3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2012; 9(8):655-664. doi:10.7150/ijms.5004 This issue Cite
Research Paper
Intracranial Pressure Response to Non-Penetrating Ballistic Impact: An Experimental Study Using a Pig Physical Head Model and Live Pigs
State Key Laboratory of Trauma, Burns and Combined Injury, Research Institute of Surgery, Daping Hospital, Third Military Medical University, Chongqing 400042, China.
* Equal study contribution.
Received 2012-8-9; Accepted 2012-9-6; Published 2012-9-24
Abstract
This study was conducted to characterize the intracranial pressure response to non-penetrating ballistic impact using a "scalp-skull-brain" pig physical head model and live pigs. Forty-eight ballistic tests targeting the physical head model and anesthetized pigs protected by aramid plates were conducted with standard 9 mm bullets at low (279-297 m/s), moderate (350-372 m/s), and high (409-436 m/s) velocities. Intracranial pressure responses were recorded with pressure sensors embedded in similar brain locations in the physical head model and the anesthetized pigs. Three parameters of intracranial pressure were determined from the measured data: intracranial maximum pressure (Pmax), intracranial maximum pressure impulse (PImax), and the duration of the first positive phase (PPD). The intracranial pressure waves exhibited blast-like characteristics for both the physical model and l live pigs. Of all three parameters, Pmax is most sensitive to impact velocity, with means of 126 kPa (219 kPa), 178 kPa (474 kPa), and 241 kPa (751 kPa) for the physical model (live pigs) for low, moderate, and high impact velocities, respectively. The mean PPD becomes increasingly short as the impact velocity increases, whereas PImax shows the opposite trend. Although the pressure parameters of the physical model were much lower than those of the live pigs, good correlations between the physical model and the live pigs for the three pressure parameters, especially Pmax, were found using linear regression. This investigation suggests that Pmax is a preferred parameter for predicting the severity of the brain injury resulting from behind armor blunt trauma (BABT).
Keywords: Intracranial pressure response, Non-penetrating ballistic impact, Pig physical head model, Live pigs
Introduction
Although ballistic helmets can prevent the penetration of ballistic projectiles, bullets, or shrapnel into the head, damage to the head caused by non-penetrating ballistic impact resulting from rapid deformation of the helmet is still difficult to prevent completely [1,2]. Non-penetrating injuries to the head behind a protective covering, which are included in behind armor blunt trauma (BABT), have not been researched as extensively as thoracic BABT [3]. Nonetheless, the BABT head injury pattern has been observed on human cadaver heads and dry skulls protected by polyethylene or aluminum plates when impacted by a non-penetrating 9 mm round [4,5]. As revealed by Sarron's reports, non-penetrating ballistic impact to the head while wearing protection resulted in skin lacerations, extensive skull fractures and brain damage, which were associated with increased pressure in the brain. This evidence suggested that intracranial pressure waves are the likely mechanism behind such injuries. However, the intracranial pressure response of brain tissue to non-penetrating ballistic impact resulting from BABT has been poorly documented to date.
The aim of this study was to characterize intracranial pressure on brain tissue during non-penetrating ballistic impact to provide a better understanding of the biomechanics of BABT of the head. A physical model of the pig head and live pigs were used as objects of ballistic tests to acquire pressure data. Three characteristic parameters of the pressure wave were considered: intracranial maximum pressure, intracranial maximum pressure impulse, and the duration of the first positive phase. The sensitivity of pressure parameters to impact velocity was examined. Moreover, the intracranial pressure in the physical head model was compared with that in the live pigs.
Materials and Methods
Preparation of the pig physical head model and experimental animals
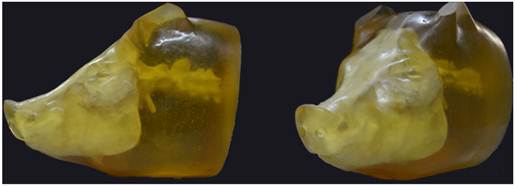
A "scalp-skull-brain" pig physical head model was developed to measure the intracranial pressure response to non-penetrating ballistic impact (Fig. 1). The pig physical head was molded from the approximate geometry of a white pig weighing 45.36 kg, which was provided by the Experimental Animal Center, Daping Hospital, Third Military Medical University (Chongqing, China). The scalp was made of polyurethane elastomer, while the brain was produced with silicone gel. Thermosetting resin mixed with calcium phosphate and fiberglass was used as the skull material.
Twenty-four white pigs weighing 43 ± 3 kg were divided randomly into three groups (n=8) for intracranial pressure tests for ballistic impacts at low, moderate, and high velocities. Animals were anaesthetized by transvenous injection of pentobarbital sodium (40 mg/kg) at the beginning of the experiment. All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the Experimental Animal Center, Daping Hospital, Third Military Medical University.
The pig physical head model.
Ballistic impact
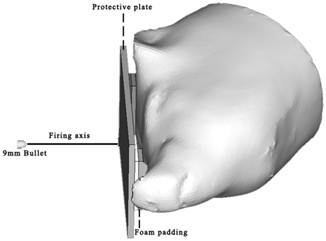
Aramid plates (200 × 150 × 9 mm) were used to represent helmet protection and induce BABT in a pig physical head model and living pigs. To simulate the realistic space between the head and a ballistic helmet, a 12 mm gap between the protective plate and the subject was created by placing two pieces of foam padding, which is used specifically for helmets, on the nasion and the occiput of each subject. The nominal target location on each test specimen was the midpoint on the line drawn laterally between the eyes. The subject with ballistic protection was held in a lateral position, ensuring that the firing axis was perpendicular to the protective plate (Fig. 2). The ammunitions used for ballistic impact were 9 mm bullets at low, moderate, and high velocities of approximately 280 m/s, 360 m/s, and 420 m/s, respectively. These velocities were selected from preliminary experiments to provide varying injury risks with no chance of penetrating the protective plates. Each shot aimed at the point on the protective plate that was aligned with the nominal impact location on the subject. The ballistic impacts were conducted eight times at each velocity (low, moderate, and high) on both the physical head model and the live pigs (each live animal was impacted only once). Each plate was used only once in all tests.
Schematic representation of the ballistic impact scenario for both the pig physical head model and live pigs.
Recording of intracranial pressure
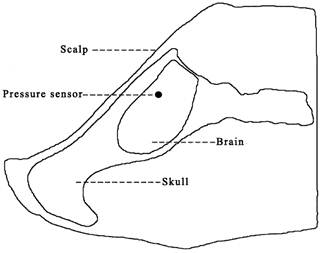
A piezoresistive pressure sensor (EZM-093-500(sg), Saiying Electronics, Anhui, China) was installed inside the brain of each test specimen both in the physical head model and in the anesthetized pigs (Fig. 3). The sensor was inserted into the brain parenchyma through a occipital approach and positioned approximately 12 mm under the parietal bone, facing the point of impact. The location of the sensor was controlled by a scheduled operation procedure performed by one person to ensure as much uniformity as possible among individual subjects. Signals were acquired using a TST6150 numerical recording system (Test Electronics, Chengdu, China) and analyzed with DAP6.01. The data were used to calculate the maximum value of the first positive phase of the post-impact curve of intracranial pressure versus time (Pmax), the duration of the first positive phase (PPD), and the integral of the intracranial pressure curve over the duration of PPD (PImax).
Data analysis
Statistical analyses of intracranial pressure parameters were performed using SPSS 17.0 statistical software. One-way analysis of variance (ANOVA) was conducted on Pmax, PImax, and PPD for comparison between different impact velocities. A probability value of <0.05 was considered significant. In addition, linear regressions were performed to determine not only the sensitivity of intracranial pressure parameters to ballistic impact velocity but also the correlation of all three pressure parameters between the physical head model and the live pigs.
Illustration of the location of the pressure sensor in the brain of a test specimen.
Results
Intracranial pressure of the physical head model and the live pigs
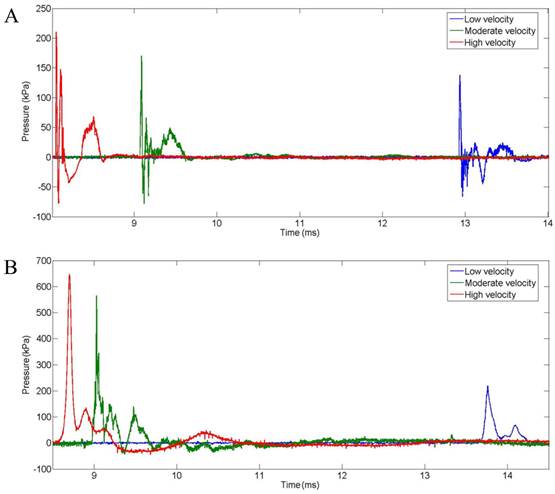
Intracranial pressure time histories were recorded for each ballistic test. As shown in Fig. 4 AB, the typical waveforms captured by the pressure sensors in the brain of the pig physical head model and live pigs exhibited blast-like features characterized by a steep shock front followed by a near-exponential decay. The waveforms of intracranial pressure corresponding to the different ballistic impact velocities display similar agreement.
The calculated Pmax, PImax, and PPD values for each test are included in Table 1. For all three pressure parameters, the values for the physical head model were significantly lower than those for the live pigs. The Pmax means were 126 kPa, 178 kPa, and 241 kPa for the physical head model and 219 kPa (p<0.01), 474 kPa (p<0.01), and 751 kPa (p<0.01) for the live pigs under low, moderate, and high impact velocities, respectively. The PPD was also significantly different between the pig physical head model and the live pigs, with means of 0.039 ms, 0.032 ms, and 0.028 ms for the physical head model and 0.311 ms (p<0.01), 0.238 ms (p<0.01), and 0.181 ms (p<0.01) for the live pigs under low, moderate, and high impact velocities, respectively. As the maximum value of the integral of the intracranial pressure curve within PPD, PImax showed magnified differences between the pig physical head model and the live pigs relative to PPD, with means of 1.83 kPa ms, 3.46 kPa ms, and 4.54 kPa ms for the physical head model and 64.50 kPa ms (p<0.01), 88.11 kPa ms (p<0.01), and 132.78 kPa ms (p<0.01) for the live pigs under low, moderate, and high impact velocities, respectively.
Intracranial pressure records
| Target | Impact velocity (m/s) | Pmax (kPa) | PImax (kPa ms) | PPD (ms) |
|---|---|---|---|---|
| physical model/ low velocity | ||||
| 1 | 279 | 112 | 1.65 | 0.025 |
| 2 | 287 | 107 | 1.70 | 0.044 |
| 3 | 292 | 119 | 1.53 | 0.026 |
| 4 | 297 | 138 | 2.06 | 0.034 |
| 5 | 296 | 149 | 2.31 | 0.030 |
| 6 | 295 | 125 | 1.76 | 0.058 |
| 7 | 289 | 128 | 2.03 | 0.045 |
| 8 | 297 | 130 | 1.62 | 0.046 |
| physical model/ moderate velocity | ||||
| 9 | 366 | 188 | 3.84 | 0.036 |
| 10 | 356 | 172 | 3.87 | 0.049 |
| 11 | 355 | 171 | 5.04 | 0.029 |
| 12 | 359 | 185 | 4.59 | 0.047 |
| 13 | 350 | 171 | 2.19 | 0.027 |
| 14 | 361 | 167 | 2.36 | 0.024 |
| 15 | 360 | 165 | 3.21 | 0.022 |
| 16 | 365 | 203 | 2.59 | 0.022 |
| physical model/ high velocity | ||||
| 17 | 409 | 215 | 3.28 | 0.023 |
| 18 | 411 | 210 | 4.99 | 0.020 |
| 19 | 429 | 279 | 3.97 | 0.054 |
| 20 | 417 | 218 | 7.16 | 0.048 |
| 21 | 420 | 238 | 3.38 | 0.017 |
| 22 | 419 | 250 | 5.15 | 0.024 |
| 23 | 426 | 253 | 4.39 | 0.019 |
| 24 | 422 | 268 | 3.96 | 0.015 |
| live pigs/ low velocity | ||||
| 25 | 282 | 178 | 51.27 | 0.257 |
| 26 | 295 | 241 | 47.79 | 0.202 |
| 27 | 289 | 190 | 82.48 | 0.665 |
| 28 | 280 | 182 | 82.15 | 0.143 |
| 29 | 291 | 212 | 50.08 | 0.251 |
| 30 | 293 | 289 | 48.27 | 0.184 |
| 31 | 296 | 269 | 74.29 | 0.506 |
| 32 | 288 | 193 | 79.64 | 0.283 |
| live pigs / moderate velocity | ||||
| 33 | 371 | 583 | 134.49 | 0.446 |
| 34 | 366 | 424 | 70.45 | 0.093 |
| 35 | 370 | 439 | 70.03 | 0.164 |
| 36 | 372 | 566 | 59.50 | 0.143 |
| 37 | 368 | 532 | 120.88 | 0.269 |
| 38 39 | 360 365 | 404 416 | 84.68 72.17 | 0.158 0.437 |
| 40 | 363 | 428 | 92.68 | 0.192 |
| live pigs / high velocity | ||||
| 41 | 422 | 705 | 153.29 | 0.218 |
| 42 | 431 | 892 | 149.72 | 0.203 |
| 43 | 434 | 832 | 148.52 | 0.177 |
| 44 | 419 | 653 | 153.35 | 0.092 |
| 45 | 429 | 701 | 138.03 | 0.247 |
| 46 | 430 | 740 | 148.19 | 0.176 |
| 47 | 436 | 837 | 100.53 | 0.132 |
| 48 | 420 | 647 | 70.63 | 0.203 |
Sensitivity of pressure parameters to impact velocity
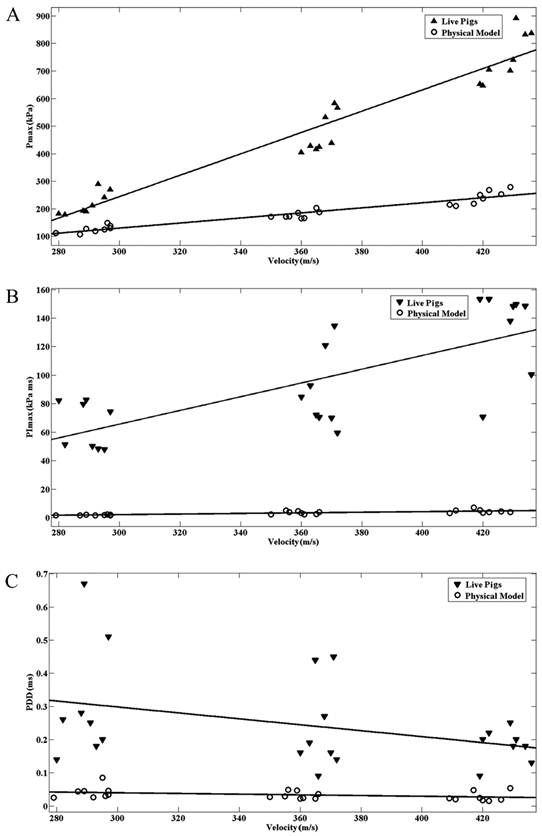
ANOVAs were used to compare pressure parameters (Pmax, PImax, PPD) at different ballistic impact velocities. Data from the physical head model and live pigs were examined. The Pmax, PImax, and PPD values obtained from the physical head model and live pigs are plotted against impact velocity in Fig. 5A,B,C. Of the three parameters, Pmax was the most sensitive to impact velocity, corresponding to the ANOVA finding that differences in Pmax between different impact velocities are significant (p < 0.01) for both the physical head model and live pigs. PImax, which exhibited significant differences between different ballistic impact velocities, increased with the impact velocity. However, the PPD values under different impact velocities did not show statistically significant differences (p>0.05) for either the physical head model or the live pigs, although there was a non-significant decrease in PPD with increasing impact velocity. The intracranial pressure response of the live pigs is more sensitive to impact velocity compared with that of physical head.
Correlations
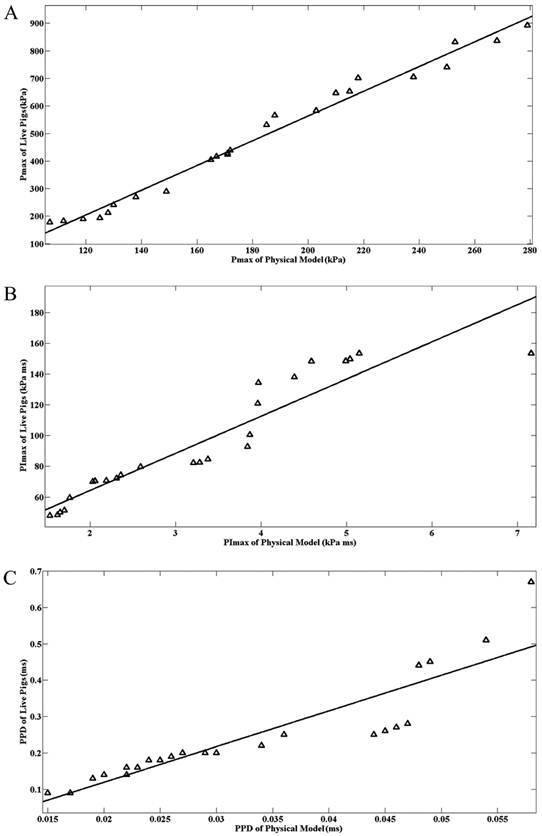
The correlation parameters between the physical head model and live pigs for intracranial pressure parameters are provided in Table 2. For each correlation, data from the physical head model and live pigs under low, moderate, and high impact velocities were used. Fig. 6A,B,C show the correlations of Pmax, PImax, and PPD between the physical head model and live pigs. Of the three pressure parameters, Pmax of the physical head model correlated best with that of live pigs (R2=0.9785, p<0.001).
Correlations of intracranial pressure between the physical head model and live pigs (N=24)
| R2 | p | |
|---|---|---|
| Pmax | 0.9785 | <0.001 |
| PImax | 0.8769 | <0.001 |
| PPD | 0.8942 | <0.001 |
Intracranial pressures in the brain of the pig physical head model(A) and a live pig(B) with a protective aramid plate following the impact of 9 mm bullets at low, moderate, and high velocities.
Intracranial maximum pressure (Pmax)(A) and Intracranial maximum pressure impulse (PImax)(B) of the pig physical head model and the live pigs for different impact velocities. Duration of the first positive phase (PPD) of the intracranial pressure wave in the pig physical head model and the live pigs for different impact velocities(C).
Correlation of the intracranial maximum pressure (Pmax)(A) and intracranial maximum pressure impulse (PImax)(B) between the pig physical head model and live pigs. Correlation of the duration of the first positive phase (PPD) of the intracranial pressure wave between the pig physical head model and live pigs(C).
Discussion
Non-penetrating injuries to the head resulting from BABT have been studied in human cadavers, dry skulls filled with silicone [4,5], and finite element (FE) models of the human head [6]. Although these types of models have accurate anatomy or similar anthropometry to humans, their lack of living physiology remains a main limitation for further investigation of the post-injury physiological response to BABT. Animals possessing living physiology are the only viable subjects in which to study the medical outcomes of non-penetrating injuries to the head, including not only anatomical features but also physiopathological changes, and can therefore help correlate biomechanical response data with the resulting BABT injuries to the head. In our study, we used pigs as ballistic subjects and focused on the brain's intracranial pressure response to non-penetrating impacts to the protected head. In addition, a pig physical head model was also used because of its reusability, which may contribute to providing comparatively repeatable response data. As previously reported, BABT injury to the head may be characterized physically by skin lacerations, skull fractures, and brain damage. Many authors indicate that the skin and scalp absorb 13% to 20% of incident energy. A study of transcranial transmission of blast waves revealed that blast waves pass through the thicker cranium of pigs while maintaining about two-thirds of their original magnitude [7]. Thus, the structural configuration of the "scalp-skull-brain" for the pig physical head model developed in this study is suitable for investigating the biomechanical response of BABT to the head.
High intracranial pressure was observed in the brain as a result of exposure to non-penetrating ballistic impact. However, the pressure waves propagating through the tissue have some of the characteristics of blast waves. Because of these pressure waves, the peak pressure, duration, and impulse can be regarded as contributors to injury [8]. Thus, three parameters, Pmax, PImax, and PPD, were extracted from the recorded pressure curve to describe the characteristics of the intracranial pressure response. Pmax is the maximal instantaneous pressure recorded in the brain, corresponding to local compression of the surrounding tissue under skull deflection. PImax corresponds to the first positive area under the intracranial pressure curve, and refers to the amount of energy transferred to the head. It depends not only on Pmax but also on PPD, which is defined as the duration of the first positive phase. In a previous study on the dynamic effects of projectiles on cadaveric skulls protected by a helmeted plate, the risk of skull fractures correlated well with brain parenchymal pressures [5], indicating that intracranial pressure is an appropriate predictor for studying the effects of cephalic impacts behind helmets. Injury criteria based on intracranial pressure have not yet been determined for BABT injuries to the head. However, conclusions from the related research areas of blast-induced traumatic brain injury (bTBI) and thoracic BABT are also instructive. A study focusing on the biomechanical assessment of brain response to blast pressure waves suggested that the maximum intracranial pressure can be compared with the threshold injury pressure and used to assess the severity of brain injury and the occurrence of cerebral contusion [9]. The intrathoracic pressure impulse was also found to be a useful predictor for pulmonary contusion volume after ballistic blunt impact thoracic trauma [10]. Further investigations are needed to clarify the function of intracranial pressure as an injury criterion for BABT injuries to the head.
To obtain a large range of intracranial pressure responses, both the physical head model and live pigs were exposed to ballistic impacts of standard 9 mm bullets at low (279-297 m/s), moderate (350-372 m/s), and high (409-436 m/s) velocities. Non-penetrating ballistic impacts produced lower intracranial pressures (mean Pmax=126 kPa, 178 kPa, and 241 kPa for low, moderate, and high impact velocities, respectively) in the surrogate and higher intracranial pressures in the living specimens (mean Pmax=219 kPa, 474 kPa, and 751 kPa for low, moderate, and high impact velocities, respectively). For comparison, Sarron's study [5] conducted on helmeted human cadaveric skulls reported intracranial pressures of 892±222 kPa after impact by non-penetrating 9 mm rounds at a velocity of 410.1±5.5 m/s. The higher intracranial pressures measured in this study may have resulted from the lack of scalp and anatomical differences in the human cadaveric skull subjects. Moreover, Pmax for both the pig physical head model and live pigs was quite sensitive to impact velocity, indicating that Pmax is a promising parameter for predicting brain injury severity resulting from BABT.
The PPD characteristics of the intracranial pressure response to non-penetrating ballistic impact have yet to be detailed. However, the data from several other BABT studies can be used as a point of reference. Bass et al. [11] confirmed that the impact duration is less than 300 μs when a helmet-head system is impacted by a 9 mm bullet. This impact duration is comparable to the PPD values of intracranial pressure in the brains of live pigs (mean PPD=311 μs, 238 μs, and 181 μs for low, moderate, and high impact velocities, respectively) in our study. The mean PPD values of intracranial pressure in the brain of the physical head model are 42 μs, 32 μs, and 28 μs for low, moderate, and high impact velocities, respectively, which are much lower than those for the live pigs. This order of magnitude for PPD has been observed in the pressure responses of internal organs to non-penetrating ballistic impacts conducted on a physical human surrogate torso model (HSTM) [12]. According to the description of the HSTM, the internal organs were made of silicone gel, which was similar to that used to make the brain of pig physical head model. Thereby, the similarity in the material composition is likely to be the main cause of the similarity of PPD values of pressure response in the HSTM and pig physical head model. Despite the fact that PPD decreased with increasing impact velocity, statistical analysis revealed that PPD was relatively insensitive to impact velocity for both the physical head model and live pigs. It is quite likely that the differences between the impact velocities are too small to produce statistically significant differences in PPD. Considering that the PPDs for the live pigs were much shorter than the overpressure durations of short-duration blasts (< 30 ms), which has a substantial effect on the peak overpressure tolerance, the exceptionally short PPD of the intracranial pressure waves will most likely be only a minor factor in BABT head injuries.
The difference in PImax between the pig physical head model and the live pigs was increased by integral calculation of intracranial pressure vs. time during PPD. However, the PImax for the live pigs was more sensitive to impact velocity than that for the pig physical head model.
Although considerable differences existed in the intracranial pressure parameters between the pig physical head model and the live pigs, especially in PImax, linear regression has revealed correlations in the intracranial pressure parameters between the two subject types. Of the three pressure parameters, the Pmax of the physical head model correlated best with that of live pigs. This finding suggests that it is feasible to predict the value of Pmax in a live pig using its physical surrogate.
As we know, human surrogates have seen increasing use in obtaining mechanical responses to blunt ballistic impacts, which could improve the prediction of injury and the evaluation of protective equipment performance against projectiles [13]. However, discrepancies in the mechanical responses of surrogates compared with humans are inevitable owing to the simplification of human anatomic structure and differences between the material properties of biosimulants and living tissue [14]. Therefore, knowledge of the correlation between the mechanical responses for human surrogates and live pigs is critical for the better understanding and appropriate usage of surrogate response data. Unfortunately, such information remains unavailable because response data pertaining to humans in physiological conditions are understandably inaccessible. Animals, long used as live pigs for ballistic tests, are the only viable subjects for studying a living body's mechanical responses to ballistic impact and therefore contribute to interpreting the biomechanical correlation between physical surrogates and live pigs. In this study, we developed a physical model of the pig head to measure intracranial pressure responses to non-penetrating ballistic impacts and investigate the correlation between the physical surrogate and living specimens. We hope our work may help predict the biomechanical responses of living animals to blunt ballistic impact based on surrogate tests and also provide insight into controlling for differences between living humans and surrogates when developing response and injury targets.
Despite the good correlation of Pmax between the physical head model and the live pigs, the limitation of the physical model in lack of viscoelasticity for the brain material induced considerable differences of PPD and PImax between the physical head model and live pigs. Of course, an effort should be made to adjust the material properties of the physical model to correct its response and provide better correlation with the live pigs.
Acknowledgements
This work was supported by the State key Laboratory of Trauma, Burns, and Combined injury (Grant no. SKLZZ201101). We thank Mr. Liangchao Zhang and Mr. Bo Zhang for their valuable and skillful assistance during the animal experiments and ballistic testing.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Carey ME. Analysis of wounds incurred by U.S. Army Seventh Corps personnel treated in Corps hospitals during Operation Desert Storm, February 20 to March 10, 1991. J Trauma. 1996;40(Suppl 3):S165-S169
2. Balandina DV, Bolotnikb NN, Pilkey WD. Capabilities of helmets for preventing head injuries induced by ballistic impacts. SHOCK VIB. 2004;11:547-562
3. Cannon L. Behind armour blunt trauma: an emerging problem. J R Army Med Corps. 2001;147(1):87-96
4. Sarron JC, Caillou JP, Cunha JD. et al. Consequences of Nonpenetrating Projectile Impact on a Protected Head: Study of Rear Effects of Protections. J Trauma. 2000;49(5):923-929
5. Sarron JC, Dannawi M, Faure A. et al. Dynamic Effects of a 9 mm Missile on Cadaveric Skull Protected by Aramid, Polyethylene or Aluminum Plate: An Experimental Study. J Trauma. 2004;57(2):236-243
6. Lee HP, Gong SW. Finite element analysis for the evaluation of protective functions of helmets against ballistic impact. Comput Methods Biomech Biomed Engin. 2010;13(5):537-550
7. Bauman RA, Ling G, Tong L. et al. An introductory characterization of a combat-casualty relevant swine model of closed head injury resulting from exposure to explosive blast. J Neurotrauma. 2009;26(6):841-860
8. Bass CR, Panzer MB, Rafaels KA. et al. Brain Injuries from Blast. Ann Biomed Eng. 2012;40(1):185-202
9. Chafi MS, Karami G, Ziejewski M. Biomechanical Assessment of Brain Dynamic Responses Due to Blast Pressure Waves. Ann Biomed Eng. 2010;38(2):490-504
10. Prat N, Rongieras F, Voiglio E. et al. Intrathoracic pressure impulse predicts pulmonary contusion volume in ballistic blunt thoracic trauma. J Trauma. 2010;69(4):749-755
11. Bass C, Bolduc M, Waclawik S. Development of a non-penetrating, 9 mm, ballistic helmet trauma test method. Proceedings of the Personal Armour Systems Symposium 2002. 2002:18-22
12. Roberts JC, Merkle AC, Biermanna PJ. et al. Computational and experimental models of the human torso for non-penetrating ballistic impact. J Biomech. 2007;40(1):125-136
13. Bass CR, Salzar RS, Lucas SR. et al. Injury risk in behind armor blunt thoracic trauma. Int J Occup Saf Ergon. 2006;12(4):429-442
14. Crandall JR, Bose D, Forman J. et al. Human Surrogates for Injury Biomechanics Research. Clin Anat. 2011;24(3):362-371
Author contact
![]() Corresponding author: Jing Chen, PhD. State Key Laboratory of Trauma, Burns and Combined Injury, Research Institute of Surgery, Daping Hospital, Third Military Medical University, No.10, Changjiang Zhilu, Chongqing 400042, China. Tel: +86 023 68757462. Fax: +86 023 68710162. E-mail: chenjing9811tmmu.edu.cn
Corresponding author: Jing Chen, PhD. State Key Laboratory of Trauma, Burns and Combined Injury, Research Institute of Surgery, Daping Hospital, Third Military Medical University, No.10, Changjiang Zhilu, Chongqing 400042, China. Tel: +86 023 68757462. Fax: +86 023 68710162. E-mail: chenjing9811tmmu.edu.cn

 Global reach, higher impact
Global reach, higher impact