3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2013; 10(10):1406-1411. doi:10.7150/ijms.6565 This issue Cite
Research Paper
Identification and Determination of Antibiotic Susceptibilities of Brucella Strains Isolated from Patients in Van, Turkey by Conventional and Molecular Methods
1. Microbiology Laboratory, Van Education and Training Hospital, Van, Turkey;
2. Department of Medical Microbiology, Yuzuncu Yil University, Faculty of Medicine, Van, Turkey;
3. Microbiology Laboratory, Mengucek Gazi Education and Training Hospital, Erzincan, Turkey;
4. Department of Family Medicine, Diskapi Yildirim Beyazit Education and Training Hospital, Ankara, Turkey;
5. Bacterial Zoonoses Research Reference Laboratory, Turkey Public Health Agency, Ankara, Turkey.
Received 2013-4-26; Accepted 2013-8-13; Published 2013-8-22
Abstract
Purpose: Brucellosis is a worldwide zoonotic disease and still constitutes a major public health problem. In this study, we aimed to identify biovars of Brucella strains isolated from clinical specimens taken from brucellosis patients from the Eastern Anatolia region as well determine the susceptibility of these isolates to tigecycline and azithromycin, drugs that may serve as alternatives to the conventional drugs used in the therapy.
Materials and methods: Seventy-five Brucella spp. isolates were included in the study. All strains were identified by both conventional and molecular methods. Brucella Multiplex PCR kit (FC-Biotech, Code: 0301, Turkey) and B. melitensis biovar typing PCR kit (FC-Biotech, Code: 0302, Turkey) were used for molecular typing. Antimicrobial susceptibilities of all strains were determined by E-tests.
Results: By conventional biotyping, 73 strains were identified as B. melitensis biovar 3 and two strains as B. abortus biovar 3. Molecular typing results were compatible with conventional methods. The MIC50 and MIC90 values of doxycycline were 0.047 and 0.094; tigecycline 0.094 and 0.125; trimethoprim/sulfamethoxazole 0.064 and 0.19; ciprofloxacin 0.19 for both; streptomycin 0.75 and 1; rifampin 1 and 2 and azithromycin 4 and 8. According to the MIC values, doxycycline was found to be the most effective antibiotic, followed by tigecycline, trimethoprim-sulfamethoxazole and ciprofloxacin.
Conclusion: Currently recommended antibiotics for the treatment of brucellosis such as doxycycline, rifampin, streptomycin, trimethoprim-sulfamethoxazole and ciprofloxacin were found to be still effective. While our results showed that tigecycline can be used an alternative agent in the treatment of brucellosis, azithromycin has not been confirmed as an appropriate agent for the treatment.
Keywords: Brucella, biotyping, antibiotic sensitivity, E-test.
Introduction
Gram-negative bacteria from genus Brucella are the causative agent of brucellosis.1 Brucellosis is a serious public health problem worldwide, especially in the Middle East, Mediterranean countries, South America and Central Asia with 500,000 new cases reported every year.2-4 Although the prevalence of brucellosis has decreased in many developed countries due to various eradication programs, it still remains an important endemic disease in Turkey, where 10,000 human brucellosis cases are reported annually.5,6 The most common symptoms of brucellosis are recurring fever, chills, shivering, fatigue, body aches, joint and back pain, anorexia and weakness. During the course of the disease, complications like spondylitis, wedge-shaped vertebral collapse, meningitis, pancarditis, bronchopneumonia, acute respiratory distress syndrome, unilateral epididymo-orchitis and uveitis can be encountered.1,7,8
Together with the isolation and identification of Brucella strains, conventional and molecular biotyping is very important for epidemiologic studies and eradication programs.9 According to the World Health Organization's recommendations for the treatment of human brucellosis published in 1986, the gold standard for brucellosis therapy is doxycycline-rifampin combination for six weeks or a combination of 6 week doxycycline and 2-3 weeks streptomycin.10 The recommended drug combinations are widely used in Turkey for the treatment of brucellosis without serious complication. However, some studies have shown that the drug regimens recommendations of WHO are not implemented in clinical practice and relapses due to inadequate therapy can occur in 5-10% of patients. Factors such as high incidence of relapse, resistance to rifampin (especially in regions with endemic tuberculosis) and the ototoxic and nephrotoxic side effects of streptomycin have led to the investigation of new therapeutic options for brucellosis.11,12 Accordingly, fluoroquinolones and macrolides are presented as new alternative antimicrobial agents for treatment.13 It has been shown that tigecycline, a novel antibiotic with activity against multidrug resistant bacteria, could be a potentially promising agent for brucellosis treatment.14,15
In this study, we aimed to identify Brucella strains isolated from various clinical specimens using conventional and molecular methods and to determine their antimicrobial susceptibilities to doxycycline, rifampin, streptomycin, trimethoprim-sulfamethoxazole, azithromycin, tigecycline and ciprofloxacin using E-tests.
Materials and Methods
Brucella strains
A total of 75 Brucella strains, isolated in our laboratory from various clinical specimens between January 2009 and June 2011, were included in the study. Brucella strains were isolated from blood (n = 71), CSF (n = 2), pleural (n = 1) and peritoneal fluid (n = 1). Samples were collected with syringes under sterile conditions from the patients prediagnosed with brucellosis displaying symptoms such as fever, chills, shivering, fatigue, body aches, headache, joint and back pain. Blood samples were collected into adult blood culture bottles and other sterile body fluids (CSF, pleural and peritoneal fluid) into pediatric blood culture bottles. The samples were incubated for at least 21 days in the automated blood culture system (BacT/Alert, Biomerieux, France). Positive signals were recorded and the samples were inoculated with sterile loop onto sheep blood agar, Eosin Methylene Blue (EMB) agar and Brucella agar and incubated for 48-72 h at 37oC.
After incubation, identification at species level was performed using colony morphology, Gram staining, growth characteristics, oxidase, catalase, movement and polyvalent antisera agglutination methods. Samples with strains identified as Brucella spp. were stored in 10% skim milk and preserved at -80oC until conventional biotyping, molecular typing and antibiotic susceptibility tests were performed.
Biovar Determination by Conventional Methods
Strains identified as Brucella spp. were analyzed by conventional biotyping methods including the CO2 requirement, H2S production, urease activity, sensitivity to thionin and basic fuchsin, phage sensitivity and agglutination with monospecific A and M antisera.16
Molecular Identification and Brucella melitensis Molecular Typing
DNA isolation: Brucella DNA was isolated by heat-lysis method. Strains were suspended in 500 μl TE buffer (10 mM Tris, pH 8.0,1 mM EDTA) and bacterial suspensions were exposed to 100ºC for 10 min to ensure cell lysis. After that, samples were centrifuged at 12,000 rpm for 10 min and an upper clear phase containing DNA was taken.
For the species identification and molecular typing of the Brucella strains, a two-stage method was utilized. A Brucella Multiplex PCR kit (FC Biotek Code: 0301, Ankara, Turkey) was used for differentiation of six different species in the Brucella genus. We added 3-5 µl of the DNA sample to 45 µl of amplification mixture. For amplification, 40 PCR cycles were performed. The amplification products were separated by 1.5% agarose gel electrophoresis and evaluated with standard molecular weight samples.
In the second stage, samples identified as B. melitensis were analyzed using a Brucella melitensis biovar typing PCR kit (FC-Biotek, Code: 0302, Ankara, Turkey) for biovar differentiation of B. melitensis biovar 1, 2 and 3 in a single PCR tube.17 For this purpose, 2 μl of DNA extract was added to 45 μl of amplification mixture, and 40 PCR cycles were performed. The PCR products (10 μl) were separated by 1.3% agarose gel electrophoresis and visualized with the AlphaImager 2000 (Alpha Innotech Corporation, San Leandro, CA).
Determination of Antimicrobial Susceptibility
In vitro determination of antibiotic efficacy against Brucella strains is based on the Minimal Inhibitory Concentration (MIC) values. For this purpose, methods such as microbroth dilution, agar dilution and E-test can be used. In our study, the E-test method was utilized as it is a reliable, reproducible, less labor-intensive, less time-consuming and more practical micro-dilution method.13 Susceptibility to doxycycline, streptomycin, rifampin, trimethoprim-sulfamethoxazole, ciprofloxacin, tigecycline and azithromycin were assessed.
Suspension of the growing bacterial colonies in Mueller-Hinton broth (Oxoid®, Hampshire, UK) with a turbidity equivalent to that of a 0.5 McFarland standard was inoculated by rolling a sterile swab over 5% sheep blood-enriched Mueller-Hinton agar (Oxoid®, Hampshire, UK) plates. The plates were incubated at 37°C for 48h.
Determination of the MIC was performed in accordance with the recommended reference values of the Clinical Laboratory Standards Institute's (CLSI) guidelines for Brucella and slowly growing bacteria species. Tigecycline is not defined in the same guidelines and FDA recommendation of ≤0.25 as sensitive was utilized as interpretive MIC breakpoints for tigecycline.18,19
In every stage of this study, B. melitensis (bv1: 16M, ATCC 23456; bv3: Ether, NCTC 10505), B. abortus (bv1: NCTC 10863; bv3: ATCC 23448), B. suis 1330 bv1 (NCTC 10316), E. coli ATCC 25922 and S. aureus ATCC 29213 strains were used for the quality control.
This study was approved by the Non-Pharmacological Clinical Research Ethics Committee of the Yuzuncu Yil University Medical Faculty (approval No:12.01.2010/06).
Results
Sample Distribution
A total of 75 Brucella strains including 44 isolated from female and 31 from male patients were analyzed in this study. Samples had been sent from Infectious Disease (n = 38), Pediatrics (n = 17), Internal Medicine (n = 12), General Surgery (n = 2), Thoracic Surgery (n = 2), Orthopedics (n = 2), Gynecology (n = 1) and Brain Surgery (n = 1) departments of Yuzuncu Yil University, Faculty of Medicine, Training and Research Hospital.
Conventional and Molecular Typing Results
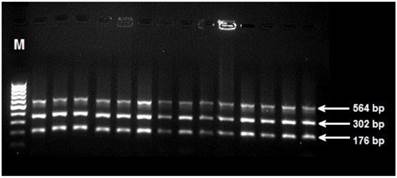
Seventy-three (97.3%) of Brucella strains were identifed as B. melitensis biovar 3 and the rest of them (2.7%) were identified as B. abortus biovar 3 by conventional biotyping. The results of agarose gel electrophoresis and multiplex PCR were in accordance with conventional biotyping. Agarose gel electrophoresis image of B. melitensis strains is shown in Figure 1.
Agarose gel electrophoresis of Brucella melitensis strains. Strains identified as Brucella melitensis by B. melitensis biovar typing PCR kit (FC-Biotek, Code: 0302, Ankara, Turkey). (M: DNA molecular marker, bp: base pair).
Results of Antibiotic Susceptibility Testing
According to antibiotic susceptibility testing by E-test, all strains were found to be susceptible to doxycycline, tigecycline, trimethoprim-sulfamethoxazole, ciprofloxacin, streptomycin. Forty of the 73 B. melitensis strains were susceptible to rifampin, 33 strains were intermediate-resistant to rifampin and 34 strains were resistant to azithromycin. The two strains identified as B. abortus were resistant to azithromycin; one was sensitive, and another intermediate-resistant to rifampin. The antibiotic susceptibilities of Brucella strains are shown in Table 1.
According to MIC50 and MIC90 values, doxycycline was the most effective antibiotic against B. melitensis strains. After doxycycline, the most effective antibiotics were tigecycline, trimethoprim-sulfamethoxazole and ciprofloxacin, respectively. The highest MIC50 and MIC90 values had azithromycin, rifampin and streptomycin, respectively. The MIC ranges and MIC50 and MIC90 values of the antibiotics used in this study are given in Table 2.
The distribution of antibiotic susceptibility of Brucella strains.
| Antibiotics | B. melitensis | B. abortus | The limit of sensitivity | ||||
|---|---|---|---|---|---|---|---|
| (S) | (I) | (R) | (S) | (I) | (R) | ||
| Doxycycline | 73 | - | - | 2 | - | - | ≤1 |
| Tigecycline | 73 | - | - | 2 | - | - | ≤0,25 |
| TMP-SXTa | 73 | - | - | 2 | - | - | ≤2/38 |
| Ciprofloxacin | 73 | - | - | 2 | - | - | ≤1 |
| Streptomycin | 73 | - | - | 2 | - | - | ≤8 |
| Rifampin | 40 | 33 | - | 1 | 1 | ≤1* | |
| Azithromycin | 39 | - | 34 | - | - | 2 | ≤4 |
TMP-SXT: Trimethoprim/sulfamethoxazole, S: Sensitive, I: Intermediate sensitive, R: Resistant, *: Rifampin I: 2, R: ≥4.
The MIC values (µg/ml) for Brucella melitensis strains (n:73).
| Antibiotics | MIC results (µg/ml) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.016 | 0.023 | 0.032 | 0.047 | 0.064 | 0.094 | 0.125 | 0.19 | 0.25 | 0.38 | 0.5 | 0.75 | 1 | 1.5 | 2 | 3 | 4 | 6 | 8 | 12 | 16 | ||
| Doxycycline | 7 | 12 | 27a | 18 | 8b | 1 | ||||||||||||||||
| Tigecycline | 5 | 23 | 27 | 14 | 4 | |||||||||||||||||
| TMP-SXT | 2 | 4 | 11 | 10 | 12 | 13 | 13 | 1 | 3 | 1 | 3 | |||||||||||
| Ciprofloxacin | 22 | 46 | 4 | 1 | ||||||||||||||||||
| Streptomycin | 27 | 24 | 19 | 3 | ||||||||||||||||||
| Rifampin | 1 | 3 | 14 | 22 | 21 | 11 | 1 | |||||||||||||||
| Azithromycin | 1 | 1 | 3 | 5 | 11 | 18 | 18 | 9 | 6 | 1 | ||||||||||||
MIC: Minimum inhibitory concentration, TMP-SXT: Trimethoprim/sulfamethoxazole, a : MIC50, b : MIC90.
Discussion
Brucella melitensis is the most frequently reported cause of human brucellosis worldwide and it is commonly isolated from patients. In several countries, it is reported as endemic.1 In Turkey, concordantly, B. melitensis is the main cause of human brucellosis.7 In most of the studies conducted both abroad and in Turkey, B. melitensis has been reported as the only isolated species.20-22 However, the results of some studies have confirmed the presence of B. abortus in Turkey.23 In previous biotyping studies, it has been shown that the most common variation is B. melitensis biovar 3.9 From the 75 strains isolated in our study, 73 were identified as B. melitensis biovar 3 and two strains as B. abortus biovar 3.
As Brucella spp. are intracellular microorganisms, at least one antibiotic from the combination used for the treatment should be an antibiotic with a good intracellular penetration.24 Doxycycline is ranked as a gold standard drug by the World Health Organization (WHO) and it became the most commonly prescribed tetracycline derivative in the treatment of Brucella infections because of its superior pharmacokinetics profile.8 In many studies, doxycycline was found to be the most effective antibiotic against Brucella strains.13,21,25 In our study as well, doxycycline was compared to other antibiotics used in therapy and was found to be the most effective antibiotic with the lowest MIC50 and MIC90 values.
Aminoglycosides combined with tetracyclines are still commonly used for brucellosis treatment. The most preferred aminoglycoside for brucellosis is streptomycin. Other studies from different parts of the world have also determined streptomycin as one of the most effective antibiotics in treatment of brucellosis. However, as streptomycin exerts toxic effects upon the eighth nerve and carries the risk of the side effect of ototoxicity during therapy, an alternative treatment regimen is suggested.8,26 MIC values for streptomycin obtained in the present study were higher when compared with other tested antibiotics tested and in accordance with results from previous studies from Turkey.13,22,27
A combination of rifampin and doxycycline is the best oral therapy for brucellosis nowadays.28 In previous studies, rifampin has been presented together with less active antibiotics and existence of intermediate-sensitive strains has been reported. Aliskan et al.29 found that, in an endemic region, from 65 isolates containing B. melitensis strains isolated from bone marrow and blood, 8 showed intermediate sensitivity to rifampin. In our study, the MIC90 value of the rifampin was higher compared to the other tested antibiotics, and from a total of 75 strains, 34 were found to have intermediate sensitivity to rifampin. The emergence of strains of intermediate sensitivity is likely due to the frequent usage of rifampin as an antitubercular agent in long-term, multi-drug tuberculosis therapy in Turkey, which is accepted as an endemic region for tuberculosis.7
Trimethoprim-sulfamethoxazole is another agent recommended for the treatment of brucellosis. It is used in combination with rifampin in pregnant women and children under 8 years old, who cannot use tetracycline. A combination of trimethoprim-sulfamethoxazole, doxycycline and rifampin is successfully used in the treatment of Brucella endocarditis, which is the brucellosis complication with the highest mortality rate.30 Previous studies have identified trimethoprim-sulfamethoxazole as an effective antibiotic with low MIC levels.20 Aliskan et al.29 reported trimethoprim-sulfamethoxazole as the most effective antimicrobial agent with the lowest MIC50 and MIC90 values. In our study, trimethoprim-sulfamethoxazole was found to be the most effective antibiotic after doxycycline according to the MIC50 and it was the third most effective antibiotic, after doxycycline and tigecycline according to the MIC90.
Azithromycin is another antibiotic with good tissue penetration features that can be used in combination with rifampin, especially during pregnancy.24 In many studies, azithromycin was found to be an effective antibiotic against Brucella strains.27,31 In the present study, however, azithromycin's MIC values were high. Additionally, when compared to other tested antibiotics, azithromycin's activity was the lowest and resistance was reported in 36 strains. This might be due to the regional differences in antimicrobial susceptibilities between countries.
Ciprofloxacin and ofloxacin are the leading quinolones effective in the treatment of brucellosis. Fluoroquinolones easily penetrate into the cells and they are efficient against Brucella spp.1,8,12 Previous studies confirmed fluoroquinolones as effective against Brucella.5,20,21,28 The MIC values of ciprofloxacin obtained in our study were found to be similar to the results of previous studies. Due to high relapse rate associated with ciprofloxacin monotherapy, it should be used only in combined regimens.28
A semi-synthetic member of a new generation tetracycline group, tigecycline, may be of importance for the treatment of brucellosis in the future.14 Tigecycline's activity against Brucella strains is similar to that of tetracycline; however, the development of resistance to this antimicrobial agent is more difficult. From a pharmacological point of view, monotherapy for brucellosis with tigecycline appears possible.12 With the exception of nausea and vomiting, tigecycline's side effects are mild and the only restrictive factor for its usage is that administration can be provided only by intravenous infusion which requires the hospitalization of patients.15 Additionally, the high cost of tigecycline therapy is yet another factor that limits its wider usage. The reported results suggest tigecycline as a promising therapeutic option although it is not as effective as doxycycline.5,13,32 In the present study, tigecycline was found to be the most effective antibiotic after doxycycline. According to this result, tigecycline is an appropriate agent for the treatment of brucellosis. However, its administration by intravenous infusion and the consequent need for hospitalization of patients limits its usage to cases with no response to treatment or to complicated cases.
In conclusion, our results have revealed that B. abortus antigens in addition to B. melitensis should be used in conventional and molecular biotyping of brucellosis in eastern Turkey. The World Health Organization's recommendations for brucellosis treatment published in 1986 still appear to be effective in Turkey. Although azithromycin does not seem to be an appropriate agent for the treatment of brucellosis in our region due to its high MIC values and presence of resistant strains, tigecycline could be considered in cases with no response to treatment or in complicated cases.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Corbel MJ. Brucellosis in humans and animals. World Health Organization. 2006:14-17
2. Elfaki MG, Uz-Zaman T, Al-Hokail AA, Nakeeb SM. Detection of Brucella DNA in sera from patients with brucellosis by polymerase chain reaction. Diagn Microbiol Infect Dis. 2005;53:1-7
3. Godfroid J. Brucellosis in wildlife. Rev Sci Tech. 2002;21:277-286
4. Sauret JM, Vilissova N. Human brucellosis. J Am Board Fam Pract. 2002;15:401-406
5. Turan H, Arslan H, Uncu H, Azap Ö, Şerefhanoğlu K. In vitro activity of tigecycline against Brucella strains: a comparative study with doxycycline, ciprofloxacin and rifampin. İnfeksiyon Derg. 2007 21:147-151
6. Yüce A, Alp Çavuş S. [Brucellosis in Turkey: an overview]. Klimik Derg. 2006;19:87-97
7. Baysal B. Ustaçelebi S. Temel ve Klinik Mikrobiyoloji. Ankara: Güneş Kitabevi Ltd. Sti. 1999:571-577
8. Anğ Ö, Yumuk Z. Bruselloz. Çev: Madkour's Brucellosis. In: (ed.) Madkour MM. Nobel Tıp Kitabevleri. İstanbul. 2008
9. Şimşek H, Erdenliğ S, Oral B, Tülek N. Determination of type-biotype and epidemiological investigation of Brucella isolates from humans. Klimik Derg. 2004;17:103-106
10. World Health Organization. Joint FAO/WHO Expert Committee on Brucellosis. Sixth report, Technical Report Series 740. Geneva: WHO. 1986
11. Ariza J, Bosilkovski M, Cascio A, Colmenero JD, Corbel MJ, Falagas ME. et al. Perspectives for the treatment of brucellosis in the 21st century: the Ioannina recommendations. PLoS Med. 2007;4:317
12. Pappas G, Solera J, Akritidis N, Tsianos E. New approaches to the antibiotic treatment of brucellosis. Int. J. Antimicrob Agents. 2005;26:101-105
13. Bayram Y, Korkoca H, Aypak C, Parlak M, Cikman A, Kilic S. et al. Antimicrobial susceptibilities of Brucella ısolates from various clinical speciemens. Int J Med Sci. 2011;8:198-202
14. Pappas G, Papadimimitriou P, Christou L, Akritidis N. Future trends in human brucellosis treatment. Expert Opin. Investig Drugs. 2006;15:1141-1149
15. Zer Y, Namıduru M, Çam R. [In-vitro activities of tigecycline for Brucella melitensis strains isolated from blood cultures]. ANKEM Derg. 2007;21:42-45
16. Alton GG, Jones LM, Pietz DE. Laboratory Techniques in Brucellosis; 2 ed, Ser No.55. Geneva: World Health Organization. 1975:1-163
17. Büyük F, Celebi O, Şahin M, Ünver A, Tazegül E. [Brucella and Campylobacter mixed infection in two different sheep and goat herds]. Kafkas Univ Vet Fak Derg. 2011;17(Suppl A):177-180
18. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; Twentieth informational supplement; CLSI document M 100-S20. Wayne, Pennsylvania, USA: CLSI. 2010
19. Tygacil. Highlights of prescribing information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021821s021lbl.pdf
20. Marianelli C, Graziani C, Santangelo C, Xibilia MT, Imbriani A, Amato R. et al. Molecular epidemiological and antibiotic susceptibility characterization of Brucella isolates from humans in Sicily, Italy. J Clin Microbiol. 2007;45:2923-2928
21. Bodur H, Balaban N, Aksaray S, Yetener V, Akinci E, Colpan A. et al. Biotypes and antimicrobial susceptibilities of Brucella isolates. Scand J Infect Dis. 2003;35:337-338
22. Tanyel E, Coban AY, Koruk ST, Simsek H, Hepsert S, Cirit OS. et al. Actual antibiotic resistance pattern of Brucella melitensis in central Anatolia. An update from an endemic region. Saudi Med J. 2007;28:1239-1242
23. Kuloğlu F, Erdenliğ S, Akata F, Tansel O, Gürcan S, Tuğrul HM. Species and biovar distribution of Brucella isolates in Trakya University Hospital Between 1997-2002. Mikrobiyol Bul. 2004;38:187-191
24. Eşel D, Sümerkan B, Ayangil D, Telli M. [Comparison of agar dilution method and E-test in the determination of antibiotic ausceptibility of Brucella melitensis strains]. ANKEM Derg. 2004;18:196-199
25. Trujillano-Martín I, García-Sánchez E, Martínez IM, Fresnadillo MJ, García-Sánchez JE, García-Rodríguez JA. In vitro activities of six new fluoroquinolones against Brucella melitensis. Antimicrob. Agents Chemother. 1999;43:194-195
26. Rubinstein E, Lang R, Shasha B, Hagar B, Diamanstein L, Joseph G. et al. In vitro susceptibility of Brucella melitensis to antibiotics. Antimicrob Agents Chemother. 1991;35:1925-1927
27. Akova M, Gür D, Livermore DM, Kocagöz T, Akalin HE. In vitro activities of antibiotics alone and in combination against Brucella melitensis at neutral and acidic pHs. Antimicrob. Agents Chemother. 1999;43:1298-3000
28. Kılıç D, Kurt H, Sözen TH, Kandilci S. Antibiotic susceptibility and clinical evaluation of Brucella spp isolated from blood cultures. İnfeksiyon Derg. 1994 8:59-62
29. Alişkan H, Turunç T, Demiroğlu YZ, Çolakoğlu S, Arslan H. Investigation of in vitro antibiotic susceptibility of Brucella melitensis. Mikrobiyol Bul. 2008;42:125-129
30. McLean DR, Russell N, Khan MY. Neurobrucellosis: Clinical and therapeutic features. Clin Infect Dis. 1992;15:582-590
31. Yamazhan T, Aydemir Ş, Tünger A, Serter D, Gökengin D. In vitro activities of various antimicrobials against Brucella melitensis strains in the Agean Region in Turkey. Med Princ Pract. 2005;14:413-416
32. Kılıç S, Dizbay M, Cabadak H. In vitro activity of tigecycline, tetracycline and fluoroquinolones against Brucella melitensis. Chemotheraphy. 2008;20:33-37
Author contact
![]() Corresponding author: Cenk Aypak, Department of Family Medicine, Diskapi Education and Research Hospital, 06110, Ankara, Turkey. Tel:+90 5056452780; Fax: +90 3123170287 E-mail: cenkaypakcom.
Corresponding author: Cenk Aypak, Department of Family Medicine, Diskapi Education and Research Hospital, 06110, Ankara, Turkey. Tel:+90 5056452780; Fax: +90 3123170287 E-mail: cenkaypakcom.

 Global reach, higher impact
Global reach, higher impact