3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2013; 10(6):691-698. doi:10.7150/ijms.6170 This issue Cite
Research Paper
Wnt10b Promotes Differentiation of Mouse Hair Follicle Melanocytes
1. Bioengineering College, Chongqing University, Chongqing 400044, PR China;
2. Department of Cell Biology, Third Military Medical University, Chongqing 400038, PR China;
3. Department of Genetics, Institute of Basic Medicine, Medical University of Chongqing, Chongqing 400016, PR China.
Received 2013-2-28; Accepted 2013-3-20; Published 2013-4-5
Abstract
Previous research has revealed that Wnt10b activates canonical Wnt signaling, which is integral to melanocyte differentiation in hair follicles (HFs). However, the function of Wnt10b in HF melanocytes remains poorly understood. We determined using Dct-LacZ transgenic mice that Wnt10b is mainly expressed near and within melanocytes of the hair bulbs during the anagen stage of the hair cycle. We also found that Wnt10b promotes an increase in melanocyte maturation and pigmentation in the hair bulbs of the mouse HF. To further explore the potential functions of Wnt10b in mouse HF melanocytes, we infected iMC23 cells with Ad-Wnt10b to overexpress Wnt10b. We demonstrated that Wnt10b promotes the differentiation of melanocytes by activating canonical Wnt signaling in melanocytes.
Keywords: Wnt10b, Wnt/β-catenin signaling, Hair follicle, Melanocyte, Differentiation.
Introduction
Melanocytes are melanin-producing cells that play important roles in synthesizing melanin for pigmentation and protecting individuals from harmful UV rays [1-4]. Melanocytes originate from the neural crest and migrate through the dermis to the epidermis, finally settling in the hair follicle (HF) [5-7]. Melanocyte stem cells (McSCs) are undifferentiated and unpigmented melanocytes that reside in the bulge-subbulge area and provide the cellular source for mature pigment-producing melanocytes in the hair matrix [8-10]. The McSCs are activated at the beginning of the anagen stage of the hair follicle cycle and replenish the hair matrix melanocytes, which undergo apoptosis at the catagen stage of the hair follicle cycle and are re-activated upon initiation of the anagen phase. McSCs in the hair follicle also possess a cyclic behavior that is related to hair regeneration cycles [11, 12].
Wnt/β-catenin signaling plays a crucial role in melanocyte development. Defects in Wnt1, Wnt3a or β-catenin expression inhibit melanocyte formation from the neural crest [13, 14]. Consistent with this observation, inhibition of the Wnt pathway decreases pigment cells in zebrafish [15], and Wnt signaling promotes the differentiation of neural crest cells to melanocytes in mouse [16]. The relationship between Wnt/β-catenin signaling and microphthalmia-associated transcription factor (MITF) is a critical feature in the development and pigmentation of melanocytes [17-20]. Previous work has revealed that β-catenin reduces the dendricity of melanocytes by regulation of Rho family GTPases and mitogen-activated protein kinase (MAPK) pathways [21]. However, the specific Wnt proteins that are involved in melanocyte pigmentation in adult hair follicles remain poorly defined. To date, approximately 19 unique Wnt ligands have been identified. Wnt10b is a ligand that activates canonical Wnt/β-catenin signaling [22]. Canonical Wnt/β-catenin signaling leads to the stabilization and accumulation of β-catenin, which translocates into the nucleus and activates transcription of specific targets. Previously, it has been reported that Wnt10b is related to hair follicle cycling during which it likely plays a role in the cyclic behavior of melanocytes in the hair follicle. Piul Rabbani et al. have demonstrated that expression of Wnt10b in keratinocytes activates Wnt signaling in neighboring melanocytes and that activation of Wnt/β-catenin signaling induces McSC differentiation [23].
These data revealed that Wnt10b activates canonical Wnt signaling, which is a critical signaling pathway in HF melanocytes. However, the role of Wnt10b in HF melanocytes remains poorly defined. In this study, we detected the expression of Wnt10b in HF melanocytes and investigated the activity of Wnt10b in mouse melanocyte cells.
Materials and Methods
Mice
Dct-LacZ transgenic mice were kindly provided by Prof. Ian Jackson [24]. The colony was backcrossed with C57BL/6J mice that were obtained from the Laboratory Animal Center of the Third Military Medical University, Chongqing, China. All of the protocols that involved mice were approved by the medical ethics committee of the Third Military Medical University.
X-gal staining and immunohistochemistry
The acquired skins were fixed in 4% paraformaldehyde at 4℃ for 1 h, then stained with X-gal staining solution (Beyotime, China) at room temperature for 24 h, and post-fixed overnight in 4% paraformaldehyde at 4℃. After sequential dehydration in ethanol and xylene, the skins were embedded in paraffin and sliced into 5 μm sections for immunohistochemistry. Sections were then deparaffinized, rehydrated and microwaved for antigen retrieval. After blocking endogenous peroxidase activity and non-specific biotin binding, sections were incubated with rabbit anti-Wnt10b antibody (Sigma, USA) at 4℃ overnight, and signals were detected using appropriate secondary antibodies that were conjugated with DAB (Zhongshan Golden bridge, China). After gradual dehydration, the sections were mounted with neutral gum (Zhongshan Golden bridge) and observed using a microscope.
Isolation of total RNA and RT-PCR
We harvested total RNA from mouse dorsal skin samples in different phases using Trizol (Invitrogen, USA), and we reverse transcribed the total RNA with ReverTra Ace reverse transcriptase (TOYOBO, Japan) for cDNA synthesis. Semi-quantitative PCR was performed using primers for Wnt10b and GAPDH [25]. The primers for Wnt10b were 5'-CTGCGGATGGAAGGGTAG-3' and 5'-GGGACTGAGCCAGGAACA-3'. The primers for GAPDH were 5'-ACCACAGTCCATGCCATCAC-3' and 5'-TCCACCACCCTGTTGCTGTA-3'.
Hematoxylin and eosin staining
Skin samples were first fixed in 4% paraformaldehyde at 4℃ overnight. After sequential dehydration in ethanol and xylene, the skin was embedded in paraffin, deparaffinized and rehydrated. The sections were subsequently stained with hematoxylin (Zhongshan Golden bridge, Beijing, China) for 1 minute then rinsed with water. In a second step, the sections were stained with eosin (Zhongshan Golden bridge) for 30 seconds and rinsed with water. After gradual dehydration, the sections were mounted with neutral gum (Zhongshan Golden bridge) and observed using a microscope.
Cell and whisker follicle culture
iMC23 cells were kindly provided by Ke Yang [26]. HEK 293, JB6 and iMC23 cells were cultured in DMEM/high-glucose media (Hyclone, Shanghai, China) with 10% FBS (Gibco, USA) and incubated in an atmosphere containing 5% CO2 in air at 37℃.
We cut the vibrissa pads from the Dct-LacZ transgenic mice at P2 and isolated the whisker follicles from the vibrissa pads under a stereo microscope. The whisker follicles were plated into 48-well plates and cultured in Williams E media (Invitrogen, Shanghai, China) [27].
Tyrosinase activity assay
IMC23 cells in 6-well plates were infected with Ad-Wnt10b or Ad-GFP for 72 h, trypsinized and counted. Cells (1x105) were treated with 200 μl 1% TritonX-100/PBS at -80℃ for 30 min and thawed at 37℃. Cell extracts were then clarified by centrifugation. The supernatants (50 μl) were transferred into 96-well plates and 10 μl 2 mg/ml L-Dopa (Sigma, USA) was added to each well. After incubation for 2 h at 37℃, the absorbance at 490 nm was measured. The experiments were performed three times [25].
Western blot analysis
1. IMC23 cells were lysed with PIPA lysis buffer (Beyontime, China) for 5 min on ice. 2. Total protein was collected by centrifugation. 3. The total protein concentration of each sample was determined using the Enhanced BCA Protein Assay kit (Beyontime). 4. Proteins were denatured by boiling. 5. Protein (50 µg per lane) was loaded onto a 10% SDS-PAGE gel and transferred onto a PVDF membrane. 6. The resulting membranes were blocked with 5% fat-free milk for 1 h. 7. Membranes were probed with rabbit anti-tyrosinase antibody (1:1,000; Bioworld, USA), goat anti-TRP1 antibody (1:1,000; Santa Cruz Biotechnology, Inc., USA), or rabbit anti-β-catenin antibody (1:1,000; Abcam, USA) at 4˚C overnight. 8. Membranes were incubated with HRP-conjugated secondary antibody (1:1,000; Beyotime, China) for 1 h. 9. Bands were visualized on the membranes using an ECL western blotting detection system and X-ray film.
Top-Luc analysis
iMC23 cells were seeded into 25 cm2 flasks and transfected with 2 μg DNA per flask of a Tcf4/Lef1 reporter plasmid. At 16 h after transfection, cells were replated onto 24-well plates and infected with Ad-GFP or Ad-Wnt10b 4 h after replating. At 36 h after infection, the cells were lysed, and lysates were harvested for luciferase assays with Promega's Luciferase Assay Kit (Promega, Madison, WI, USA). Each assay condition was performed in triplicate [28].
Statistical analysis
Data were presented as the means +SD from three independent experiments. All statistical analyses were performed using a t-test, and p values < 0.05 were considered to be statistically significant.
Results
Wnt10b expression near melanocytes during the hair cycle
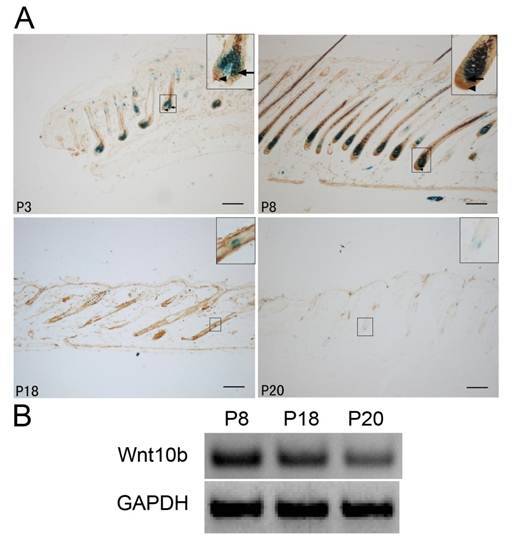
We used Dct-LacZ transgenic mice and X-gal staining to identify LacZ-positive melanocytes in HFs of dorsal skin sections (Fig. 1A).
In the anagen stage (P3 and P8), Wnt10b was expressed mainly in cells localized to hair bulbs. In the catagen stage (P18), Wnt10b expression gradually decreased. In the telogen stage (P20), only a low level of Wnt10b expression was detected in the hair follicle.
Notably, in the anagen stage (P3 and P8), Wnt10b was mainly expressed in the vicinity of melanocytes in the hair bulbs. A low level of Wnt10b expression was also detected in the melanocytes themselves (Fig. 1A).
RT-PCR of Wnt10b mRNA in the dorsal skin of Dct-LacZ transgenic mice also indicated changes in Wnt10b expression during the hair cycle (Fig. 1B).
Expression of Wnt10b in hair follicles in various phases: (A) LacZ-positive melanocytes and Wnt10b-positive cells are visible at the same time in dorsal skin sections from Dct-LacZ transgenic mice at various stages. In the anagen stage (P3 and P8), Wnt10b was detected in some hair bulb melanocytes (arrows), but more Wnt10b was detected in the vicinity of melanocytes in the hair bulbs (arrowheads). At P8 (the anagen stage), Wnt10b expression increased significantly, while at P20 (the telogen stage) only a low level of Wnt10b protein was detected. Similar results were observed for LacZ levels. (B) RT-PCR was used to detect the expression of Wnt10b mRNA in the dorsal skin of Dct-LacZ transgenic mice. Scale bars represent 100 μm.
Wnt10b induces melanogenesis in the dorsal skin or whisker follicles
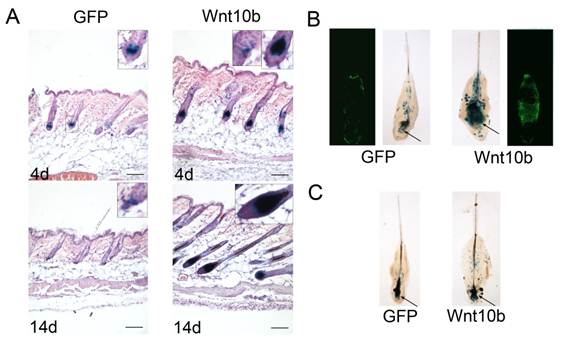
To investigate what influences Wnt10b in melanocytes, we treated the Dct-LacZ transgenic mice with intradermal injections of Ad-Wnt10b and an Ad-GFP vehicle control in the dorsal skin at the telogen stage. Following the injections (4 d or 14 d), we stained sections of the dorsal skin with X-gal and eosin-hematoxylin. LacZ-positive melanocytes were detected at higher levels in the Ad-Wnt10b-treated follicles relative to the Ad-GFP-treated follicles at 4 d and 14 d post injection. We also found that more pigmentation was present in the Ad-Wnt10b-treated follicles compared with the Ad-GFP-treated follicles at both time points (Fig. 2A).
Whisker follicles from the Dct-LacZ transgenic mice were also treated with Ad-Wnt10b, the Ad-GFP vehicle control (Fig. 2B) or with GFP or Wnt10b protein that was harvested from JB6 cells that were treated with Ad-GFP and Ad-Wnt10b (Fig. 2C). Whole whisker follicles were stained with X-gal. Similar to the results for the HFs, LacZ-positive melanocytes were detected at higher levels in the Ad-Wnt10b-treated follicles relative to the Ad-GFP-treated follicles both through these two ways.
In conclusion, the results suggest that Wnt10b is capable of promoting melanogenesis in the dorsal skin or the whisker follicles.
Wnt10b promotes the differentiation of melanocytes in iMC23 cells
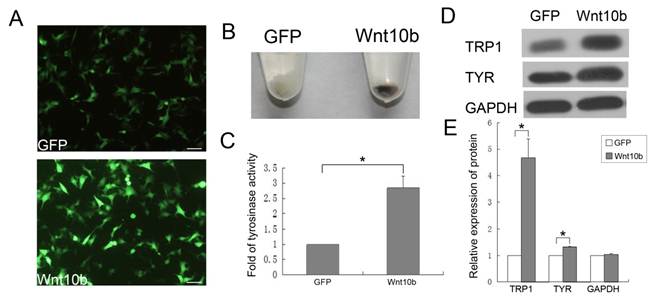
To prove that Wnt10b promotes the differentiation of melanocytes, we employed a mouse melanocyte cell line, iMC23 [26], as an in vitro cell model. IMC23 cells were infected with an Ad-GFP vehicle control or Ad-Wnt10b (Fig. 3A).
IMC23 cells that were infected with Ad-GFP or Ad-Wnt10b were harvested after 72 h. Ad-Wnt10b-infected cells displayed increased pigmentation relative to the vehicle control (Fig. 3B). This result indicates that Wnt10b promotes melanogenesis of melanocytes in vitro. Because the iMC23 cells are immature melanocytes, they are unable to synthesize melanin. However, infection with Ad-Wnt10b enabled melanin synthesis by these cells, so we infer that Wnt10b promotes iMC23 cell differentiation into mature melanocytes.
We also detected tyrosinase activity of the iMC23 cells that were infected with the Ad-GFP vehicle control or Ad-Wnt10b for 72 h (Fig. 3C). Tyrosinase is a crucial regulatory enzyme that is uniquely expressed in melanocytes and catalyzes the rate-limiting initial events in melanin synthesis. Therefore, tyrosinase activity is a marker for pigmentation and melanocyte maturation [25]. An obvious increase in tyrosinase activity was detected in the Ad-Wnt10b-infected iMC23 cells compared with the Ad-GFP-infected cells. These results provide additional evidence that Wnt10b promotes iMC23 differentiation into mature melanocytes.
Wnt10b increases the number of melanocytes: (A) Dorsal skin sections from Dct-LacZ transgenic mice were stained with X-gal and eosin-hematoxylin. Staining was performed after injecting Ad-GFP or Ad-Wnt10b into the dorsal skin and waiting 4 or 14 d. LacZ-positive melanocytes were detected at higher levels in the Ad-Wnt10b-treated follicles compared with the Ad-GFP-treated follicles at both time points. (B) Whole-mount X-gal staining of whisker follicles that were acquired from Dct-LacZ transgenic mice at P2 and treated with Ad-GFP or Ad-Wnt10b for. LacZ-positive mature melanocytes were detected at higher levels in the hair bulb of the Ad-Wnt10b-treated follicles relative to the Ad-GFP-treated follicles (arrows). (C) Whole-mount X-gal staining of whisker follicles that were acquired from Dct-LacZ transgenic mice at P2 and treated with GFP or Wnt10b protein that was harvested from JB6 cells infected with Ad-GFP or Ad-Wnt10b. LacZ-positive mature melanocytes were detected at higher levels in the hair bulb of the Wnt10b protein-treated follicles compared with the GFP-treated follicles (arrows). Scale bars represent 100 μm.
To further confirm the role of Wnt10b in melanocyte differentiation, we measured the levels of a differentiation-related protein (TRP1, tyrosinase) [9, 29-31] and GAPDH in iMC23 cells that were infected with Ad-GFP or Ad-Wnt10b by quantitative western blots (Fig. 3D, E). The blots revealed that TRP1 levels increased significantly in the Ad-Wnt10b-infected iMC23 cells compared with the Ad-GFP-infected cells. Tyrosinase exhibited less of an increase in the Ad-Wnt10b-infected cells compared with the Ad-GFP-infected cells.
These data provide compelling evidence that Wnt10b promotes the differentiation of melanocytes.
Wnt10b promotes the differentiation of melanocytes by activating canonical Wnt signaling in melanocytes
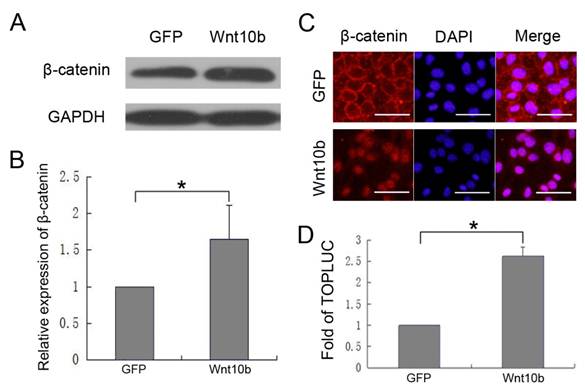
To investigate whether Wnt10b promotes melanocyte differentiation by activating canonical Wnt signaling in melanocytes, we measured β-catenin and GAPDH levels in iMC23 cells that were infected with Ad-GFP or Ad-Wnt10b by quantitative western blot (Fig. 4A, B). The results revealed that β-catenin increased significantly in the Ad-Wnt10b-infected iMC23 cells compared with the Ad-GFP-infected cells. These results indicate that β-catenin accumulates to a higher level in the iMC23 cells that were infected with Ad-Wnt10b relative to the control cells, so Wnt10b may activate canonical Wnt signaling to enable melanocyte differentiation.To further test this hypothesis, iMC23 cells were first infected with Ad-GFP or Ad-Wnt10b for 72 h. Cells were then fixed and subjected to immunofluorescence staining with DAPI and an anti-β-catenin antibody. Ad-GFP-infected cells served as a control (Fig. 4C). β-catenin was uniquely expressed on the cytomembranes of iMC23 cells that were infected with Ad-GFP, while β-catenin was detected in the nucleus of the cells that were infected with Ad-Wnt10b. β-catenin nuclear translocation is a critical step in canonical Wnt signaling, therefore these data support the idea that Wnt10b promotes melanocyte differentiation by activating canonical Wnt signaling in melanocytes.
iMC23 cells were also transfected with a Tcf4/Lef1 reporter prior to infection with Ad-GFP or Ad-Wnt10b. At 36 hrs post infection, cells were collected and luciferase assays were performed (Fig. 4D). Tcf4/Lef1 reporter activity of the iMC23 cells that were infected with Ad-Wnt10b was notably increased compared with cells that were infected with Ad-GFP. Because the Tcf4/Lef1 construct reports on vital factors downstream of canonical Wnt signaling [32-35], these results demonstrate that Wnt10b activates canonical Wnt signaling in melanocytes.
These results demonstrate that Wnt10b promotes melanocyte differentiation by activating canonical Wnt signaling in melanocytes.
Wnt10b influences the pigmentation of iMC23 cells and enhances the melanogenesis of melanocytes in mouse hair follicles by promoting the differentiation of McSCs: (A) After infecting iMC23 cells with Ad-GFP or Ad-Wnt10b for 24 h, GFP and Wnt10b fluorescence was detected. (B) The iMC23 cells infected with Ad-GFP or Ad-Wnt10b were harvested after 72 h. (C) Fold change in tyrosinase activity of iMC23 cells that were infected with Ad-GFP or Ad-Wnt10b. (D) Western blots of a differentiation-related protein and GAPDH in iMC23 cells that were infected with Ad-GFP or Ad-Wnt10b. (E) Quantification of Western blots of a differentiation-related protein and GAPDH in iMC23 cells that were infected with Ad-GFP or Ad-Wnt10b. Scale bars represent 100 μm. Data are reported as averages +SD. These data were representative results of three independent experiments. Asterisks indicate statistical significance (P<0.05).
Wnt10b promotes melanocyte differentiation by activating canonical Wnt signaling in melanocytes: (A) Western blots of β-catenin and GAPDH in iMC23 cells that were infected with Ad-GFP or Ad-Wnt10b. (B) Quantification of Western blots of β-catenin in iMC23 cells that were infected with Ad-GFP or Ad-Wnt10b. (C) Immunofluorescence of β-catenin in iMC23 cells that were infected with Ad-GFP and Ad-Wnt10b for 72 h. (D) iMC23 cells were transfected with a Tcf4/Lef1 reporter and infected with Ad-GFP or Ad-Wnt10b. At 36 h post infection, the cells were harvested for luciferase assays. Scale bars represent 100 μm. Data are reported as averages +SD. These data were representative results of three independent experiments. Asterisks indicate statistical significance (P<0.05).
Discussion
Previous research has revealed that Wnt10b activates canonical Wnt signaling, which is closely related to melanocytes in the HF. However, the function of Wnt10b in HF melanocytes remains unknown. In this study, we detected Wnt10b expression in HF melanocytes and investigated the functions of Wnt10b in mouse melanocytes.
Although Wnt10b was previously shown to be expressed in the HF [36, 37], the function of Wnt10b in hair follicle melanocytes is poorly understood. Previous research showed that melanocytes proliferate and produce melanin during the anagen stage, undergo apoptosis in the catagen stage, and are absent throughout the telogen stage [12]. Wnt10b undergoes a dramatic up-regulation at the onset of the anagen stage [38]. In the anagen stage, we showed that the increased pigmentation of melanocytes in hair bulbs correlated with Wnt10b expression in keratinocytes around melanocytes in hair bulbs (Fig. 1). Considering that Wnt10b is a paracrine protein, it might act on melanocytes in the hair bulb following its secretion by nearby keratinocytes. Because hair bulb melanocytes are mature melanocytes, we hypothesized that Wnt10b expressed in keratinocytes around hair bulb melanocytes activates melanogenesis in hair follicles.
To examine the effects of Wnt10b on melanocytes, we treated the dorsal skin or whisker follicles of Dct-LacZ transgenic mice with Ad-Wnt10b. We found that Ad-Wnt10b addition promotes an increase in the number of mature melanocytes, as well as their pigmentation, in hair bulbs (Fig. 2). These results suggest that Wnt10b is capable of promoting melanocyte differentiation.
To further confirm the function of Wnt10b in melanocytes, we analyzed tyrosinase activity and melanin content in iMC23 cells, which are melanoblast-like cells that do not undergo melanogenesis. The results revealed that Wnt10b significantly increased tyrosinase activity and melanin synthesis and also up-regulated tyrosinase and TRP1 expression. These proteins function downstream in the melanin biosynthetic pathway, so the data provide additional evidence that Wnt10b promotes the differentiation of melanocytes.
Previous research has shown that suppression of the Wnt/β-catenin signaling pathway inhibits melanocyte pigmentation [39]. In addition, synthetic activation of Wnt/β-catenin signaling in embryonic skin is accompanied by epidermal hyperpigmentation [40]. Consistent with these results, we demonstrated that Wnt10b promotes the differentiation of melanocytes by activating canonical Wnt signaling in melanocytes (Fig. 4). Our results are consistent with previous studies of Wnt10b in canonical Wnt signaling of HFs [23, 36].
Human skin and mouse skin are different in that epidermal melanocytes cannot survive in mouse epidermis after the neonatal stage, but the epidermal melanocytes in human are derived from the amelanotic melanocytes in the ORS of human hair follicle, which show similar distribution patterns and cellular behaviors as mouse melanocyte stem cells [41]. Follicular repigmentation of vitiligo skin after narrow band UVB therapy and of adult Krt14-SCF transgenic mouse skin treated with Kit-blocking antibodies suggest that repigmentation of the skin starts from the orifices of hair follicles.
In fact, the effects of Wnt signaling on the proliferation and differentiation of human epidermal melanocytes have been shown in some papers. In human, high levels of DKK1 (an inhibitor of the canonical Wnt signaling pathway) secreted by fibroblasts in the dermis inhibits melanocyte growth, pigmentation, and melanosome transfer in keratinocytes induces a less pigmented palms skin [42, 43]. In human melanocyte and melanoma, melanogenesis stimulation by a-melanocyte-stimulating hormone (a-MSH) induces phosphorylation of β-catenin-Ser675 and stabilization of β-catenin protein, which suggested Wnt signaling could play a key role in the physiological regulation of human epidermal melanogenesis [44]. However, the exact mechanisms behind the Wnt mediated effects on melanocytes in human epidermis have not been elucidated.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81071309) and the Natural Science Foundation of Chongqing (No. cstc2012jjA0335). We thank Dr. T-C. He (Chicago University) for adenovirus production and technical assistance. We thank Prof. Ian Jackson (MRC Human Genetics Unit) for the Dct-LacZ transgenic mice.
Abbreviations
HF: hair follicle; X-gal: 5-bromo-4-chloro-3-indolyl-β-D-galactoside; McSC: melanocyte stem cell; Ad: adenovirus; MITF: microphthalmia-associated transcription factor; DCT: dopachrome tautomerase; TRP1: tyrosinase-related protein 1.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiological reviews. 2004;84:1155-228 doi:10.1152/physrev.00044.2003
2. Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ. Hair follicle pigmentation. The Journal of investigative dermatology. 2005;124:13-21 doi:10.1111/j.0022-202X.2004.23528.x
3. Yamaguchi Y, Brenner M, Hearing VJ. The regulation of skin pigmentation. The Journal of biological chemistry. 2007;282:27557-61 doi:10.1074/jbc.R700026200
4. Park HY, Kosmadaki M, Yaar M, Gilchrest BA. Cellular mechanisms regulating human melanogenesis. Cellular and molecular life sciences: CMLS. 2009;66:1493-506 doi:10.1007/s00018-009-8703-8
5. Mayer TC. The migratory pathway of neural crest cells into the skin of mouse embryos. Dev Biol. 1973;34:39-46
6. Jordan SA, Jackson IJ. MGF (KIT ligand) is a chemokinetic factor for melanoblast migration into hair follicles. Dev Biol. 2000;225:424-36 doi:10.1006/dbio.2000.9856
7. Thomas AJ, Erickson CA. The making of a melanocyte: the specification of melanoblasts from the neural crest. Pigment cell & melanoma research. 2008;21:598-610 doi:10.1111/j.1755-148X.2008.00506.x
8. Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M. et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854-60 doi:10.1038/416854a
9. Osawa M, Egawa G, Mak SS, Moriyama M, Freter R, Yonetani S. et al. Molecular characterization of melanocyte stem cells in their niche. Development. 2005;132:5589-99 doi:10.1242/dev.02161
10. Nishimura EK. Melanocyte stem cells: a melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment cell & melanoma research. 2011;24:401-10 doi:10.1111/j.1755-148X.2011.00855.x
11. Slominski A, Paus R, Plonka P, Chakraborty A, Maurer M, Pruski D. et al. Melanogenesis during the anagen-catagen-telogen transformation of the murine hair cycle. The Journal of investigative dermatology. 1994;102:862-9
12. Tobin DJ, Slominski A, Botchkarev V, Paus R. The fate of hair follicle melanocytes during the hair growth cycle. The journal of investigative dermatology Symposium proceedings / the Society for Investigative Dermatology, Inc [and] European Society for Dermatological Research. 1999;4:323-32
13. Ikeya M, Lee SM, Johnson JE, McMahon AP, Takada S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 1997;389:966-70 doi:10.1038/40146
14. Hari L, Brault V, Kleber M, Lee HY, Ille F, Leimeroth R. et al. Lineage-specific requirements of beta-catenin in neural crest development. The Journal of cell biology. 2002;159:867-80 doi:10.1083/jcb.200209039
15. Dorsky RI, Moon RT, Raible DW. Control of neural crest cell fate by the Wnt signalling pathway. Nature. 1998;396:370-3 doi:10.1038/24620
16. Dunn KJ, Williams BO, Li Y, Pavan WJ. Neural crest-directed gene transfer demonstrates Wnt1 role in melanocyte expansion and differentiation during mouse development. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10050-5
17. Steingrimsson E, Moore KJ, Lamoreux ML, Ferre-D'Amare AR, Burley SK, Zimring DC. et al. Molecular basis of mouse microphthalmia (mi) mutations helps explain their developmental and phenotypic consequences. Nature genetics. 1994;8:256-63 doi:10.1038/ng1194-256
18. Dorsky RI, Raible DW, Moon RT. Direct regulation of nacre, a zebrafish MITF homolog required for pigment cell formation, by the Wnt pathway. Genes & development. 2000;14:158-62
19. Takeda K, Yasumoto K, Takada R, Takada S, Watanabe K, Udono T. et al. Induction of melanocyte-specific microphthalmia-associated transcription factor by Wnt-3a. The Journal of biological chemistry. 2000;275:14013-6
20. Schepsky A, Bruser K, Gunnarsson GJ, Goodall J, Hallsson JH, Goding CR. et al. The microphthalmia-associated transcription factor Mitf interacts with beta-catenin to determine target gene expression. Mol Cell Biol. 2006;26:8914-27 doi:10.1128/MCB.02299-05
21. Kim JH, Sohn KC, Choi TY, Kim MY, Ando H, Choi SJ. et al. Beta-catenin regulates melanocyte dendricity through the modulation of PKCzeta and PKCdelta. Pigment cell & melanoma research. 2010;23:385-93 doi:10.1111/j.1755-148X.2010.00695.x
22. Ouji Y, Yoshikawa M, Shiroi A, Ishizaka S. Promotion of hair follicle development and trichogenesis by Wnt-10b in cultured embryonic skin and in reconstituted skin. Biochemical and biophysical research communications. 2006;345:581-7 doi:10.1016/j.bbrc.2006.04.142
23. Rabbani P, Takeo M, Chou W, Myung P, Bosenberg M, Chin L. et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145:941-55 doi:10.1016/j.cell.2011.05.004
24. Mackenzie MA, Jordan SA, Budd PS, Jackson IJ. Activation of the receptor tyrosine kinase Kit is required for the proliferation of melanoblasts in the mouse embryo. Dev Biol. 1997;192:99-107 doi:10.1006/dbio.1997.8738
25. Guo H, Yang K, Deng F, Ye J, Xing Y, Li Y. et al. Wnt3a promotes melanin synthesis of mouse hair follicle melanocytes. Biochemical and biophysical research communications. 2012;420:799-804 doi:10.1016/j.bbrc.2012.03.077
26. Yang K, Chen J, Jiang W, Huang E, Cui J, Kim SH. et al. Conditional immortalization establishes a repertoire of mouse melanocyte progenitors with distinct melanogenic differentiation potential. The Journal of investigative dermatology. 2012;132:2479-83 doi:10.1038/jid.2012.145
27. Lu Z, Hasse S, Bodo E, Rose C, Funk W, Paus R. Towards the development of a simplified long-term organ culture method for human scalp skin and its appendages under serum-free conditions. Experimental dermatology. 2007;16:37-44 doi:10.1111/j.1600-0625.2006.00510.x
28. Tang N, Song WX, Luo J, Luo X, Chen J, Sharff KA. et al. BMP-9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signalling. Journal of cellular and molecular medicine. 2009;13:2448-64 doi:10.1111/j.1582-4934.2008.00569.x
29. Shibahara S, Yasumoto K, Amae S, Udono T, Watanabe K, Saito H. et al. Regulation of pigment cell-specific gene expression by MITF. Pigment cell research / sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society. 2000;13(Suppl 8):98-102
30. Steingrimsson E, Copeland NG, Jenkins NA. Melanocytes and the microphthalmia transcription factor network. Annual review of genetics. 2004;38:365-411 doi:10.1146/annurev.genet.38.072902.092717
31. Zhao H, Eling DJ, Medrano EE, Boissy RE. Retroviral infection with human tyrosinase-related protein-1 (TRP-1) cDNA upregulates tyrosinase activity and melanin synthesis in a TRP-1-deficient melanoma cell line. The Journal of investigative dermatology. 1996;106:744-52
32. Huelsken J, Behrens J. The Wnt signalling pathway. Journal of cell science. 2002;115:3977-8
33. Katoh M, Katoh M. WNT signaling pathway and stem cell signaling network. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:4042-5 doi:10.1158/1078-0432.CCR-06-2316
34. MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Developmental cell. 2009;17:9-26 doi:10.1016/j.devcel.2009.06.016
35. De Sousa EMF, Medema JP. Axing Wnt signals. Cell research. 2012;22:9-11 doi:10.1038/cr.2011.141
36. Li YH, Zhang K, Ye JX, Lian XH, Yang T. Wnt10b promotes growth of hair follicles via a canonical Wnt signalling pathway. Clinical and experimental dermatology. 2011;36:534-40 doi:10.1111/j.1365-2230.2011.04019.x
37. Li YH, Zhang K, Yang K, Ye JX, Xing YZ, Guo HY. et al. Adenovirus-Mediated Wnt10b Overexpression Induces Hair Follicle Regeneration. The Journal of investigative dermatology. 2012 doi:10.1038/jid.2012.235
38. Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE. et al. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mechanisms of development. 2001;107:69-82
39. Cho M, Ryu M, Jeong Y, Chung YH, Kim DE, Cho HS. et al. Cardamonin suppresses melanogenesis by inhibition of Wnt/beta-catenin signaling. Biochemical and biophysical research communications. 2009;390:500-5 doi:10.1016/j.bbrc.2009.09.124
40. Zhang Y, Andl T, Yang SH, Teta M, Liu F, Seykora JT. et al. Activation of beta-catenin signaling programs embryonic epidermis to hair follicle fate. Development. 2008;135:2161-72 doi:10.1242/dev.017459
41. Commo S, Bernard BA. Melanocyte subpopulation turnover during the human hair cycle: an immunohistochemical study. Pigment cell research / sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society. 2000;13:253-9
42. Yamaguchi Y, Itami S, Watabe H, Yasumoto K, Abdel-Malek ZA, Kubo T. et al. Mesenchymal-epithelial interactions in the skin: increased expression of dickkopf1 by palmoplantar fibroblasts inhibits melanocyte growth and differentiation. The Journal of cell biology. 2004;165:275-85 doi:10.1083/jcb.200311122
43. Yamaguchi Y, Passeron T, Hoashi T, Watabe H, Rouzaud F, Yasumoto K. et al. Dickkopf 1 (DKK1) regulates skin pigmentation and thickness by affecting Wnt/beta-catenin signaling in keratinocytes. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22:1009-20 doi:10.1096/fj.07-9475com
44. Bellei B, Pitisci A, Catricala C, Larue L, Picardo M. Wnt/beta-catenin signaling is stimulated by alpha-melanocyte-stimulating hormone in melanoma and melanocyte cells: implication in cell differentiation. Pigment cell & melanoma research. 2011;24:309-25 doi:10.1111/j.1755-148X.2010.00800.x
Author contact
![]() Corresponding author: yanglibiocom (L.Y.); yangke0210com (K.Y.); Tel.: +86-130-683-129-97 (L.Y.); +86-139-837-742-10 (K.Y.).
Corresponding author: yanglibiocom (L.Y.); yangke0210com (K.Y.); Tel.: +86-130-683-129-97 (L.Y.); +86-139-837-742-10 (K.Y.).

 Global reach, higher impact
Global reach, higher impact