6 M) induced JNK phosphorylation (p < 0.01 versus control, Fig. 7B), which was attenuated by the PKC-δ inhibitor rottlerin (3×10-6 M; p < 0.01 versus 10-6 M DMT alone, Fig. 7B). DMT (10-6 M) induced PKC-δ expression in vascular smooth muscle cells transfected with control siRNA (p < 0.001 versus untreated cells, Fig. 8). In addition, the DMT-induced PKC-δ expression was attenuated in vascular smooth muscle cells transfected with PKC-δ siRNA but not in the cells transfected with control siRNA (p < 0.001, Fig. 8). PKC-δ expression was attenuated in vascular smooth muscle cells transfected with PKC-δ siRNA but not in the cells transfected with control siRNA (p < 0.001, Fig. 8).
Discussion
The results of the present study suggest that the DMT-induced contraction in the isolated endothelium-denuded rat aorta involves the PKC-δ-dependent JNK-mediated pathway. The major findings of this in vitro study are that the PKC-δ inhibitor rottlerin attenuated 1) DMT-induced contraction; 2) DMT-induced PKC-δ expression; and 3) DMT-induced JNK phosphorylation. In addition, the transfection of vascular smooth muscle cells with PKC-δ siRNA attenuated DMT-induced PKC-δ expression.
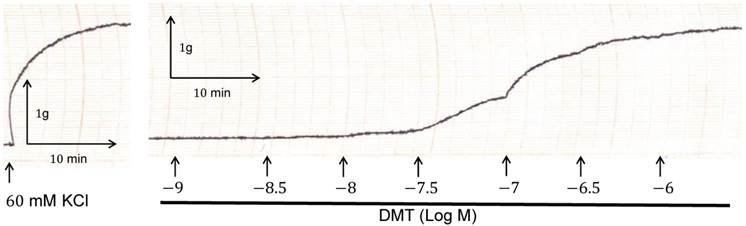
Trace showing the change in tension in endothelium-denuded rat aorta in response to isotonic 60 mM KCl, and in response to dexmedetomidine (DMT).
A: Trace showing the change in tension in endothelium-denuded rat aorta in response to isotonic 60 mM KCl or dexmedetomidine (DMT) after pretreatment with 10-5 M chelerythrine. B: The effect of chelerythrine on DMT-induced concentration-response curves in the isolated endothelium-denuded rat aorta. All the data are expressed as the mean ± SEM (control: n = 7, 10-5 M chelerythrine: n = 7, and 3×10-5 M chelerythrine: n = 5) and as the percentage of the maximal contraction induced by isotonic 60 mM KCl. N indicates the number of rats from which the descending thoracic aortic rings were derived. *p < 0.001 versus 10-9 M DMT in the control. †p < 0.01 and ‡p < 0.001 versus control. #p < 0.001 versus 10-5 M chelerythrine.
The effect of Go6976 (A), safingol (B), and ruboxistaurin (C) on dexmedetomidine (DMT)-induced concentration-response curves in the isolated endothelium-denuded rat aorta. All the data are expressed as the mean ± SEM (n = 5) and as the percentage of the maximum contraction induced by isotonic 60 mM KCl. N indicates the number of rats from which the descending thoracic aortic rings were derived.
A: Trace showing the change in tension in endothelium-denuded rat aorta in response to isotonic 60 mM KCl or dexmedetomidine (DMT) after pretreatment with 2 × 10-5 M rottlerin. B: The effect of rottlerin on DMT-induced concentration-response curves in the isolated endothelium-denuded rat aorta. All the data are expressed as the mean ± SEM (n = 5) and as the percentage of the maximum contraction induced by isotonic 60 mM KCl. N indicates the number of rats from which the descending thoracic aortic rings were derived. *p < 0.001 and †p < 0.01 versus control.
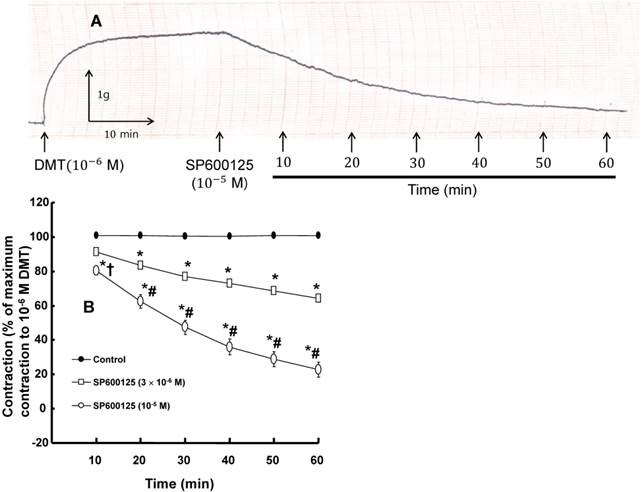
A: Trace showing the change in tension in response to SP600125 (10-5 M) in endothelium-denuded rat aorta precontracted with 10-6 M dexmedetomidine (DMT). B: The effect of SP600125 on the DMT-induced contraction in the endothelium-denuded rat aorta. All the data are expressed as the mean ± SEM (n = 7) and as the percentage of the maximal contraction induced by DMT (10-6 M). N indicates the number of rat thoracic aortic rings. *p < 0.001 versus control. †p < 0.05 and #p < 0.001 versus 3×10-6 M SP600125.
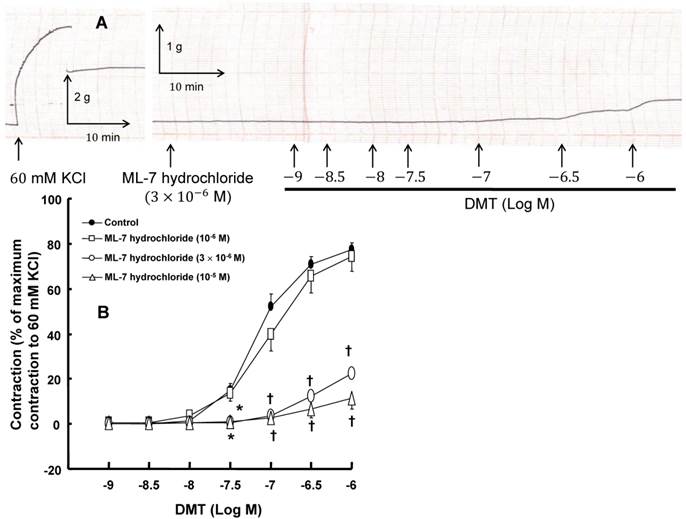
A: Trace showing the change in tension in endothelium-denuded rat aorta in response to isotonic 60 mM KCl or dexmedetomidine (DMT) after pretreatment with 3 × 10-6 M ML-7 hydrochloride. B: The effect of ML-7 hydrochloride on the DMT-induced contraction in the endothelium-denuded rat aorta. All the data are expressed as the mean ± SEM (control: n = 8, 10-6 M ML-7 hydrochloride: n = 7, 3 × 10-6 M ML-7 hydrochloride: n = 6, and 10-5 M ML-7 hydrochloride: n = 6) and as the percentage of the maximal contraction induced by isotonic 60 mM KCl. N indicates the number of rat thoracic aortic rings. *p < 0.05 and †p < 0.001 versus control.
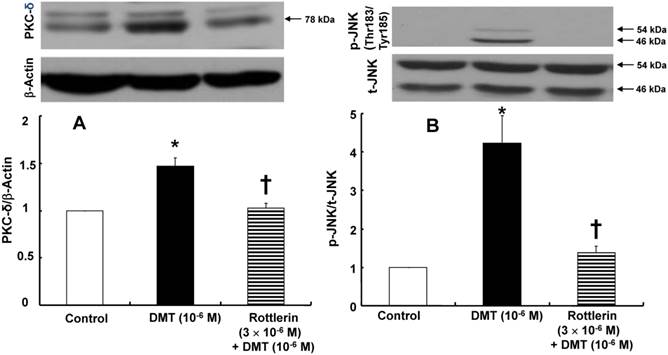
A: The effect of dexmedetomidine (DMT) on the protein kinase C (PKC)-δ expression (n = 4) in rat aortic vascular smooth muscle cells (VSMCs). The VSMCs were treated with 10-6 M DMT alone for 5 min and 10-6 M DMT for 5 min after pre-treatment with 3×10-6 M rottlerin for 1 h for PKC-δ expression. The data are expressed as the mean ± SEM. N indicates the number of independent experiments. *p < 0.001 versus control. †p < 0.01 versus 10-6 M DMT alone. B: The effect of rottlerin on the DMT-induced c-Jun NH2-terminal kinase (JNK, n = 3) phosphorylation in rat aortic VSMCs. The VSMCs were treated with 10-6 M DMT alone for 10 min and 10-6 M DMT for 10 min after pre-treatment with 3×10-6 M rottlerin for 1 h. The data are expressed as the mean ± SEM. N indicates the number of independent experiments. *p < 0.01 versus control. †p < 0.01 versus 10-6 M DMT alone. p-JNK: phosphorylated JNK, t-JNK: total JNK.
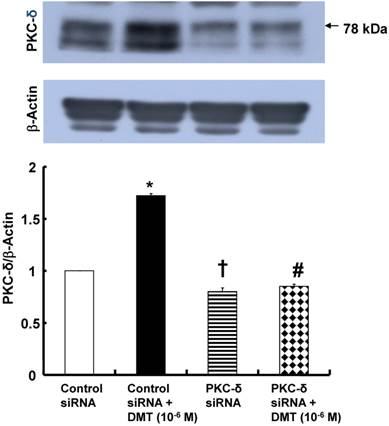
The effect of dexmedetomidine (DMT) on the protein kinase C (PKC)-δ expression (n = 4) in rat aortic vascular smooth muscle cells (VSMCs) transfected with PKC-δ siRNA or control siRNA. The VSMCs were treated with 10-6 M DMT for 5 min. The data are expressed as the mean ± SEM. N indicates the number of independent experiments. *p < 0.001 and †p < 0.001 versus untreated VSMCs transfected with control siRNA. #p < 0.001 versus DMT-treated VSMCs transfected with control siRNA.
PKC-α, -β, -δ, -ε and -ζ are involved in the pathway that mediates vascular smooth muscle contraction [13]. Consistent with previous reports, the pan-PKC inhibitor chelerythrine inhibited the DMT-induced contraction in a concentration-dependent manner (Fig. 2), which suggests that the DMT-induced contraction may be mediated by the PKC-dependent pathway [11,15]. Go6976 is a partially selective inhibitor of conventional PKC, including PKC-α and -β [26,27]. Go6976 had no effect on the DMT-induced contraction, which suggests that the DMT-induced contraction does not involve the pathway mediated by PKC-α and -β. The selective PKC-β inhibitor ruboxistaurin had no effect on the DMT-induced contraction, which suggests that the DMT-induced contraction does not involve the PKC-β-mediated pathway [28]. The PKC-α inhibitor safingol also had no effect on the DMT-induced contraction, which suggests that the PKC-α-mediated pathway does not contribute to the DMT-induced contraction [22]. Rottlerin, which is a highly selective PKC-δ inhibitor (3-6 µM) that also inhibits other PKC isoforms at higher concentrations, attenuated the DMT-induced contraction in a concentration-dependent manner [14]. Taken together, these results suggest that the DMT-induced contraction appears to be mediated primarily by the PKC-δ-dependent pathway.
Myosin light chain kinase, which is activated by Ca2+-calmodulin, contributes to vascular smooth muscle contractions via the phosphorylation of the 20 kDa myosin regulatory light chain [29]. The myosin light chain kinase inhibitor ML-7 hydrochloride attenuated the DMT-induced contraction, which suggests that the DMT-induced contraction involves the activation of the myosin light chain kinase. The DMT-induced contraction is dependent mainly on calcium influx via the voltage-operated calcium channels [17]. In addition, DMT increases the intracellular calcium concentration in the rat aorta [11]. The DMT-induced increase in intracellular calcium levels (by calcium influx) appears to be associated with myosin light chain kinase activation, which contributes to the contraction triggered by DMT [11,17,29].
We have previously reported that DMT induces PKC phosphorylation in rat aortic vascular smooth muscle cells, which was also confirmed in the current study (data not shown) [11,15]. DMT also induced PKC-δ expression, which was attenuated by rottlerin. These results are consistent with the finding that rottlerin attenuated the DMT-induced contraction in the current isometric tension study. In addition, the DMT-induced contraction is mediated by JNK via the 5-lipoxygenase pathway [12,24]. Consistent with previous reports, JNK phosphorylation was induced by DMT, and the JNK inhibitor SP600125 inhibited the DMT-induced contraction, which suggests that the DMT-induced contraction involves the JNK-mediated pathway [12,24]. The mitogen-activated protein kinase would be a downstream signaling molecule of PKC that contributes to vascular smooth muscle contraction [27]. It has been reported that the activation of extracellular signal-regulated kinase is mediated by PKC-δ activation via interleukin-1 beta in vascular smooth muscle [30]. The attenuation of DMT-induced JNK phosphorylation in rat aortic vascular smooth muscle cells by rottlerin in the current study is consistent with previous reports [27,30]. Given both previous reports and the current findings that rottlerin inhibited DMT-induced JNK phosphorylation (Fig. 7B) and SP600125 inhibited DMT-induced contraction (Fig. 5), the PKC-δ-dependent JNK-mediated pathway appears to contribute to the DMT-induced contraction [14,26,30]. Consistent with the results of the current isometric tension measurement, the transfection of vascular smooth muscle cells with PKC-δ siRNA significantly decreased DMT-induced PKC-δ expression compared with the cells transfected with control siRNA. Several PKC isoforms, including conventional, novel, and atypical PKC, induce contractions via either the stimulation of the calcium channel or the inhibition of the potassium channel [13]. The PKC isoforms also contribute to the enhanced phosphorylation of 20 kDa myosin regulatory light chain mediated by the inhibition of myosin light chain phosphatase via the phosphorylation of the phosphorylation-dependent inhibitory protein of myosin light chain phosphatase, which leads to enhanced contraction [13,29]. The DMT-induced contraction involves caldesmon phosphorylation mediated by a pathway involving JNK and PKC [15]. Taking into consideration previous reports, further research regarding the cellular signal pathway downstream of the PKC-δ-dependent JNK phosphorylation that contributes to the DMT-induced contraction is needed to elucidate the associated cellular signal pathway [13,15].
This in vitro study of DMT-induced vasoconstriction evoked by the PKC-δ-mediated pathway has several limitations. First, we used the aorta in this study, which is regarded as a conduit vessel, whereas the peripheral vascular resistance that contributes to blood pressure is determined mainly by the small resistance arteriole [31]. Second, the aorta was endothelium-denuded in this study. DMT induces endothelial nitric oxide release; therefore, the DMT-induced vasoconstriction observed in the current study using an endothelium-denuded aorta would be attenuated in an in vivo state [18,32,33]. Despite these limitations, the vasoconstriction evoked by the DMT-induced PKC-δ-mediated pathway may contribute to severe hypertension or transiently increased blood pressure as observed in patients taking high doses of DMT [3-5,7-9,34].
In conclusion, these results suggest that the DMT-induced contraction in the isolated endothelium-denuded rat aorta appears to be mediated by a pathway that involves PKC-δ-dependent JNK phosphorylation.
Supplementary Material
Table S1.
Abbreviations
DMT: dexmedetomidine; JNK: c-Jun-NH2-terminal kinase; PKC: protein kinase C.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013R1A1A2057459).
Competing Interests
Ju-Tae Sohn is currently funded by a grant (2013R1A1A2057459) from the National Research Foundation of Korea (RNF). The remaining authors have declared that they have no competing interests.
References
1. Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specificity and potency of medetomidine as an alpha 2-adrenoceptor agonist. Eur J Pharmacol. 1988;150:9-14
2. Guimarães S, Moura D. Vascular adrenoceptors: an update. Pharmacol Rev. 2001;53:319-56
3. Schmeling WT, Kampine JP, Roerig DL, Warltier DC. The effects of the stereoisomers of the alpha 2-adrenergic agonist medetomidine on systemic and oronary hemodynamics in conscious dogs. Anesthesiology. 1991;75:499-511
4. Lawrence CJ, Prinzen FW, de Lange S. Hemodynamic and coronary vascular effects of dexmedetomidine in the anesthetized goat. Acta Anaesthesiol Scand. 1997;41:830-836
5. Jalonen J, Halkola L, Kuttila K, Perttilä J, Rajalin A, Savunen T, Scheinin M, Valtonen M. Effects of dexmedetomidine on coronary hemodynamics and myocardial oxygen balance. J Cardiothorac Vasc Anesth. 1995;9:519-524
6. Flacke WE, Flacke JW, Bloor BC, McIntee DF, Sagan M. Effects of dexmedetomidine on systemic and coronary hemodynamics in the anesthetized dog. J Cardiothorac Vasc Anesth. 1993;7:41-49
7. Erkonen G, Lamb F, Tobias JD. High-dose dexmedetomidine-induced hypertension in a child with traumatic brain injury. Neurocrit Care. 2008;9:366-369
8. Mason KP, Zurakowski D, Zgleszewski S, Prescilla R, Fontaine PJ, Dinardo JA. Incidence and predictors of hypertension during high-dose dexmedetomidine sedation for pediatric MRI. Paediatr Anaesth. 2010;20:516-523
9. Shah S, Sangari T, Qasim M, Martin T. Severe hypertension and bradycardia after dexmedetomidine for radiology sedation in a patient with acute transverse myelitis. Paediatr Anaesth. 2008;18:681-682
10. Peltonen JM, Pihlavisto M, Scheinin M. Subtype-specific stimulation of [35S]GTPgammaS binding by recombinant alpha2-adrenoceptors. Eur J Pharmacol. 1998;355:275-279
11. Kim JG, Sung HJ, Ok SH, Kwon SC, Cheon KS, Kim HJ, Chang KC, Shin IW, Lee HK, Chung YK, Sohn JT. Calcium sensitization involved in dexmedetomidine-induced contraction of isolated rat aorta. Can J Physiol Pharmacol. 2011;89:681-689
12. Ok SH, Jeong YS, Kim JG, Lee SM, Sung HJ, Kim HJ, Chang KC, Kwon SC, Sohn JT. c-Jun NH2-terminal kinase contributes to dexmedetomidine-induced contraction in isolated rat aortic smooth muscle. Yonsei Med J. 2011;52:420-428
13. Ward JP, Knock GA, Snetkov VA, Aaronson PI. Protein kinases in vascular smooth muscle tone-role in the pulmonary vasculature and hypoxic pulmonary vasoconstriction. Pharmacol Ther. 2004;104:207-231
14. Das Evcimen N, King GL. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res. 2007;55:498-510
15. Baik J, Ok SH, Cho H, Yu J, Kim W, Nam IK, Choi MJ, Lee HK, Sohn JT. Dexmedetomidine-induced contraction involves phosphorylation of caldesmon by JNK in endothelium-denuded rata aortas. Int J Biol Sci. 2014;10:1108-1115
16. Ok SH, Han JY, Lee SH, Shin IW, Lee HK, Chung YK, Choi MJ, Sohn JT. Lipid emulsion-mediated reversal of toxic-dose aminoamide local anesthetic-induced vasodilation in isolated rat aorta. Korean J Anesthesiol. 2013;64:353-359
17. Ok SH, Bae SI, Shim HS, Sohn JT. Dexmedetomidine-induced contraction of isolated rat aorta is dependent on extracellular calcium concentration. Korean J Anesthesiol. 2012;63:253-259
18. Kim HJ, Sohn JT, Jeong YS, Cho MS, Kim HJ, Chang KC, Shin MK, Park CS, Chung YK. Direct effect of dexmedetomidine on rat isolated aorta involves endothelial nitric oxide synthesis and activation of the lipoxygenase pathway. Clin Exp Pharmacol Physiol. 2009;36:406-412
19. Jin L, Teixeira CE, Webb RC, Leite R. Comparison of the involvement of protein kinase C in agonist-induced contractions in mouse aorta and corpus cavernosum. Eur J Pharmacol. 2008;590:363-368
20. Rainbow RD, Norman RI, Everitt DE, Brignell JL, Davies NW, Standen NB. Endothelin-I and angiotensin II inhibit arterial voltage-gated K+ channels through different protein kinase C isoenzymes. Cardiovasc Res. 2009;83:493-500
21. Allahdadi KJ, Duling LC, Walker BR, Kanagy NL. Eucapnic intermittent hypoxia augments endothelin-1 vasoconstriction in rats: role of PKCdelta. Am J Physiol Heart Circ Physiol. 2008;294:H920-H927
22. Sodha NR, Feng J, Clements RT, Bianchi C, Boodhwani M, Ramlawi B, Mieno S, Khabbaz KR, Sellke FW. Protein kinase C alpha modulates microvascular reactivity in the human coronary and skeletal microcirculation. Surgery. 2007;142:243-252
23. Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106:1319-1331
24. Ok SH, Byon HJ, Jin H, Kim HJ, Kim W, Nam IK, Eun SY, Sohn JT. Dexmedetomidine-induced contraction involves c-Jun NH2-terminal kinase phosphorylation via activation of the 5-lipoxygenase pathway in the isolated endothelium-denuded rat aorta. Clin Exp Pharmacol Physiol. 2014;41:1014-1022
25. Eun SY, Ko YS, Park SW, Chang KC, Kim HJ. IL-1β enhances vascular smooth muscle cell proliferation and migration via P2Y2 receptor-mediated RAGE expression and HMGB1 release. Vascul Pharmacol. 2015 doi: 10.1016/j.vph.2015.04.013
26. Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marmé D, Schächtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J Biol Chem. 1993;268:9194-9197
27. Ding RQ, Tsao J, Chai H, Mochly-Rosen D, Zhou W. Therapeutic potential for protein kinase C inhibitor in vascular restenosis. J Cardiovasc Pharmacol Ther. 2011;16:160-167
28. Jirousek MR, Gillig JR, Gonzalez CM, Heath WF, McDonald JH 3rd, Neel DA, Rito CJ, Singh U, Stramm LE, Melikian-Badalian A, Baevsky M, Ballas LM, Hall SE, Winneroski LL, Faul MM. (S)-13-[(dimethylamino)methyl]-10,11,14,15-tetrahydro 4,9:16, 21-dimetheno-1H, 13H-dibenzo[e,k]pyrrolo[3,4 h][1,4,13]oxadiazacyclohexadecene-1,3(2H)-d ione (LY333531) and related analogues: isozyme selective inhibitors of protein kinase C beta. J Med Chem. 1996;39:2664-2671
29. Akata T. Cellular and molecular mechanisms regulating vascular tone. Part 2: regulatory mechanisms modulating Ca2+ mobilization and/or myofilament Ca2+ sensitivity in vascular smooth muscle cells. J Anesth. 2007;21:232-242
30. Ginnan R, Guikema BJ, Singer HA, Jourd'heuil D. PKC-delta mediates activation of ERK1/2 and induction of iNOS by IL-1beta in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2006;290:C1583-C1591
31. Christensen KL, Mulvany MJ. Location of resistance arteries. J Vasc Res. 2001;38:1-12
32. Lu D, Kassab GS. Role of shear stress and stretch in vascular mechanobiology. J R Soc Interface. 2011;8:1379-1385
33. Shafaroudi MM, McBride M, Deighan C, Wokoma A, Macmillan J, Daly CJ, McGrath JC. Two "knockout" mouse models demonstrate that aortic vasodilatation is mediated via alpha2a-adrenoceptors located on the endothelium. J Pharmacol Exp Ther. 2005;314:804-810
34. Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134-1142
Author contact
![]() Corresponding author: Ju-Tae Sohn, Department of Anesthesiology and Pain Medicine, Gyeongsang National University Hospital, Jinju, Republic of Korea. TEL: +82-55-750-8586; FAX: +82-55-750-8142; E-mail: jtsohngsnu.ac.kr
Corresponding author: Ju-Tae Sohn, Department of Anesthesiology and Pain Medicine, Gyeongsang National University Hospital, Jinju, Republic of Korea. TEL: +82-55-750-8586; FAX: +82-55-750-8142; E-mail: jtsohngsnu.ac.kr

 Global reach, higher impact
Global reach, higher impact