Impact Factor
ISSN: 1449-1907
Int J Med Sci 2015; 12(6):468-477. doi:10.7150/ijms.11210 This issue Cite
Research Paper
Enhanced Expression of Sodium Hydrogen Exchanger (NHE)-1, 2 and 4 in the Cervix of Ovariectomised Rats by Phytoestrogen Genistein
1. Dept of Physiology, Faculty of Medicine, University of Malaya, 50603 Lembah Pantai, Kuala Lumpur, Malaysia.
2. Dept of Molecular Medicine, Faculty of Medicine, University of Malaya, 50603 Lembah Pantai, Kuala Lumpur, Malaysia.
Received 2014-11-30; Accepted 2015-4-7; Published 2015-6-2
Abstract
Restoring the pH of cervicovaginal fluid is important for the cervicovaginal health after menopause. Genistein, which is a widely consumed dietary health supplement to overcome the post-menopausal complications could help to restore the cervicovaginal fluid pH. We hypothesized that genistien effect involves changes in expression of NHE-1, 2 and 4 proteins and mRNAs in the cervix. This study investigated effect of genistein on NHE-1, 2 and 4 protein and mRNA expression in the cervix in order to elucidate the mechanisms underlying possible effect of this compound on cervicovaginal fluid pH after menopause. Methods: Ovariectomised adult female rats received 25, 50 and 100 mg/kg/day genistein for seven consecutive days. At the end of the treatment, animals were sacrificed and cervix was harvested. Expression of Nhe-1, 2 and 4 mRNA were analyzed by Real-time PCR while distribution of NHE-1, 2 and 4 protein were observed by immunohistochemistry. Results: Treatment with 50 and 100 mg/kg/day genistein caused marked increase in the levels of expression and distribution of NHE-1, 2 and 4 proteins in the endocervical epithelia. Levels of Nhe-1, 2 and 4 mRNA in the cervix were also increased. Coadministration of ICI 182 780 and genistein reduced the expression levels of NHE-1, 2 and 4 proteins and mRNAs in the cervix. Conclusions: Enhanced expression of NHE-1, 2 and 4 proteins and mRNAs expression in cervix under genistein influence could help to restore the cervicovaginal fluid pH that might help to prevent cervicovaginal complications related to menopause.
Keywords: genistein, NHE-1, 2 and 4, cervix
Introduction
Phytoestrogen genistein, which can be found in soy-based food products, is widely consumed as a health supplement by the post-menopausal women [1]. Genistein has been proven useful in reducing the risk of cardiovascular diseases after menopause [2], protects against post-menopausal osteoporosis [3], relieves post-menopausal symptoms such as hot flushes [4] and overcoming post-menopausal female reproductive complications such as altered cervical and vaginal fluid pH, vaginal dryness and cervical atrophy [1]. Despite of these health benefits, over-consumption of genistein could predispose the uterus to neoplasia [5] and triggers deranged development of the female reproductive tract in fetus [6]. The effect of genistein is mainly attributed to its ability to bind to estrogen receptor [7], which made this compound an alternative treatment to alleviate post-menopausal complications related to estrogen deficiency.
Cervix, which connects vagina and the uterus, functions to restore the sperm prior to entry into the uterine lumen [8]. Cervix produces mucus which consistency changes throughout the female reproductive cycle. pH of the cervical secretion changes throughout the cycle, being high before ovulation due to increased in HCO-3 content [9]. The alkaline cervical fluid pH is necessary for sperm capacitation [10] and aids in the expansion of polyanionic macromolecular mucins, [9]. pH of the cervical fluid may influence the vaginal fluid pH. Genistein, which shares similar characteristics to estrogen could help to restore the cervical fluid pH after menopause, thus this could influence the vaginal fluid pH. As the results, the overall effect of genistein on cervicovaginal fluid pH could help to reduce the cervicovaginal complications related to estrogen deficiency.
Sodium-proton exchanger (NHE), which is involved in the H+ flux across the absorptive and secretory epithelia has been reported to participate in H+ secretion that aid in reabsorption of HCO3- in the kidney's ascending loop of Henle [11]. NHE-2 and NHE-3 was found to participate in Na+ reabsorption in exchanged with H+ secretion during acidification of the epididymal fluid. NHE has also been proposed to participate in uterine fluid pH regulation under the estrogen influence [12]. The mechanisms by which NHE might be involved in increasing the pH of uterine fluid are not fully understood, however this membrane transporter could help to ensure a continuous HCO3- secretion into the lumen by facilitating HCO3-regeneration in the epithelial cells. In view that genistein has been shown to cause increased in uterine fluid pH [13], therefore this compound might directly or indirectly affect the cervical NHE expression and activity. Currently, the effect of genistein on cervical fluid pH is unknown. We hypothesized that genistein could affect expression of NHE in the cervix that might contribute towards restoration of cervical fluid pH which could indirectly influence the vaginal fluid pH. This study therefore investigated effect of genistein on NHE-1, 2 and 4 expression (similar isoforms expressed in the uterus) in the cervix of ovariectomised rats represents post-menopause model. Changes in expression of these isoforms under genistein influence could explain ability of this compound to restore the cervicovaginal fluid pH after menopause.
Materials and methods
Animals and hormones treatment
Adult female Sprague-Dawley (SD) rats weighing 200 - 225 g were obtained from Animal House, Faculty of Medicine, University of Malaya and were kept in a clean and well ventilated environment: temperature was kept 23 ± 2 C with 12 h light: 12 h dark cycle and 30 - 70% humidity. The animals had free access to soy-free diet (Harlan, Germany) and tap water ad libitum. All experimental procedures were approved by the Faculty of Medicine ethics committee with ethics number: 2013-07-15/FIS/R/NS. Genistein was purchased from LC laboratories (Woburn, MA, USA) with 99% purity. All other chemicals were of analytical grades. Ovariectomy was performed under isoflurane anesthesia two weeks prior to the treatment to remove the effect of endogenous sex-steroids. The rats were given intramuscular injection of 0.1 ml kombitrim antibiotic to prevent post-surgical wound infection. Animals were divided into the following groups (n=6 per groups):
Group 1: seven days treatment with peanut oil (control)
Group 2, 3 & 4: seven days treatment with 25, 50 and 100 mg/kg/ day genistein respectively
Additional groups received estrogen receptor blocker (ICI 182 780) only or 100 mg/kg/day genistein with ICI 182 780
A day after the last treatment, animals were sacrificed via cervical dislocation. Abdominal cavity was cut open and cervix was removed for tissue analyses.
Quantification of Nhe-1, 2, and 4 isoforms mRNA by Real Time PCR
Tissues were rinsed with 0.1% phosphate buffer and kept in RNALater solution (Ambion, Austin, TX, USA). Total RNAs were extracted by using RNeasy plus Mini Kit (Qiagen, Hilden, Germany) with their purity and concentration were assessed by determining the 260/280 UV absorption ratios (Gene Quant 1300, UK). The extracted RNAs were run on agarose gel to check for their integrity. RNAs were reversely transcribed into cDNA using a high capacity RNA-to-cDNA kit (Applied Biosystems, USA). One step Real Time PCR was performed to evaluate gene expression with the application of TaqMan®RNA-to-CT 2-Step Kit. The amplified region of cDNA was probed with a fluorescence-labeled probe. Gapdh was used as reference or house-keeping gene as its expression was the most stable in the endometrium throughout the oestrus cycle [14].
PCR program included 15 min, 48 C reverse transcriptase, 10 min, 95 C activation with ampliTaq gold DNA polymerase, denaturing at 95 C, 15s and annealing at 60 C for 1 min. Denaturing and annealing were performed for 40 cycles. Measurements were normalized with GenEx (MultiD, Sweden) followed by Data Assist v3 (Applied Biosystems, USA) software. The latter was used to calculate the RNA fold changes. All experiments were carried out in triplicates. TaqMan® primers and probes were obtained from pre-designed assays (Applied Biosystems, USA) with Nhe- 1, 2 and 4 assay numbers are Rn01418250, Rn006888610 and Rn01437220-m1 respectively while the assay number for Gapdh is Rn99999916-s. Data was analyzed according to Comparative Ct (2-ΔΔCt) method. Relative quantity of the target in each sample was determined by comparing the normalized target quantity of genes to normalized target quantity of reference.
Immunoperoxidase and immunofluorescence detection of NHE-1, 2 and 4 isoforms protein
Cervix were fixed in 10% formalin overnight prior to processing and dehydrated through increasing concentrations of ethanol, cleared in chloroform and blocked in paraffin wax. Tissues were then sectioned into 5 µm thicknesss, deparaffinized in xylene, rehydrated in reducing concentrations of ethanol. Tri-EDTA buffer (10mM Tris Base, 1mM EDTA solution, 0.05% Tween 20, pH 9.0) was used for antigen retrieval. 1% H2O2 in methanol was used to neutralize the endogenous peroxidase. Sections were blocked in donkey serum (sc-2044) to prevent non-specific antibody binding prior to incubation with goat polyclonal NHE-1 (sc-33325), NHE-2 (sc-16099) and NHE-4 (sc-16104) primary antibodies (Santa Cruz Biotechnology, CA, USA) at a dilution of 1:100 in blocking serum. Sections were then incubated at 4 C overnight. 24 h later, the sections were rinsed three times in PBS, five min each and incubated with biotinylated secondary antibody for 1 h at room temperature. Localization of proteins was made by DAB (3,3'-Diaminobenzidine) (Santa Cruz, CA, USA) staining, which gave dark-brown stains at the site of the binding of primary antibody linked to secondary antibody conjugated with HRP complex (Immunocruz, ABC staining system, Santa Cruz, CA, USA). The sections were rinsed five min each with deionized water and counterstained with hematoxylin to visualize the nuclei. The slides were dehydrated with different dilution of ethanol and xylene and were covered with a drop of DPX neutral mounting medium (Labchem Inc, Georgetown, ON, USA).
For immunofluorescence staining, the sections were blocked in 10% normal donkey serum (Sc-2044) (Santa Cruz Biotechnology, CA, USA) prior to incubation with NHE-1, 2 and 4 primary antibodies at dilution as above (Santa Cruz Biotechnology, CA) with 1.5% normal blocking serum at room temperature for one h. After three times rinsing with PBS, the sections were incubated with IgG-fluorochrome-conjugated donkey anti-goat secondary antibody (Sc-2024) (Santa Cruz Biotechnology, CA, USA) at a dilution of 1:250 in PBS with 1.5% normal blocking serum at room temperature for 45 min. The slides were rinsed three times with PBS and were mounted with Ultracruz mounting medium (Santa Cruz Biotechnology, CA, USA). The slides were counterstained to visualize the nuclei.
Evaluation of immunoperoxidase and immunofluorescence staining intensity
The slides were viewed under Nikon Eclipse 80i microscope (SEO Enterprises Inc, Lakeland, FL, USA) with attached Nikon DS Ri1 12 megapixel camera (Nikon, Tokyo, Japan). Immunoperoxidase and immunofluoresence images were captured under standardized condition of illumination. The photographs were taken at a fixed exposure time. The tiff images (1280 × 1024 pixels) were taken at objective lens magnification of 40×. By using NIS-Element AR program (Nikon Instruments Inc, Melville, NY, USA), the exposure time and sensitivity were set prior to image capturing. Slide with no tissue (blankfield) was viewed under the microscope and an auto white balance was performed. Areas of interest on the images were selected and total counts (spots with dark-brown stained/ fluorescence signals) were obtained. The mean intensity of dark brown stain/ signals (which could be restricted) was determined which represents the average amount of protein in the tissues. Average intensity was obtained from four different sections of four different rats receiving similar treatment.
Statistical analyses
Statistical differences were evaluated by one-way ANOVA. A probability level of less than 0.05 (p<0.05) was considered as significant. Post‐hoc statistical power analysis was performed for all experiments and values were > 0.8 which indicate adequate sample size. Meanwhile, Shapiro-Wilk test was performed and all values obtained were >0.05 which indicate data normality. For mRNA quantification, mean value for each group was obtained from six (6) rats while for protein expression and histology, mean value for each group was obtained from four (4) rats.
Results
Distribution of NHE-1 protein in endocervix
Figures 1a and 2a show distribution of NHE-1 in endocervical epithelia of genistein-treated ovariectomised rats. NHE-1 protein was highly distributed at the apical membrane of epithelia lining the endocervical lumen in 50 and 100 mg/kg/day genistein treated rats as compared to control. Lower signals/ staining were observed in rats which received 25 mg/kg/day genistein treatment as compared to 50 and 100mg/kg/day genistein treatments.
Distribution of NHE-2 protein in endocervix
Figures 3a and 4a show distribution of NHE-2 protein in endocervical epithelia of genistein-treated ovariectomised rats. The highest NHE-2 protein distribution was observed in rats which received 50 and 100 mg/kg/day genistein treatments. Low distribution was observed in rats which received 25 mg/kg/day genistein treatment.
Distribution of NHE-4 protein in endocervix
Figures 5a and 6a show distribution of NHE-4 protein in the luminal epithelia of endocervix in genistein-treated ovariectomised rats. The distribution was the highest at the apical membrane of endocervical epithelia of 50 and 100 mg/kg/day genistein-treated rats. Lower distribution was observed in rats which received 25 mg/kg/day genistein treatment as compared to 50 and 100mg/kg/day genistein treatments.
Levels of expression of NHE-1, 2 and 4 protein in the luminal epithelia of endocervix
Quantitative analyses of fluorescence signals (figure 1b, 3b and 5b) and dark-brown staining (figure 2b, 4b and 6b) revealed that the highest levels of expression of NHE-1, 2 and 4 protein were observed in the luminal epithelia of the endocervix of 100 mg/kg/day genistein treated rats, followed by 50mg/kg/day genistein-treated rats. Low levels of expression was observed in rats receiving 25 mg/kg/day genistein treatment.
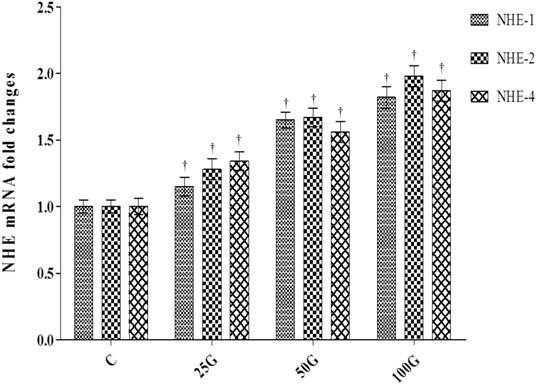
Expression levels of Nhe-1, 2 and 4 mRNA in cervix
Figure 7 shows the levels of expression of Nhe-1, 2 and 4 mRNAs in cervical tissue homogenates of ovariectomized rats receiving genistein treatment. The levels of expression of NHE-1, 2 and 4 mRNAs were the highest in cervix of rats which received 100 mg/kg/day genistein treatment. Significantly lower mRNA expression levels were observed following 50 and 25 mg/kg/day genistein treatments (p<0.05 as compared to 100 mg/kg/day genistein treatment). The levels of mRNA expression in 25mg/kg/day genistein treated rats were higher than control.
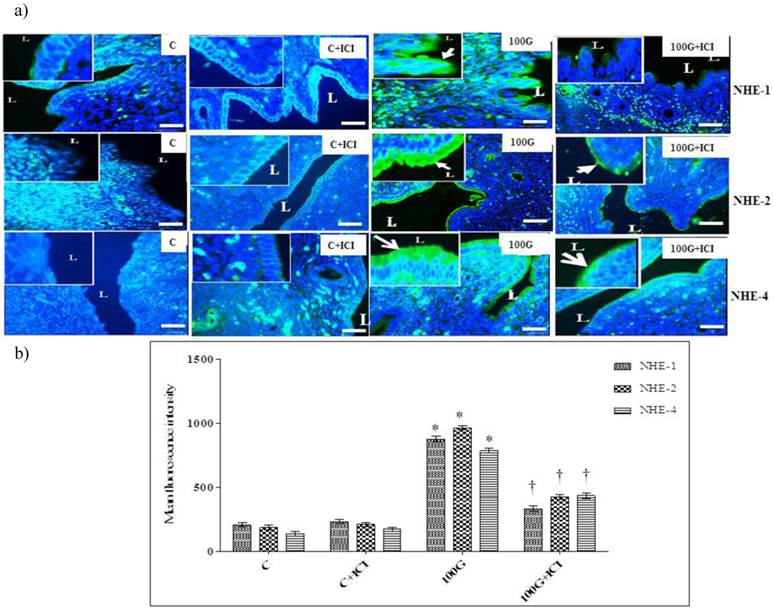
Effects of ICI 182780 on NHE-1, 2 and 4 protein distribution and expression
Figure 8a shows distribution of NHE-1, 2 and 4 proteins in endocervical epithelia while figure 8b shows quantitative analysis of the intensity of fluorescence signals in rats receiving 100 mg/kg/day genistein with or without ICI 182 780 treatment. Our findings indicated that the intensity of fluorescence signals was significantly reduced following concomitant 100 mg/kg/day genistein and ICI 182 780 treatment as compared to 100mg/kg./day genistein-only treatment.
(a) Immunofluorescence images of NHE-1 in cervix. High intensity signals were seen at apical membrane of endocervical epithelia under 100 mg/kg/day genistein. Lower signals were seen at apical membrane of endocervical epithelia following treatment with 50 and 25 mg/.kg/day genistein (b) Quantitative analyses of fluorescence signals for NHE-1 in endocervical epithelia under genistein influence. The fluorescence signal intensity was highest in 100 mg/kg/day genistein treated group. Signal intensity in all groups of treatment was higher than control. C: control, 25G: 25 mg/kg/day genistein, 50G: 50 mg/kg/day genistein, 100G: 100 mg/kg/day genistein. Arrows pointing toward NHE-1. † p<0.05 as compared to C. scale bar: 50 µm
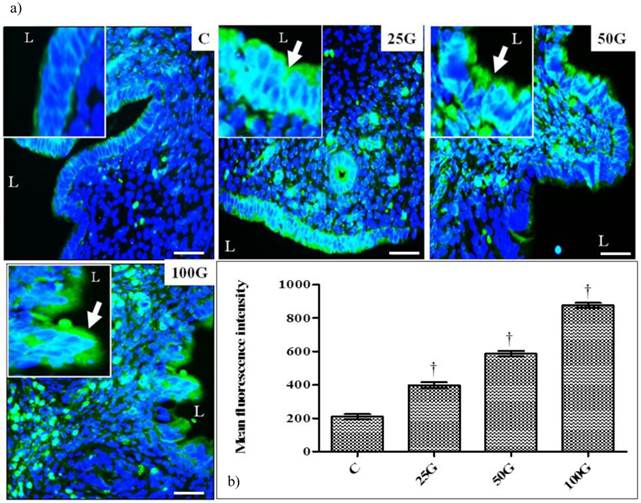
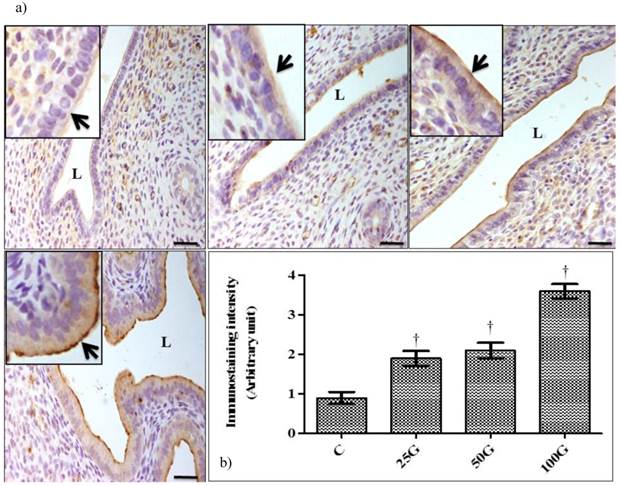
(a) Immunoperoxidase images of NHE-1 distribution in cervix. High intensity dark-brown staining could be seen at the apical membrane of endocervical epithelia in 50 and 100 mg/kg/day genistein treatment groups. Mild staining was seen in 25 mg/kg/day genistein treatment group. (b) Quantitative analyses of peroxidase staining for NHE-1 in endocervical epithelia. The intensity of peroxidase staining was the highest in 100 mg/kg/day genistein treatment group. Staining intensity in all groups was higher than control. C: control, 25G: 25 mg/kg/day genistein, 50G: 50 mg/kg/day genistein, 100G: 100 mg/kg/day genistein. Arrows pointing toward NHE-1. † p<0.05 as compared to C. scale bar: 50 µm
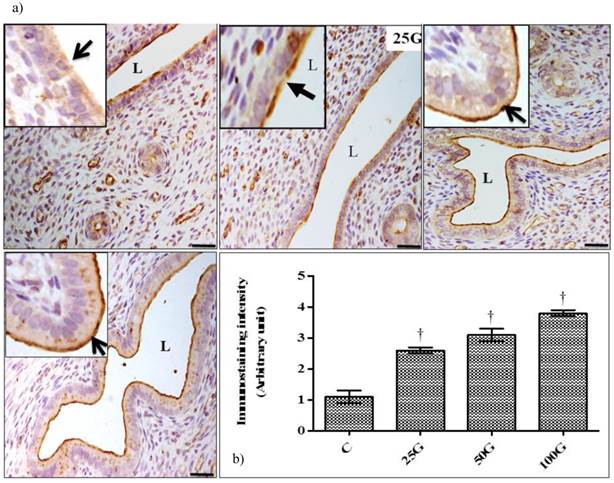
(a) Immunofluorescence images of NHE-2 distribution in cervix. High intensity fluorescence signals were observed at the apical membrane of endocervical epithelia in 100 mg/kg/day genistein treatment group. Moderate signal was seen in 25 and 50 mg/kg/day genistein treatment groups. (b) Quantitative analyses of fluorescence signals for NHE-2 in endocervical epithelia under genistein influence. Fluorescence signal intensity was the highest in 100 mg/kg/day genistein treatment group. Signal intensity in all groups of treatments was higher than control. C: control, 25G: 25 mg/kg/day genistein, 50G: 50 mg/kg/day genistein, 100G: 100 mg/kg/day genistein. Arrows pointing toward NHE-2. † p<0.05 as compared to C. scale bar: 50 µm
(a) Immunoperoxidase images of NHE-2 distribution in cervix. High intensity staining were observed at the apical membrane of endocervical epithelia in 100 and 50 mg/kg/day genistein treatment groups. (b) Quantitative analyses of peroxidase staining for NHE-2 in endocervical epithelia. The intensity of peroxidase staining was the highest in 100 mg/kg/day genistein treatment group. Staining intensity in all groups was higher than control. C: control, 25G: 25 mg/kg/day genistein, 50G: 50 mg/kg/day genistein, 100G: 100 mg/kg/day genistein. Arrows pointing toward NHE-2 isoform. † p<0.05 as compared to C. scale bar: 50 µm
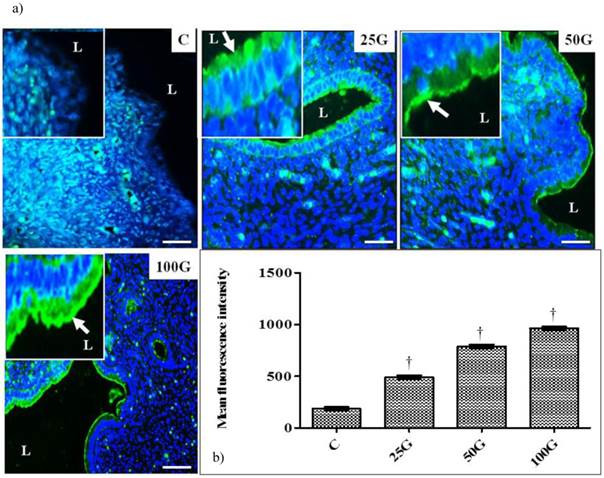
(a) Immunofluorescence images of NHE-4 distribution in cervix. High intensity signals could be seen at the apical membrane of endocervical epithelia in 50 and 100 mg/kg/day genistein treatment groups. (b) Quantitative analyses of fluorescence signals for NHE-4 in endocervical epithelia under genistein influence. Fluorescence signal intensity was the highest in 100 mg/kg/day genistein treatment group. Signal intensity in all groups of treatment was higher than control. C: control, 25G: 25 mg/kg/day genistein, 50G: 50 mg/kg/day genistein, 100G: 100 mg/kg/day genistein. Arrows pointing toward NHE-4 isoform. † p<0.05 as compared to C. scale bar: 50 µm
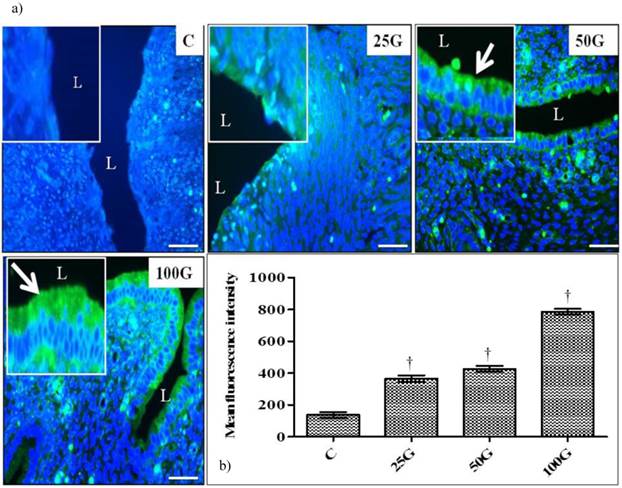
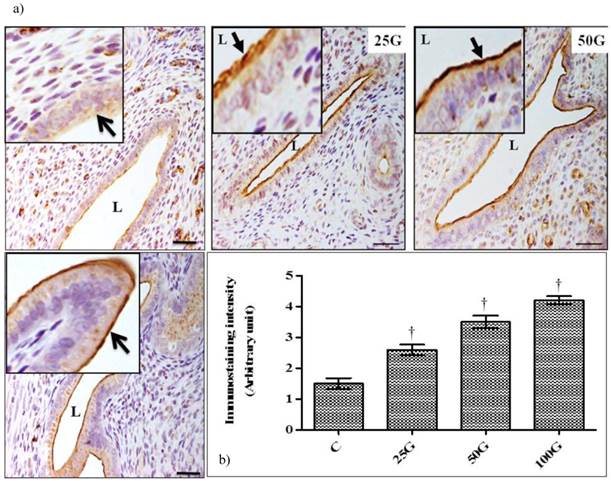
(a) Immunoperoxidase images of NHE-4 distribution in cervix. High intensity staining were observed at the apical membrane of endocervical epithelia in 100 and 50 mg/kg/day genistein treatment groups. (b) Quantitative analyses of peroxidase staining for NHE-4 in endocervical epithelia. The intensity of peroxidase staining was the highest in 100 mg/kg/day genistein treatment group. Staining intensity in all groups was higher than control C: control, 25G: 25 mg/kg/day genistein, 50G: 50 mg/kg/day genistein, 100G: 100 mg/kg/day genistein. Arrows pointing toward NHE-4. † p<0.05 as compared to C. scale bar: 50 µm
Expression of NHE-1, 2 and 4 mRNA in the cervix of genistein-treated ovariectomised rats. The highest mRNA levels for NHE-1, 2 and 4 were noted in 100 mg/kg/day genistein treatment group. C: control, 25G: 25 mg/kg/day genistein, 50G: 50 mg/kg/day genistein, 100G: 100 mg/kg/day genistein † p<0.05 as compared to C.
Effect of ICI 182780 on NHE-1, 2 and 4 mRNA expression
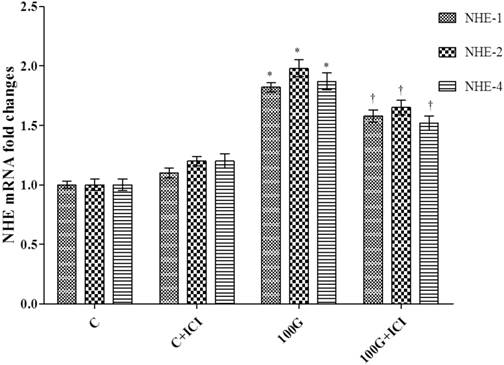
Figure 9 shows levels of expression of NHE-1, 2 and 4 mRNAs in the cervical tissue homogenates of rats receiving 100 mg/kg/day genistein with or without ICI 182 780. Our findings indicated that administration of ICI 182780 in 100mg/kg/day genistein treated rats significantly reduced the levels of expression all three NHE isoforms mRNAs as compared to 100mg/kg/day genistein-only treatment.
(a) Effect of ICI 182780 on distribution of NHE-1, 2 and 4 protein in cervix (b) Intensity of fluorescence signal in endocervical epithelium. Distribution and expression levels of NHE-1, 2 and 4 proteins in endocervical epithelia were markedly reduced in the group which received concomitant 100 mg/kg/day genistein and ICI 182780 treatment. No significant difference was noted between ICI 182780 only treatment and control. 100G: 100 mg/kg/day genistein. Arrows pointing toward NHE. * p<0.05 as compared to C. † p<0.05 as compared to 100G+ICI 182780.
Effect of ICI 182780 on NHE-1, 2 and 4 mRNA levels in cervical tissue homogenates. Expression of NHE-1, 2 and 4 mRNA was significantly reduced in rats receiving concomitant 100 mg/kg/day genistein and ICI 182 780 treatment as compared to 100 mg/kg/day genistein-only treatment. No significant difference was noted between ICI 182780-only treatment and control. 100G: 100 mg/kg/day genistein. * p<0.05 as compared to C. † p<0.05 as compared to 100G+ICI 182780.
Discussion
This study has confirmed genistein upregulation of NHE isoforms expression in the cervix of rats that might contribute towards the beneficial effect of this compound in preventing cervicovaginal complications after menopause as a result of altered cervicovaginal fluid pH. We have shown that administration of genistein at 50 and 100 mg/kg/day could enhance the expression of NHE-1, 2 and 4 in the cervix of sex-steroid deficient rats involving the estrogen receptor mediated pathway. Restoring the pH of cervical fluid could prevent pathologies in the cervix and vagina as alteration in cervical fluid pH might predispose cervix to pre-malignant changes, therefore increasing the risk of carcinoma [15]. Additionally, restoring the pH of the cervical fluid could help to restore the pH of vaginal fluid, that is essential for maintaining equilibrium of the vaginal flora [16]. This could prevent vaginal infection which is one of the most frequent complication of menopause [17].
Cervix is a sex-steroid responsive organ that responses to estrogen treatment. Estrogen stimulates increased in cervical fluid secretion [18], Both estrogen and progesterone affect the consistency of cervical mucus [8]. Estrogen has been shown able to influence the membrane transport processes in human cervical cell line in culture [19] and induces increase in cervical HCO3- secretion which contributed towards the alkaline cervical fluid pH [8]. Genistein, which is structurally related to estrogen and is widely consumed as a health supplement by the post-menopausal women was reported able to increase the pH of uterine fluid in post-menopausal rat model [13]. In view of this, there is a possibility that genistein could affect the cervical fluid pH. In this study, there were evidences that genistein could affect the pH of cervical fluid in view that it was able to upregulate the expression of NHE isoforms 1, 2 and 4 in the epithelia lining the endocervical lumen. Increased in apical membrane expression of NHE could either help to increase the excretion of H+ or to continuously maintain the excretion of HCO3- into the cervical lumen. This however warrants further investigations.
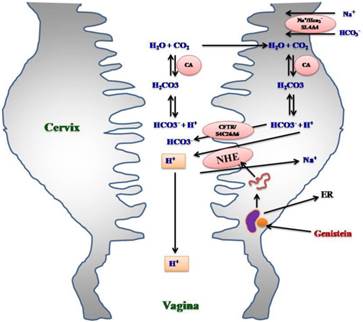
The involvement of NHE in the regeneration of HCO3- has been shown in the kidney [20] where high levels of NHE expression has been reported at the apical membrane which mediate H+ efflux that could neutralize the HCO3- in the tubular fluid [20]. As proposed in the kidneys and uterus, H+ will combine with HCO3- to form H2CO3 through the action of transmembrane carbonic anhydrase [21]. H2CO3 will then dissociates into CO2 and H2O which diffuse into the cells to form H2CO3 through the action of intracellular carbonic anhydrase [21]. Within the cells, H2CO3 will dissociate into H+ and HCO3-. In the kidneys, HCO3- is reabsorbed into the plasma via basolateral membrane HCO3- transporters [22], while, in uterus, high expression of HCO3- transporters or examples CFTR [7] and SLC26A6 [13] at the apical membrane of the endometrial luminal epithelia assist in HCO3- extrusion into the lumen. [22] There is a possibility that H+ excreted in the uterus through NHE might assist in HCO3- recycling that ensure continuous supply of HCO3- uterine lumen [13]. We speculated that similar mechanisms could occur in the cervix in which upregulation of NHE 1, 2 and 4 expression at the apical membrane of endocervical epithelia might help to ensure the continuous supply of HCO3- into the cervical lumen. Alternatively, increased expression of NHE-1, 2 and 4 might also help to enhance H+ extrusion into the cervical lumen which contributed towards the reduction in cervical fluid pH. Further studies are needed to confirm changes in the cervical fluid pH under genistein influence that could support either one of these possibilities. In the case that NHE enhances H+ extrusion into the cervical lumen, reduction in cervical fluid pH could influence the pH of the vaginal fluid. High H+ content could result in low vaginal fluid pH which could reduce the risk of vaginal infection after menopause [23]. The involvement of cervical NHE in vaginal fluid acidification could compliment the direct role of V-ATPase in mediating acidification of the vaginal fluid under estrogen or compounds with estrogenic effect [24]. The two possible consequences of genistein effect on the cervix are summarized in figure 10.
Hypothetical diagram showing the involvement of NHE isoforms in mediating possible effect of genistein on cervical and vaginal fluid pH. Genistein binds to ER prior to inducing transcription of NHE-1, 2 and 4 genes in the cervix. This leads to increased expression of NHE-1, 2 and 4 proteins at the apical membrane of endocervical epithelia. NHE mediates H+ extrusion into the cervical lumen. The extruded H+ could either decrease the cervical pH which lead to a parallel decrease in vaginal pH. Alternatively, extruded H+ can combine with HCO3-, which levels presumably increase under genistein influence through CFTR and SLC26A6 channels at the apical membrane. H+ will combine with HCO3- to form H2CO3 which through the action of transmembrane CA, will generates CO2 and H2O. CO2 and H2O traverse the apical membrane and will be reconverted to H+ and HCO3- via the action of intracellular CA.. ER: estrogen receptor, NHE: sodium proton exchanger, CFTR: cystic fibrosis transmembrane regulator, SLC26A^: chloride bicarbonate exchanger, SLC4A4: sodium bicarbonate cotransporter, CA: carbonic anhydrase.
We concluded that enhanced expression of NHE 1, 2 and 4 isoforms in the endocervival epithelia could help to restore the cervicovaginal fluid pH after menopause. This might help to reduce the cervicovaginal complications related to menopause [25].
Acknowledgements
This study was funded by UMRG Grant (RG314-14AFR) University of Malaya, Kuala Lumpur, Malaysia.
Conflict of interest
The authors reported no conflict of interest in this study.
References
1. Cassidy A, Albertazzi P, Nielsen IL, Hall W, Williamson G, Tetens I, Atkins S, Cross H, Manios Y, Wolk A. Critical review of health effects of soyabean phyto-oestrogens in post-menopausal women. Proceedings of the Nutrition Society. 2006;65(01):76-92
2. Squadrito F, Altavilla D, Morabito N, Crisafulli A, D'Anna R, Corrado F, Ruggeri P, Campo GM, Calapai G, Caputi AP. The effect of the phytoestrogen genistein on plasma nitric oxide concentrations, endothelin-1 levels and endothelium dependent vasodilation in postmenopausal women. Atherosclerosis. 2002;163(2):339-347
3. Morabito N, Crisafulli A, Vergara C, Gaudio A, Lasco A, Frisina N, D'Anna R, Corrado F, Pizzoleo MA, Cincotta M. Effects of Genistein and Hormone-Replacement Therapy on Bone Loss in Early Postmenopausal Women: A Randomized Double-Blind Placebo-Controlled Study. Journal of Bone and Mineral Research. 2002;17(10):1904-1912
4. Crisafulli A, Marini H, Bitto A, Altavilla D, Squadrito G, Romeo A, Adamo EB, Marini R, D'Anna R, Corrado F. Effects of genistein on hot flushes in early postmenopausal women: a randomized, double-blind EPT-and placebo-controlled study. Menopause. 2004;11(4):400-404
5. Newbold RR, Banks EP, Bullock B, Jefferson WN. Uterine adenocarcinoma in mice treated neonatally with genistein. Cancer Research. 2001;61(11):4325-4328
6. Jefferson WN, Padilla-Banks E, Newbold RR. Disruption of the female reproductive system by the phytoestrogen genistein. Reproductive Toxicology. 2007;23(3):308-316
7. Chinigarzadeh A, Kassim NM, Muniandy S, Salleh N. Genistein-induced fluid accumulation in ovariectomised rats' uteri is associated with increased cystic fibrosis transmembrane regulator expression. Clinics. 2014;69(2):111-119
8. Johnson MH. Essential reproduction. John Wiley & Sons. 2012
9. Muchekehu RW, Quinton PM. A new role for bicarbonate secretion in cervico-uterine mucus release. The Journal of physiology. 2010;588(13):2329-2342
10. Chan HC, Ruan YC, He Q, Chen MH, Chen H, Xu WM, Chen WY, Xie C, Zhang XH, Zhou Z. The cystic fibrosis transmembrane conductance regulator in reproductive health and disease. The Journal of physiology. 2009;587(10):2187-2195
11. Good DW. Sodium-dependent bicarbonate absorption by cortical thick ascending limb of rat kidney. American Journal of Physiology-Renal Physiology 248. 1985;6:F821-F829
12. Gholami K, Muniandy S, Salleh N. Differential expression of Na+/H+-exchanger (NHE-1, 2, and 4) proteins and mRNA in rodent's uterus under sex steroid effect and at different phases of the oestrous cycle. BioMed research international. 2013 (2013)
13. Chinigarzadeh A, Kasim NF, Muniandy S, Kassim NM, Salleh N. Genistein Induces Increase in Fluid pH, Na+ and HCO3- Concentration, SLC26A6 and SLC4A4 (NBCe1)-B Expression in the Uteri of Ovariectomized Rats. International journal of molecular sciences. 2014;15(1):958-976
14. Lin P, Lan X, Chen F, Yang Y, Jin Y, Wang A. Reference gene selection for real-time quantitative PCR analysis of the mouse uterus in the pe-ri-implantation period. PloS one. 2013;8(4):e62462
15. No JH, Jo H. et al. Expression of Vascular Endothelial Growth Factor and Hypoxia Inducible Factor-1α in Cervical Neoplasia. Annals of the New York Academy of Sciences. 2009;1171(1):105-110
16. Murta EF, Perfeito PB, Oliveira TM, Michelin MA, Maluf PJ. Relation between vaginal and endocervical pH in patients undergoing cold-knife conization and hysterectomy. Arch Gynecol Obstet. 2008;277(1):43-46
17. Sánchez-Borrego R. et al. Position of the Spanish Menopause Society regarding vaginal health care in postmenopausal women. Maturitas. 2014;78(2):146-150
18. Gorodeski GI. The Cultured Human Cervical Epithelium: A New Model for Studying Paracellular Transport. Journal of the Society for Gynecologic Investigation. 1996;3(5):267-280
19. Gorodeski GI. Estrogen increases the permeability of the cultured human cervical epithelium by modulating cell deformability. American Journal of Physiology-Cell Physiology. 1998;275(3):C888-C899
20. Ford P, Rivarola V, Kierbel A, Chara O. Blot-Chabaud M, Farman N, Parisi M, Capurro C, Differential role of Na+/H+ exchange isoforms NHE-1 and NHE-2 in a rat cortical collecting duct cell line. The Journal of membrane biology. 2002;190(2):117-125
21. Gholami K, Muniandy S, Salleh N. In-Vivo Functional Study on the In-volvement of CFTR, SLC26A6, NHE-1 and CA Isoenzymes II and XII in Uterine Fluid pH, Volume and Electrolyte Regulation in Rats under Different Sex-Steroid Influence. International journal of medical sciences. 2013;10(9):1121
22. Hou J, Rajagopal M, Alan S. Claudins and the Kidney Volume 75: Annual Review of Physiology. Annual review of physiology. 2013;75:479
23. Boskey E, Telsch K, Whaley K, Moench T, Cone R. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidifi-cation. Infection and immunity. 1999;67(10):5170-5175
24. Gorodeski GI, Hopfer U, Liu CC, Margles E. Estrogen Acidifies Vaginal pH by Up-Regulation of Proton Secretion via the Apical Membrane of Vagi-nal-Ectocervical Epithelial Cells. Endocrinology. 2005;146(2):816-824
25. Bulten J, de Wilde P, Schijf C, van der Laak JA, Wienk S, Poddighe PJ, Hanselaar AG. Decreased expression of Ki-67 in atrophic cervical epithelium of post-menopausal women. The Journal of pathology. 2000;190(5):545-553
Author contact
![]() Corresponding author: E-mail: naguib.sallehcom.my; Tel.: +6-017-208-271; Fax: +6-03-7967-4775
Corresponding author: E-mail: naguib.sallehcom.my; Tel.: +6-017-208-271; Fax: +6-03-7967-4775

 Global reach, higher impact
Global reach, higher impact