3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2013; 10(8):1068-1072. doi:10.7150/ijms.5878 This issue Cite
Short Research Communication
The Corrosion Resistance of Composite Arch Wire Laser-Welded By NiTi Shape Memory Alloy and Stainless Steel Wires with Cu Interlayer in Artificial Saliva with Protein
1. Department of Orthodontic, Jilin University, No. 1500 Qinghua Road, Changchun 130021, P. R. China;
2. Key Laboratory of Automobile Materials, Ministry of Education, and Department of Materials Science and Engineering, Jilin University, NO. 5988 Renmin Street, Changchun 130025, P. R. China.
Received 2013-1-14; Accepted 2013-6-10; Published 2013-6-21
Abstract
In this paper, the corrosion resistance of laser-welded composite arch wire (CoAW) with Cu interlayer between NiTi shape memory alloy and stainless steel wire in artificial saliva with different concentrations of protein was studied. It was found that protein addition had a significant influence on the corrosion behavior of CoAW. Low concentration of protein caused the corrosion resistance of CoAW decrease in electrochemical corrosion and immersion corrosion tests. High concentration of protein could reduce this effect.
Keywords: Composite arch wire, Protein, Corrosion.
Introduction
NiTi shape memory alloy (NiTiSMA) and stainless steel (SS) arch wires have been used respectively in orthodontic clinical treatment all along. NiTiSMA has a super-elastic property, whereas it could cause disadvantageously anchorage teeth move. SS could provide adequate anchorage, but it could easily cause latent absorption of alveolar bone.[1-3]
Composite arch wire (CoAW) is a new type of arch wire solder made by NiTiSMA and SS. Its application could effectively reduce the suffering of patients and simplify treatment operation. Recently, a brand-new CoAW is developed with Cu interlayer by laser welding. Its joint bending angles could reach to 180°. Tensile strength is 520MPa and shape recovery ratio is up to 98%. This new type of CoAW could meet the requirements of clinical performance.[4]
Corrosion behavior is usually the most important property of metallic materials.[5] Intraoral structures are exposed to an ever-changing environment including saliva, foods, bacteria and their product. Protein in food and digestive enzymes make the saliva not only a single phosphate buffer, but a complex system containing protein.[6,7] Limited studies on corrosion behavior of alloys have been reported in protein-containing solutions. Brown observed inhibition of metal fretting corrosion in the presence of blood proteins.[8,9] It seems that the presence of protein may protect alloys from corrosion. The mechanisms by which proteins altered the corrosion resistance are not clear, but probably involve the adsorption of proteins onto the alloy surface.[10-12]
To understand the effect of corrosion in clinic, it is essential to become familiar with the environment in which CoAW must function and also its effect. The main interest of this article is to study the corrosion behavior of CoAW in artificial saliva (AS) with different concentrations of protein and provide a reference for the corrosion mechanism in protein-containing solution.
2. Experimental details
2.1 Materials and samples preparation
Ti-44.73 wt% NiTiSMA wire (Smart Co., Beijing), Fe-18Cr-8Ni SS (Grikin Advanced Materials Co., Ltd.) and 0.2mm pure Cu were used as base metals in this investigation. The dimensions of the wires are 30mm (length) × 0.64mm (width) × 0.48mm (thickness). The base metal was ground using SiC papers to remove oxide layer and then ultrasonically degreased in acetone. The NiTiSMA and SS wire were fixed in a self-constructed apparatus by wire-to-wire butt with pure Cu interlayer. A Nd:YAG laser welding system (JHM-1GY 300B) with a wavelength of 1064 nm was used for the welding. The optimized laser parameters used in the study were: 5.23J (laser power), 6 ms (welding time), and Φ=0.5mm.
2.2 Test solution preparation and immersing tests
The composition of AS was KCl (0.4 g/L), NaCl (0.4 g/L), CaCl2•2H2O (0.906 g/L), NaH2PO4•2H2O (0.690 g/L), Na2S•9H2O (0.005 g/L), and urea (1 g/L) and pH value was adjusted to 6.75with lactic acid. [13] The protein-containing solutions were AS with 0.05% and 0.1% bovine serum albumin (BSA). The samples were immersed in different solutions and maintained at 37℃for periods up to 28 days.
The collected solutions were individually analyzed for Cu by inductively coupled plasma-optical emission spectrometer (ICP-OES) (Optima 3300DV, Perkin Elmer, Boston, USA) and the detection limit was 0.01 ppm.
2.3. Electrochemical measurements
All electrochemical measurements were performed by CHI 920C electrochemical workstation (CH Instruments, Shanghai). The counter electrode was a rectangular platinum plate and the reference electrode was a saturated calomel electrode (SCE). After grinding with SiC papers up to 2000 grit to guarantee consistent surface roughness, all samples were embedded in cold-curing epoxy resin, with an exposed sample surface area of 20×0.64 mm2. Each group contained 5 samples.
The scanning rate was 1mV/s, starting from -1mV/SCE to remove the surface film heterogeneity. In order to evaluate the repassivation ability, cyclic potentiodynamic polarization measurements were taken between -1000 and +1000mV.
2.4. SEM surface morphology
The surface morphologies of sample was observed using environmental scanning-electron microscopy (SEM, ZEISS EVO18, Jena, Germany) equipped with energy dispersive spectrometer (EDS) analyzer (INCA-X-Max, England).
3. Results and discussion
3.1. Microstructure of the weldment
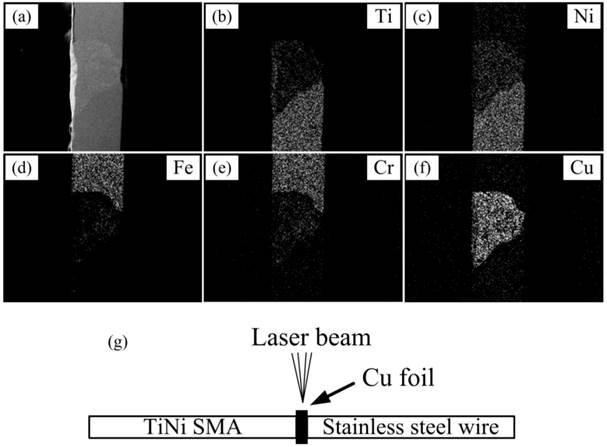
Figure 1 shows the SEM morphologies of the welding zone between the dissimilar materials. The surface of the welding zone was smooth and complete, free of any apparent pores or other defects. According to the EDS analysis, the welding zone shows a heterogeneous composition and Cu element distributes homogeneously. From SS to TiNi side, the concentrations of Fe and Cr decreased and the concentrations of Ti and Ni tended to increase.
3.2. Potentiodynamic polarization measurement
The potentiodynamic polarization behavior of CoAW is shown in Figure 2(a), and the detailed electrochemical parameters are listed in Table 1. The slope represented the oxygen consumption had a vertical stage in the cathodic section.[14,15] For the anodic polarization section, CoAW exhibited a typical passive region up to the pitting potential(Epit). The passive region of 0.05% protein AS was much larger, but that of 0.1% protein AS was nearly the same as non-protein AS. The corrosion potential (Ecorr) of 0.1% protein AS was similar as non-protein AS, but that of 0.05% protein AS was much lower. Epit represents a conservative measure of anodic pitting tendency because it gives the minimum potential below which pitting cannot be sustained. Epit in protein solutions were lower than that in non-protein solution, which means more easily tendency to corrosion.[16] The corrosion current densities of samples were all similar.
The pitting potential (Epit), corrosion potential (Ecorr) with respect to SCE and corrosion current density (Icorr) extracted from potentiodynamic polarization curve.
| Solution type | Epit(mV) | Ecorr(mV) | Icorr(μA/cm2) |
|---|---|---|---|
| Artificial saliva | 240(±19) | -765(±24) | 1.02(±0.11) |
| 0.05% Protein | 130(±16) | -855(±21) | 0.98(±0.07) |
| 0.1% Protein | 131(±18) | -768(±19) | 1.10(±0.09) |
SEM surface morphologies of the laser-welded CoAW (a-f) and schematic diagram of laser welding (g).
(a) Standard and (b) cyclic polarization curves for CoAW in different solutions.
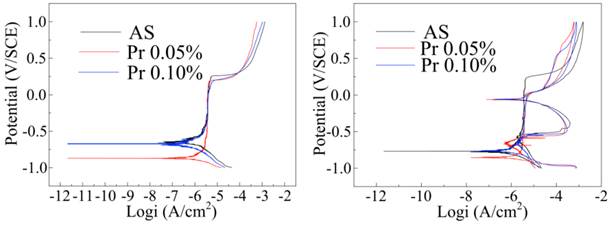
Through the potentiodynamic polarization curve, Ecorr decrease in 0.5% protein AS indicated that corrosion resistance decreased owing to a small amount of protein adding. However, Ecorr in 0.1% protein AS increased showed that high levels of protein caused corrosion resistance increased. In non protein AS, the calcium and phosphorus deposited on the surface could protect CoAW from further corrosion. Protein has an antagonism against the components in the protection film.[17,18] However, low concentration of protein is not enough to compensate the loss of protection film. As the increase of the concentration, the corrosion resistance from protein film is equal to the loss of protection film.
3.3. Cyclic potentiodynamic polarization measurement
Figure 2(b) presents typical cyclic polarization curves of CoAW in different AS solutions. The hysteresis loops occurred in the reverse anodic scan, but the loops in protein-containing AS were much smaller.
The hysteresis loops, with the Eprot above the corresponding Ecorr indicate that the specimens are capable of repassivation when the passive film is damaged.[19,20] The smaller Eprot and Ecorr distance in protein-containing AS indicates inferior protective film generated than simple AS. The reason is that negatively charged BSA deposited on the sample surface against the protection film loss as above discussed. BSA is a simple globulin, which can combine a variety of anionic, cationic and small molecules. Thus BSA could build a pellicle layer which can serve as a diffusion barrier for ionic components and reduce susceptibility to pitting attack. In vitro, proteins have been reported to increase corrosion for SS and pure metals.[21,22] Metal-protein surface complex exposure to human plasma fibronectin built by Endo could reduce the corrosion rate of Nitinol. However, it was only carried in chloric solution or cell culture medium, not in AS like this study.[23] Findings in this study further testify that protein could improve corrosion resistance in some cases.
3.4 Static corrosion test
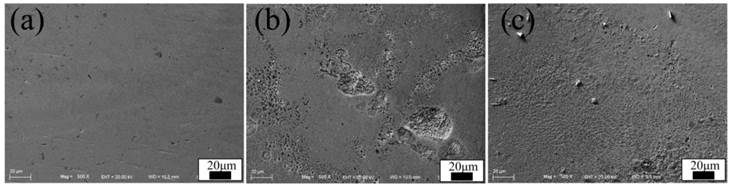
Figure 3 shows the SEM morphologies of CoAW in different solutions. The surface of CoAW in non-protein AS was smooth and no obviously corrosion defections were observed. In 0.05% protein AS, the rougher surface indicates severer corrosion. However, with the increase of protein, the surface didn't become rougher. The results of weight loss and Cu release are listed in Table 2. Both the Cu release and weight loss reached the largest value in 0.05% protein AS.
Cu release and weight loss in different solutions.
| Solution type | Cu release(μg) | Weight loss(%) |
|---|---|---|
| Artificial saliva | 0.09(±0.004) | 0.08(±0.004) |
| 0.05% Protein | 0.14(±0.008) | 0.25(±0.009) |
| 0.1% Protein | 0.10(±0.005) | 0.08(±0.003) |
The corrosion resistance of CoAW originates from the self-healing capability of passive oxide film which forms spontaneously on the surface. The passive film could rupture due to H+ ions penetrate through diffusion causing brittle regions and cracks.[24] Under normal circumstances, Cu could form a protective oxide film to prevent further corrosion. The outermost corrosion surface is mainly composed of Cu and O. Cu release was the result of exposed active metal surface, metal-ion transport and hydrolysis of the oxides.[18]
In non-protein AS, calcium and other small molecules could deposit on the oxide film, forming the barrier against further corrosion. The protein molecules deposited on the interlayer could compete with O molecule to combine Cu in protein-containing AS. But its combination product is not as stable as an oxygen-containing oxide film, leading to further destruction. Therefore, rougher surfaces were observed in 0.05% protein solution, together with more release of Cu ions and weight loss. However, with the protein concentration increase, the thickness of protein oxidation film is thickened causing corrosion resistance enhanced. The corrosion pits in 0.1% protein solution are not as much and deep as 0.05% group. For base-metal alloys, elemental release during corrosion can be affected in the presence of proteins.[25] Protein could aggravated corrosion and promoted the precipitation of harmful ions in a short period of time, but the long-term impact remains to be further studied.
For clinical applications, orthodontic arch wires confront dynamic conditions, which possibly damage the passive film causing metal surface react with oral tissue. To ensure the stable properties of CoAW, the effect of protein on corrosion behavior must be understand.[26] The results from current study are important because they reflect corrosion mechanism of CoAW and related-alloy, and provide insights into the role of proteins in corrosion process.
4. Conclusions
Under the different experimental conditions of this study, the following conclusions were drawn:
Low concentration of protein caused the corrosion resistance of CoAW samples decreased in electrochemical corrosion and immersion corrosion tests. High concentration of protein could reduce this effect.
SEM surface morphologies of CoAW immersed in artificial saliva with different protein concentrations: (a-c) 0, 0.05%, 0.1%.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Brantley WA. Orthodontic materials: scientific and clinical aspects. New York: Thieme. 2001
2. Kusy RP. A review of contemporary archwires: their properties and characteristics. Angle Orthod. 1997;67:197-207
3. Thompson SA. An overview of nickel-titanium alloys used in den-tistry. Int Endod J. 2000;33:297-310
4. Sun DQ, Li HM. A new method of TiNi shape memory alloy and austenitic stainless steel different Kind of material connection. CN Patent. 2011 CNIO215201708
5. Upadhyay D. Corrosion of alloys used in dentistry: A review. Mate Sci Eng A. 2006;432:1-11
6. Schiff N, Grosgogeat B, Michele L, Dalard F. Influence of fluoride content and pH on the corrosion resistance of titanium and its alloys. Biomaterials. 2002;23:1995-2002
7. Kwon YH, Cho HS, Noh DJ, Kim HI, Kim HK. Evaluation of the effect of fluoride-containing acetic acid on NiTi wires. J Biomed Mater. Res Part B: Appl Biomater. 2005;72:102-108
8. Lee A, Alberktsson T. The effect of serum proteins on corrosion rate in vitro. Clinical Applications of Biomaterials. New York: John Wiley & Sons. 1982
9. Brown S, Merritt K. Fretting corrosion in saline and serum. J Biomed Mater Res. 1981;15:479
10. Castner DG, Huber AE, Ratner BD. X-ray photoelectron spec-troscopy characterization of protein interactions with Co, Mo, and Ti, Trans. Soc Biomater. 1994;17:65-69
11. Goehlich V, Marek M. Corrosion behavior of Pd-Cu and Pd-Co alloys in synthetic saliva. Dent Mater. 1990;6:103-110
12. Williams DF, Skill IN, Smith R. Protein adsorption and desorption phenomena on clean metal surfaces. J Biomed Mater Res. 1985;19:313-320
13. Ahn HS, Kim MJ, Seol HJ, Lee JH, Kim Hl, Kwon YH. Effect of pH and temperature on orthodontic NiTi wires immersed in acidic fluoride solution. J Biomed Mater Res Part B: Appl Biomater. 2006;79(1):7-15
14. Ralph MD, DeBold T, Johnson MJ. Corrosion of stainless steel. OH, USA: Materials Park. 1987
15. Giacomelli FC, Giacomelli C, de Oliveira AG, Spinelli A. Effect of electrolytic ZrO2 coatings on the breakdown potential of NiTi wires used as endovascular implants. Mater Lett. 2005;59:754-758
16. Chiu KY, Cheng CF, Man HC. Corrosion behavior of AISI 316L stainless steel surface-modified with NiTi. Surf Coat Technol. 2006;200:6054-6061
17. Carroll WM, Kelly MJ. Corrosion behavior of Nitinol wires in body fluid environment. J Biomed Mater Res. 2003;67:1123-1130
18. Wever DJ, Veldhuizen AG. et al. Electrochemical and surface characterization of a nick-el-titanium alloy. Biomaterials. 1998;19:761-769
19. Rondelli G, Vicentini B. Localized corrosion behaviour in simulated human body fluids of commercial Ni-Ti orthodontic wires. Biomaterials. 1999;20:785-792
20. Anderko A, Sridhar N, Dunn DS. A general model for the re-passivation potential as a function of multiple aqueous solution species. Corros Sci. 2004;46:1583-1612
21. Zabel DD, Brown SA, Merritt K, Payer JH. AES (auger electron spectroscopy) of stainless steel corroded in saline, in serum and in vivo. J Biomed Mater Res. 1988;22:31-44
22. Clark CF, Williams DF. The effects of proteins on metallic corrosion. J Biomed Mater Res. 1982;16:125-134
23. Endo K. Chemical modification of metallic implant surface with biofunctional proteins, Molecular structure and biological activity of a modified NiTi alloy surface. Dent Mater J. 1995;14:185-188
24. Kruger J. Fundamental aspects of the corrosion of metallic implants. ASTM STP. 1979;684:107-127
25. Brown SA, Farnsworth LJ, Merritt K, Crowe TD. In vitro and in vivo metal ion release. J Biomed Mater Res. 1988;22:321-328
26. Zhang C, Sun XH. Susceptibility to stress corrosion of la-ser-welded composite arch wire in acid artificial saliva. Advances in Materials Science and Engineering. 2013;2013:738954
Author contact
![]() Corresponding author: Tel: +86 13756062697. E-mail: zc11jlu.edu.cn
Corresponding author: Tel: +86 13756062697. E-mail: zc11jlu.edu.cn

 Global reach, higher impact
Global reach, higher impact