Impact Factor
ISSN: 1449-1907
Int J Med Sci 2012; 9(8):627-633. doi:10.7150/ijms.4865 This issue Cite
Research Paper
Haematoporphyrin Based Photodynamic Therapy Combined with Hyperthermia Provided Effective Therapeutic Vaccine Effect against Colon Cancer Growth in Mice
1. Department of General Surgery, Shanghai East Hospital of Tongji University, No. 150 Jimo Road, Shanghai 200120, China
2. Department of Science and Research, Shanghai East Hospital of Tongji University, No. 150 Jimo Road, Shanghai 200120, China
Received 2012-7-12; Accepted 2012-9-5; Published 2012-9-19
Abstract
Photodynamic therapy (PDT) has become an attractive option used in tumor treatment via its direct tumoricidal activities or its immune-boosting activities. On the other hand, heat shock protein 70 has been found to be largely associated with the establishment of anti-tumor activities offered by hyperthermia treated tumor cells. In the present study, we found that injection of tumor-bearing mice with colon cancer cell line CT-26 treated with haematoporphyrin based photodynamic therapy (hematoporphyrin monomethyl ether based PDT, HMME-PDT) together with hyperthermia demonstrated the most effective therapeutic effects against tumor growth, followed by cells treated by hyperthermia alone. CT-26 cells treated only with HMME-PDT failed to provide any therapeutic effects, although significant cell death was induced by HMME-PDT. Compared to hyperthermia treatment, HMME-PDT induced more efficient surface localization of HSP70 on CT-26 cells which correlated with efficient activation of cytolytic CD8 T cells and with effective anti-tumor responses. Thus, our study demonstrated that the surface expression of HSP70 may play a more important role than the total expression or release of this molecule in the activation of immune responses. And our study offered a novel modified PDT approach to the treatment of tumor cells intrinsically low on HSP70 expression.
Keywords: photodynamic therapy, HSP70, hyperthermia, cancer vaccine, hematoporphyrin monomethyl ether
Introduction
Photodynamic therapy (PDT) has been established as a new therapy for the treatment of neoplasm and other diseases (1). Distinct from traditional radiotherapy and chemotherapy, PDT possesses unique advantages. For example, it induces extranuclear generation of photooxidative cytotoxic molecules without causing genotoxic damage (2). More importantly, PDT also induces a durable anti-tumor immune response to destroy the remaining tumor cells (3), which indicates PDT is able to enhance the immunogenicity of tumor cell. And using PDT to generate a cancer vaccine is an interesting application of this modality. Several reports demonstrated PDT generated vaccine exhibited anti-tumor effect, and some mechanisms were mentioned. For example, Gollnick used photofrin, a porphyrin-derived photosensitizer, and showed that PDT treated EMT6 tumor cells displayed protective vaccine effects against tumor growth, and the protective effects were correlated with enhanced activation of dendritic cells (4). Korbelik and his colleagues found that the BPD-PDT and Ce6-PDT could provide a therapeutic vaccine effects agaist SCCVⅡ tumor. The former one was likely resulted from increased surface expression of HSP70 and complement oponozation (5), the latter one from membrane lipid alteration and cell apoptosis (6). Treatment of liver cancer cells with hematoporphyrin monomethyl (HMME), a porphyrin-derived photosensitizer, has been shown to offer some preventive vaccine effects against liver carcinoma (7). When we attempted to injection of colon cancer CT-26 cells treated with HMME-PDT, it failed to demonstrate a therapeutic vaccine effect. One of the noted differences between Korbelik's and our study was that the SCCVⅡ cell line they used highly expressed HSP70.
Heat shock protein 70 (HSP70), as its name indicated, is normally induced by heat stress (8). In fact, HSP70 appears to be the most studied heat shock protein towards its beneficial effects offered by heat treated tumors(9). Unlike other kinds of photosensitizers used in PDT therapy which can induce the expression of HSP70, porphyrin-derived photosensitizers seemed to be less effective in the induction of HSP70 expression (10), but tends redistribute HSP70 to cell surfaces (11) which then facilitates the development of adaptive immunity (12). Therefore, in the current study we designed a novel approach to combine hyperthermia with HMME-PDT to achieve both elevated expression of HSP70 and its enhanced surface localization on treated tumor cells in hope that improved therapeutic effects would be acquired.
Materials and Methods
Animals and tumor induction in vivo
Poorly immunogenic mouse colon carcinoma cell line CT-26 and 6-8 weeks old female Balb/c mice (host for CT-26 tumor) were used in this research. Tumor was induced via injection of 2×105 CT-26 cells subcutaneously on the right lower abdomen. The tumor growth was recorded based on the diameters measured with caliper and the volume of tumor was calculated with formula as volume = 0.5×a(longest diameter)×b2(the longest diameter perpendicular to a).
Cell treatments
CT-26 cells were cultured in 37℃ and 5%CO2 in high glucose DMEM medium. For PDT treatment, CT-26 was cultured in complete medium containing hematoporphyrin monomethyl (HMME) in different dilutions for 12 hours, and then washed with PBS for 3 times. The cells were transferred to serum free medium before being irradiated with 630nm light via semi-conductor laser at a dose of 5J/cm2 (ALA-PDT KDL300) for 20 min. Therefore, HMME-PDT was used to represent the PDT treatment. For PDT combined with hyperthermia abbreviated as heat-PDT, treatment of cells at 41℃ was performed at the last third hour of HMME incubation. 1×107 PDT-treated CT-26 cells were diluted in 50μl PBS before they were used for injection. For hyperthermia stress, CT-26 cells were incubated in water bath at 41oC for 1 hour, and then incubated in normal condition for 3 hours. After cell number was evenly adjusted as that of PDT-treated cells, they were subjected to 3 cycles of freezing and thaw (- 80℃ for 20 min and 37℃ for 10 min) to eliminate living tumor cells, and this group is named as heat-F/T.
Immunization
Five days after tumor implantation when tumors usually reached approximate 3 mm in largest diameter, mice were immunized with differently treated cells by single subcutaneous peritumoral injection as described (5). Injected cells were generated from cells treated with HMME-PDT, heat-PDT or heat-F/T. PBS injection was used as a control. Measurement of tumor size was used to assess the therapeutic effects of various cell lysates.
For analyzing the activity of cytotoxicity T lymphocyte (CTL), normal mice were used after they were injected with 5×105 treated CT-26 cells by HMME-PDT, heat-PDT or heat-F/T at lower abdominal region bilaterally once a week for 3 weeks. The CTL activity was analyzed with flow cytometric analysis or with MTT assay as described below.
Flow cytometry analysis
One week after final injection, spleen and draining inguinal lymph nodes were isolated and were prepared to single-sell suspension. Draining inguinal lymph nodes cells were restimulated at 3×106/well on 24-well plate with PMA (40ng/ml, Sigma), ionomycin (100ng/ml, Sigma) and BFA (10ug/ml, BioLegend Inc,) for 6 hours. The cytokines expressed were examined based on intracellular cytokine staining protocol (13). Cell surface molecule was stained with APC-anti-CD8 (BioLegend Inc) and intracellular IFN-γwas stained with PE-anti-IFN-γ(BioLegend Inc), which together reflected the activation of cytotoxic lymphocytes (CTLs). Flow cytometry was also used to analyze the expression of HSP70 on the cell surfaces (BioLegend Inc). Same class of antibody conjugated with same kind of fluorescence was used in all flow cytometry analysis as isotype control.
MTT assay
Splenocyte suspension generated as above was analyzed for cytolytic effects by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (MTT), and splentocye from normal mice were used as control. Briefly, CT-26 target cells were incubated with splenocytes in various ratios (1:10, 1:50 and 1:100) for 72 hours in 96 wells plate. MTT was added at appropriate dilution after supernatant was discarded. After incubation for 4 hours, cells were retained and DMSO was used to dissolve the lipid-soluble MTT. Cell viability was detected by microplate reader at 495nm. The activation of cytotoxic lymphocytes was calculated by the formula CTL activation=(absorbance of treatment group - absorbance of control group)/absorbance of control group×100%. Background absorbance was subtracted from each reading. Additionally, MTT assay was also used to measure the viability of CT-26 after PDT treatment.
Western blot
Total cell lysates of treated cell were extracted by protein extraction kit from Beyotime Inc (China) and separated by electrophoresis on a 10% polyacrylamide gel. The protein were then transferred to PVDF membrane and incubated for 2 hours in TBS containing 10% nonfat milk. After blocking, membranes were incubated with rabbit polyclonal antibody specific for HSP70 (CST Inc) followed by incubation of HRP conjugated goat anti-rabbit IgG. The resulted blots were analyzed by ECL detection system (ImageQuant LAS 4000). The supernatants of treated cells were also collected to measure HSP70 release by western blot analysis. The experiment was repeated for three times.
Statistical analysis
Data comparisons were done by Student's t test or one-way ANOVA analyses. Comparison with a difference of P < 0.05 was considered significant.
Results
HMME-PDT combined with hyperthermia treatment provided the most potent therapeutic effects against CT-26 growth
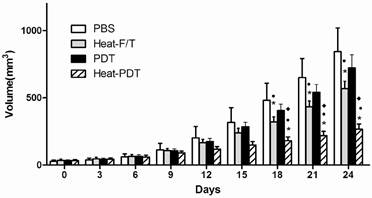
We examined if HMME-PDT would offer therapeutic effects against colon cancer and if hyperthermia modified HMME-PDT, ie, heat-PDT, would enhance the therapeutic effects of HMME-PDT. As shown in Figure 1, when tumor-bearing mice that had been implanted with CT-26 cells for approximately one week before they were injected with differentially treated cells. HMME-PDT treatment showed no significant therapeutic effects in the inhibition of tumor growth compared to PBS injection control. Up to 18 days after treated cells injection, both heat-F/T and heat-PDT treatment induced a significant tumor growth inhibition than PBS and HMME-PDT treatment. More importantly, heat-PDT treatment elicited an even more vigorous inhibition on tumor growth than heat-F/T treatment. Therefore, our finding indicated that the therapeutic vaccine effects on colon cancer cell growth by haematoporphyrin monomethyl (HMME) photosensitizer can only be established in the presence of hyperthermia treatment.
Heat-PDT provided the most potent therapeutic effect against CT-26 growth. Mice were injected with 2×105 CT-26 cells subcutaneously. 5 days later when tumor started to appear, mice were immunized with 1×107 CT-26 cells treated with HMME-PDT, Heat-PDT and Heat-F/T as described in the Methods. Mice injected with PBS were used as control. Response to the therapeutic vaccine treatment was determined by tumor volume measurement as in Methods. Each group consisted of five mice. Significance test was performed by One-way ANOVA with * representing comparison with PBS; ● with HMME-PDT and ◆ with Heat-F/T.
HSP70 expression but not the presence of cell death was associated with the anti-tumor effects
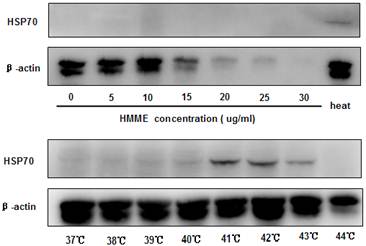
PDT has been shown to promote cell death at a dose-dependent manner and certain photosensitizers induce heat shock protein 70 (HSP70) expression in treated cells (10). Since these two parameters can both play important roles in initiating and boosting immunity, we examined if they contribute differently to the anti-tumor effects observed. When the irradiation condition of 5J/cm2 for 20 minutes was applied, HMME-PDT induced cell death at a dose-dependent fashion as revealed by MTT assay (data not show). The induction of cell death was also consistent with the loss of β-actin detection when higher dose of HMME was applied to CT-26 cells (Figure 2). No significant expression of HSP70 was found in all the doses of HMME examined in PDT-treated cells (Figure 2).
To study the impact of hyperthermia on the induction of HSP70 in CT-26 cells, cells were incubated in various temperatures for 1 hour. We found that CT-26 cells exhibited low level of HSP70 expression at 37℃. And the level of expression was increased as temperature raised (Figure 2), reaching to peak value when the temperature reached 41℃. Taken together, these data demonstrated that the induction of HSP70 played a more relevant role in conferring the therapeutic anti-tumor vaccine effects comparing to the induction of cell death. However, the mechanisms on the supreme antitumor vaccine effect provided by heat-PDT remained to be determined.
HMME-PDT promoted the release and surface redistribution of HSP70 induced by hyperthermia
Although HMME-PDT failed to induce the HSP70 expression in treated CT-26 cells (Figure 2), we examined if HMME-PDT is able to affect the release or the cell surface expression of HSP70. To detect the release of HSP70, the presence of HSP70 in the supernatants of treated cells was studied at 2 hours post irradiation by western blot analysis. As shown in Figure 3, HSP70 expression was up-regulated efficiently from cells heated at 41℃, and no HSP70 was found in the supernatants if heated cells were not subjected freezing and thaw treatment (Figure 3, lane 1). Furthermore, HMME-PDT treatment induced an elevated presence of HSP70 in the supernatants at a dose-dependent manner (Figure 3), corresponding to the cell death occurred. Therefore an enhanced release of HSP70 induced by HMME-PDT was likely a result of enhanced cell death, which could occur both in heat-PDT and heat-F/T.
Hyperthermia but not HMME-PDT induced HSP70 expression on CT-26 effectively. CT-26 cells were treated with different concentrations of HMME-PDT or cultured in different temperatures in water bath. Cell lysates were then generated to detect the expression of HSP70 with Western blot analysis. β-actin was detected for loading control. Data shown was a representative of 3 separately performed experiments.
HMME-PDT treatment led to enhanced presence of HSP70 in the supernatants. 1×106 CT-26 cells grown in medium containing different concentrations of HMME were subjected to 41 treatment for 1h first to induce HSP70 expression. 500ul supernatants were collected 2 hours after irradiation treatment. HSP70 in the supernatants was analyzed on SDS-PAGE with loading volume around 20ul. Blots were probed with antibody to HSP70. Data shown was a repeat of 3 different experiments.

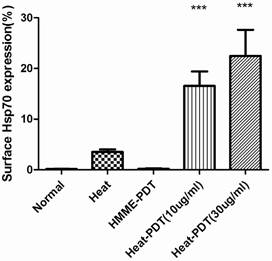
The migration of HSP70 to cell surfaces was analyzed by flow cytometric analysis. As shown in Figure 4, hyperthermia only promoted the surface migration of HSP70 slightly although it did induce the expression of this protein. In contrast, the percentage of surface HSP70 positive cells increased more significantly when CT-26 cells were treated with heat-PDT, indicating that HMME-PDT was able to promote the migration of HSP70 to the cell surfaces more efficiently than heat. Both low (10μg/ml) and high dose (30μg/ml) of HMME demonstrated this effect. No HSP70-positive cells were detected if cells were treated with HMME-PDT alone. Altogether, it is demonstrated that HMME-PDT could more efficiently promote the surface migration of HSP70 comparing to hyperthermia treatment, suggesting that the surface presence of HSP70 may exert more critical effect in the induction of anti-tumor response.
HMME-PDT enhanced HSP70 surface expression in CT-26. CT-26 cells were treated similarly as in Fig 3 with 10ug/ml HMME as low dose and 30ug/ml as high dose. Cells without any treatment or with hyperthermia treatment alone were also examined. After irradiation, cells were stained with alexfluro488-conjugated goat anti-HSP70 antibody and analyzed by flow cytometry. Significance test was performed by one way ANOVA test with * representing comparison with no treatment control.
Heat-PDT promoted CTL activation
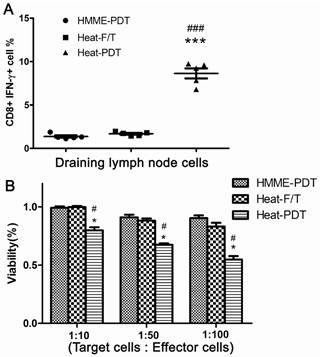
To further evaluate the most potent antitumor feature offered by heat-PDT treatment, activation of CD8 T cells was measured from mice injected with the cells treated by HMME-PDT, heat-F/T or heat-PDT. Mice treated with cells of heat-PDT, higher proportion of activated CD8 T cells in the draining inguinal lymph node were found when CTL was measured by the ability of IFN-γ production from CD8 T cells (Figure 5a). Similar finding was also obtained when CTL activities were measured by MTT assay (Figure 5b). It is indicated that this combination-based vaccine could elicit the most effective cytotoxic lymphocytes activation, corresponding well with their most potent anti-tumor therapeutic vaccine effects.
Discussion
Photodynamic therapy (PDT) is regarded as a promising therapy partly due to its reported capabilities of rapid recruitment and activation of immune cells, which in turn facilitate the development of anti-tumor adaptive immunity (14). In fact, PDT-treated tumor cells were shown to be effective in both preventive (4) and therapeutic (5) vaccines. However, in our research, no anti-tumor effect was found when cells were only treated by HMME-PDT. Two possible causes might account for this difference. SCCVⅡ cell line investigated in Gollnick's research was a breast carcinoma that expresses high level of HSP70. However, the colon cancer cell line CT-26 used in our study expresses low level of HSP70 (Figure 3). On the other hand, Ce6 photosensitizers used in Korbelik's research was able to induce raised HSP70 protein expression, which might contribute to its application in vaccine generation (15). However, the HMME photosensitizer used in our research failed to demonstrate this effect by itself independent of dosage. It is not known yet if the differences in their ability to induce the protein expression of HSP70 between HMME and Ce6 were due to its different subcellular locations, ie, HMME is mainly distributed in Golgi body, ER, mitochondria and lysosome (16), while ce6 is mainly found in lysosome and plasma membrane (17).
Heat-PDT induced the CTL response most effectively. Normal Balb/c mice were injected with 5×105 cells treated with HMME-PDT, Heat-F/T and Heat-PDT as described in the Methods. 7 days after immunization, draining inquinal lymph node cells (a) were analyzed for the induction of CTL responses by measuring the percentage of CD8+ cells capable of producing IFN-γ by FACS analysis, and splenocytes(b) were analyzed by MTT assay. FACS image was a representative image from one animal; the combined results from 5 mice were shown in dot graph. The MTT results were the mean value of 5 different mice. Significance was tested by one way ANOVA test with * representing comparison with HMME-PDT and # with Heat-F/T with p value of <0.05.
Cell death resistance and immune evasion are two important interrelated factors in tumor cell growth and metastasis (18, 19). Recently the name of immunogenic cell death was coined to define the cell death that reaches to a level which can induce both innate and adaptive immunity effectively irrespective of the form of cell death (20). Therefore, certain level of cell death is a critical endogenous alarm signal for immune system. In our study, however, although significant amount of cell death is indeed induced by HMME-PDT, no immune activation was observed. This may indicate that the quality of cell death is more relevant to the activation of immune response. Indeed, uric acid, HMGB-1, ATP, heat shock proteins and many others have been reported as effective inducing molecules for immune reaction, collectively called damaged associated molecular patterns (DAMPs). Thus, based on our study and published studies by others (21, 22), we predicted that the failure of offering productive activation of anti-tumor CTL response by cells treated with HMME-PDT is not due to lack of cell death, but a result of lack of HSP70 induction.
HSP70 is originally known as a chaperon protein (23). But it was also a critical DAMP. It can interact with natural killer (NK) cells and promote their activation (24). The presence of HSPs on the surface of cells can prevent the recognition of inhibitory receptor complex CD94/NKG2A on NK cells with its MHC class I ligands, facilitating to activation of NK cell and killing of HSP-positive tumor target cells (25). HSP70 also interacts with pattern-recognition receptors (PRR) on antigen-presenting cells like TLR-4, CD91, LOX-1 and CD40 to promote the release of released pro-inflammatory cytokines through the activation of NF-κB pathway (26, 27). Additionally, HSPs including HSP70 have been found to be important in the induction of cross-presentation of extracellular antigens to evoke CTL response (28). Our finding that injection of extracellular cell-associated antigens were ale to induce CTL responses appeared to indicate that the cross-presentation by extracellular antigens may be largely dependent on the presence of surface HSP70.
Like all the heat shock proteins, HSP70 is an intracellular protein without signal peptide, which thus is usually not secreted. In this case, HSP70 can be released as a result of cell death (21). Alternatively, HSP70 also can be release through though an endolysosomal compartment, which was similar to one used by IL-β that without out a signal peptide (29). In our results, the release of HSP70 was increased while HMME concentration was raised and more cell death was induced. On the other hand, cells were induced to express HSP70 by hyperthermia treatment without induction of cell death, no HSP70 release was detected (Figure4). Thus, the elevated releasing of HSP70 found in HMME-PDT treated cells was more likely resulted from cell death pathway. Concerning membrane localization of HSP70, the mechanisms are not clear. It was reported that lesions in the membrane of PDT-treated cells caused the inner membrane to be flopped outward and exposed the HSP70 with proteins or lipids lining the inner leaflet of on to the plasma membrane (30, 31). Furthermore, it was thought that surface localized HSPs might have a role in their efforts to stabilize damaged membranes and preserve their integrity under the oxidative stress (32). We are not certain how HMME-PDT is able to promote the membrane localization of HSP70 at present. It could be a result of HMME-PDT induced cell damages as well, since more cell surface HSP70 was found in HMME-PDT treated cells comparing to cells treated with hyperthermia alone, corresponding well with their different capacity of causing cell damages.
Cancer is the second cause of human death (33). Although surgery, chemotherapy and radiotherapy all achieved great successes in healing cancer, they all had their respective limitations. Immune therapy has its unique advantages, such as low toxicity, high efficacy, specific targeting and long duration, becomes a potential effective method (34). Vaccine especially therapeutic ones is one of the most attractive members in immune-related therapies. In this study, we provided a novel immune-based approach to increase the therapeutic efficacy on tumor vaccines with low intrinsic heat shock protein expressions. Furthermore, we uncovered the importance of surface localization of heat shock protein in the induction of cross-presentation and activation of cytotoxic T cells.
Acknowledgements
The study was supported by the grant of Scientific and Technological Project of Shanghai Science and Technology Commission (054119555).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Huang Z, Xu H, Meyers AD. et al. Photodynamic therapy for treatment of solid tumors--potential and technical challenges. Technol Cancer Res Treat. 2008;7:309-320
2. Dougherty TJ, Gomer CJ, Henderson BW. et al. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889-905
3. Korbelik M. Induction of tumor immunity by photodynamic therapy. J Clin Laser Med Surg. 1996;14:329-334
4. Gollnick SO, Vaughan L, Henderson BW. Generation of effective antitumor vaccines using photodynamic therapy. Cancer Res. 2002;62:1604-1608
5. Korbelik M, Sun J. Photodynamic therapy-generated vaccine for cancer therapy. Cancer Immunol Immunother. 2006;55:900-909
6. Korbelik M, Stott B, Sun J. Photodynamic therapy-generated vaccines: relevance of tumour cell death expression. Br J Cancer. 2007;97:1381-1387
7. Zhang HY, Ma WJ, Li YX. Generation of effective vaccines against liver cancer by using photodynamic therapy. Lasers in Medical Science. 2009;24:549-552
8. Stott B, Korbelik M. Activation of complement C3, C5, and C9 genes in tumors treated by photodynamic therapy. Cancer Immunol Immunother. 2007;56:649-658
9. Milani V, Noessner E. Effects of thermal stress on tumor antigenicity and recognition by immune effector cells. Cancer Immunology Immunotherapy. 2006;55:312-319
10. Gomer CJ, Ryter SW, Ferrario A, Rucker N, Wong S, Fisher AM. Photodynamic therapy-mediated oxidative stress can induce expression of heat shock proteins. Cancer Res. 1996;56:2355-2360
11. Korbelik M, Sun J, Cecic I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res. 2005;65:1018-1026
12. Torigoe T, Tamura Y, Sato N. Heat shock proteins and immunity: application of hyperthermia for immunomodulation. Int J Hyperthermia. 2009;25:610-616
13. Prussin C, Metcalfe DD. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995;188:117-128
14. Gollnick SO, Evans SS, Baumann H. et al. Role of cytokines in photodynamic therapy-induced local and systemic inflammation. Brit J Cancer. 2003;88:1772-1779
15. Castano AP, Gad F, Zahra T, Hamblin MR. Specific anti-tumor immune response with photodynamic therapy mediated by benzoporphyrin derivative and chlorin(6). Laser-Tissue Interaction Xiv. 2003;4961:1-9
16. Dai W, Li X, Jing Z, Liu F, Gu Y. Dynamical Change of Intracellular Distribution of Hematoporphrin Monomethyl Ether in A549 Cells. Acta Laser Biology Sinica (CHINA). 2008;17:725-728
17. Mojzisova H, Bonneau S, Vever-Bizet C, Brault D. Cellular uptake and subcellular distribution of chlorin e6 as functions of pH and interactions with membranes and lipoproteins. Biochimica Et Biophysica Acta-Biomembranes. 2007;1768:2748-2756
18. Blagosklonny MV. Cell immortality and hallmarks of cancer. Cell Cycle. 2003;2:296-299
19. Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715-727
20. Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta. 2010;1805:53-71
21. Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Inter Immunol. 2000;12:1539-1546
22. Melcher A, Todryk S, Hardwick N, Ford M, Jacobson M, Vile RG. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nature Med. 1998;4:581-587
23. Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443-451
24. Multhoff G, Mizzen L, Winchester CC. et al. Heat shock protein 70 (Hsp70) stimulates proliferation and cytolytic activity of natural killer cells. Experimental Hematology. 1999;27:1627-1636
25. Michaelsson J, Teixeira de Matos C, Achour A, Lanier LL, Karre K, Soderstrom K. A signal peptide derived from hsp60 binds HLA-E and interferes with CD94/NKG2A recognition. J Exp Med. 2002;196:1403-1414
26. Theriault JR, Mambula SS, Sawamura T, Stevenson MA, Calderwood SK. Extracellular HSP70 binding to surface receptors present on antigen presenting cells and endothelial/epithelial cells. FEBS Lett. 2005;579:1951-1960
27. Asea A, Rehli M, Kabingu E. et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028-15034
28. Nishikawa M, Takemoto S, Takakura Y. Heat shock protein derivatives for delivery of antigens to antigen presenting cells. Inter J Pharmaceut. 2008;354:23-27
29. Mambula SS, Calderwood SK. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol. 2006;177:7849-7857
30. Frasch SC, Henson PM, Nagaosa K, Fessler MB, Borregaard N, Bratton DL. Phospholipid flip-flop and phospholipid scramblase 1 (PLSCR1) co-localize to uropod rafts in formylated Met-Leu-Phe-stimulated neutrophils. J Biol Chem. 2004;279:17625-17633
31. John K, Schreiber S, Kubelt J, Herrmann A, Muller P. Transbilayer movement of phospholipids at the main phase transition of lipid membranes: implications for rapid flip-flop in biological membranes. Biophys J. 2002;83:3315-3323
32. Zhou F, Xing D, Chen WR. Dynamics and mechanism of HSP70 translocation induced by photodynamic therapy treatment. Cancer Lett. 2008;264:135-144
33. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300
34. Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6:535-545
Author contact
![]() Corresponding author: Dr. Haiyan Ge, Department of General Surgery, Shanghai East Hospital of Tongji University, No. 150 Jimo Road, Shanghai 200120, China; Tel: 0086-21-38804518; Fax: 0086-21-58798999; E-mail: gounarislcom; 08heyaomingedu.cn
Corresponding author: Dr. Haiyan Ge, Department of General Surgery, Shanghai East Hospital of Tongji University, No. 150 Jimo Road, Shanghai 200120, China; Tel: 0086-21-38804518; Fax: 0086-21-58798999; E-mail: gounarislcom; 08heyaomingedu.cn

 Global reach, higher impact
Global reach, higher impact