3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2012; 9(7):603-610. doi:10.7150/ijms.4970 This issue Cite
Research Paper
Is Helicobacter Pylori Infection Associated with Asthma Risk? A Meta-Analysis Based on 770 Cases and 785 Controls
1. Institute of Respiratory Diseases, Xinqiao Hospital, Third Military Medical University, Chongqing, China
2. Department of Environmental Hygiene, College of Preventive Medicine, Third Military Medical University, Chongqing, China
Received 2012-8-6; Accepted 2012-9-6; Published 2012-9-14
Abstract
Objective: Helicobacter pylori (H. pylori) infection has been thought to play a critical role in disorders such as gastric and lung cancer. A number of studies have been devoted to the relationship between H. pylori infection and asthma risk, which have generated inconclusive results. In this study we aimed to derive a more precise estimation of the relationship.
Methods: Meta-analyses evaluating the association of H. pylori infection and asthma risk were conducted and subgroup analyses on ethnicity and source of controls as well as CagA status were further conducted. Eligible studies were identified for the period up to Jul 2012.
Results: A total of five case-control studies comprising 770 cases and 785 controls were lastly selected for analysis. The overall data failed to indicate a significant association of H. pylori infection and asthma risk (OR=1.01; 95%CI=0.82-1.24). Likewise, in the subgroup analysis regarding ethnicity, source of controls and CagA status, no associations could be observed.
Conclusions: The pooled data failed to suggest a marked association between H. pylori infection and asthma risk. Future studies are needed to confirm this conclusion.
Keywords: Asthma, Helicobacter pylori, Infection, Meta-analysis, Risk
Introduction
Asthma is a common chronic inflammatory disease of the airways, characterized with symptoms of wheezing and shortness of breath [1]. The etiology of asthma remains largely unclear. Smoking and environmental factors as well as genetic factors are thought to be its risk factors [2, 3].
Previously, respiratory infections by microbes such as bacteria and viruses might play complex roles in the development of asthma, either triggering asthma symptom or reducing the incidence of asthma [4, 5]. Helicobacter pylori (H. pylori), a helical shaped Gram-negative bacterium, has been shown to infect various areas of stomach and duodenum and thus been reported to associate with gastric cancer risk [6]. The role for H. pylori infection in the disorders of respiratory system has been addressed for several years. H. pylori infection might have a role in the development of chronic bronchitis, bronchiectasis, lung cancer and tuberculosis [7, 8]. However, the roles of H. pylori infection in the development of asthma remain controversial [7, 9].
In general, asthma is believed to be caused by exaggerated immunologic responses to antigens in the environment, which are driven by a Th2-mediated immune response. The exogenous infection and microbial substances including H. pylori infection may elicit a Th1-mediated immune response, which suppresses Th2 responses. The lack of adequate stimulation of the Th1 might result in an overactive Th2, which in turn lead to asthma [10]. Moreover, the acquisition of H. pylori may be of importance in the induction of regulatory T cells, which could effectively reduce the possibility of allergic asthma [11, 12]. Thus, one of the H. pylori factors, the neutrophil-activating factor of H. pylori (HP-NAP) which might drive Th1 polarization and display a powerful inhibition of allergic Th2 response, is used as a potential antigen for treatment of asthma [10, 13].
Recently, increasing studies have been devoted to the association of H. pylori infection with asthma risk. However, most of the studies were cross-sectional, with the results conflicting [14, 15]. Elucidating the possible associations may help us better understand the pathological mechanism of asthma and therefore contribute to its prevention.
As cross-sectional designs may have been underpowered in evaluating the association between H. pylori infection and asthma risk, in the present study we aimed to derive a more precise estimation of the relationship via a quantitative meta-analysis of published case-control studies.
Materials and Methods
Literature search strategy
We carried out a literature search without a language limitation in the Medline, OVID, EMBASE and CNKI (China National Knowledge Infrastructure), covering all published papers up to Jul 2012, with a combination of the following keywords: helicobacter pylori, bronchus, pulmonary, allergy, asthma and atopy. If any articles in languages other than English were searched, the abstracts were screened and the full texts were further intensely reviewed for obtaining any potential information.
We evaluated potentially associated publications by checking their titles and abstracts and then procured the most relevant publications for a closer examination. Moreover, the reference lists of the selected papers were also screened for other potential articles that possibly have been missed in the initial search. Moreover, a hand search was conducted in the library of Third Military Medical University for any papers that were missed the internet search.
Inclusion and exclusion criteria
The following criteria were used for the literature selection: first, studies should concern the association of H. pylori infection with asthma risk; second, studies must be case-control studies; third, papers must offer the size of the sample, odds ratios (ORs) and their 95% confidence intervals (CIs), the genetic distribution or the information that can help infer the results. Accordingly, the following criteria for exclusion were also used: first, the design and the definition of the experiments were obviously different from those of the selected articles; second, the source of cases and controls and other essential information were not offered; third, reviews and duplicated publications. After rigorous searching, we reviewed all papers in accordance with the criteria defined above for further analysis.
Data extraction
Data were carefully extracted from all eligible publications independently by two of the authors according to the inclusion criteria mentioned above. For conflicting evaluations, an agreement was reached following a discussion. If a consensus could not be reached, another author was consulted to resolve the dispute and then a final decision was made by the majority of the votes. Extracted information was entered into a database.
Statistical analysis
The OR of asthma risk associated with status of H. pylori infection was estimated for each study. For detection of any possible sample size biases, the OR and its 95% confidence interval (CI) to each study was plotted against the number of participants respectively. An I-squared value was used as an index for the heterogeneity test, with values less than 25% indicating low, 25% to 50% indicating moderate, and greater than 50% indicating high heterogeneity [16]. A Chi-square based Q statistic test was also performed to assess heterogeneity. If the result of the Q-test was P >0.1, ORs were pooled according to the fixed-effect model (Mantel-Haenszel) [17]; otherwise, the random-effect model (DerSimonian and laird) was used [18]. The significance of the pooled ORs was determined by Z-test. Publication bias was assessed by visual inspection of funnel plots [19], in which the standard error of log (OR) of each study was plotted against its log (OR). An asymmetric plot indicates a possible publication bias. The symmetry of the funnel plot was further evaluated by Egger's linear regression test [20]. Statistical analysis was undertaken using the program STATA 11.0 software (Stata Corporation, Texas, USA).
Results
Literature search and meta-analysis databases
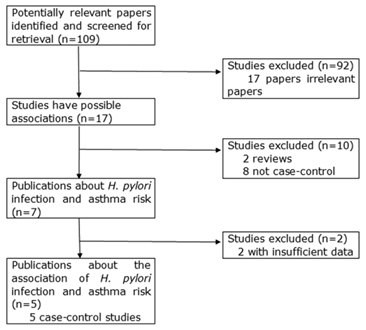
Relevant publications were retrieved and screened. As shown in Figure 1, a total of one hundred and nine potentially relevant papers were obtained and screened for retrieval. After a careful review, ninety-two irrelevant papers were excluded. In the remaining seventeen studies, two reviews [14, 21] and eight publications not being case-control design [22-29] were discarded. Then, a total of seven case-control studies were identified, of which two studies not presenting detailed raw data were further excluded [30, 31]. Finally, five case-control studies were included in this study [32-36].
All included studies were written in English. We established a database according to the extracted information from each article. The information was listed in Table 1 and 2. According to the lists, the publication year, first author and the number of cases and controls for each study were presented. In the five included case-control studies, serological methods for detecting the status of H. pylori infection were also provided.
All the cases were diagnosed as asthma. There were two groups of Asians [34, 36], one of Caucasians [35] and two of mixed ethnicities [32, 33] in the present meta-analysis. Of the included studies, two provided detailed information about CagA status [32, 34]. The controls used in one study [32] were hospital-based, while those in the remaining four were population-based.
Test of heterogeneity
For the overall data, we analyzed the heterogeneity of H. pylori positive versus H. pylori negative. The P value was 0.352 and the I-squared value was 9.5%, indicating the absence of the heterogeneity. Thus, the fixed-effect models were used. Likewise, when data were divided into subgroups, the heterogeneity was not significant (Table 3).
Case-control studies on H. pylori infection and asthma risk
| First Author | Publication Year | Study type | Number of Cases (male/female) | Number of Controls (male/female) | Type of controls | Age (mean), year (Cases/Controls) | Race origin | Country |
|---|---|---|---|---|---|---|---|---|
| Tsang | 2000 | Case-control | 90 (38/52) | 97 (46/51) | Healthy controls (PB) | 42.6/43.2 | Asian | China |
| Zhao | 2005 | Case-control | 46 (28/18) | 48 (28/20) | Healthy controls (age-, sex-, socioeconomic- matched; PB) | 51.2/50.3 | Asian | China |
| Pessi | 2005 | Case-control | 245 (93/152) | 405 (151/254) | Healthy controls (age-, gender, residence-matched; PB) | 59/60 | Caucasian | Finland |
| Annagur | 2007 | Case-control | 79 (46/33) | 36 (18/18) | Non-atopic healthy children (PB) | 9.7/10.4 | mixed | Turkey |
| Reibman | 2008 | Case-control | 318 (95/223) | 208 (69/139) | Non-asthma controls without a heavy smoking history, unstable cardiac disease, uncontrolled hypertension, neuromuscular disease or other lung disease (HB) | 34/38 | mixed | USA |
NA: not available
H. pylori infection status among asthma cases and controls included in the meta-analysis
| First author | year | Method | Cases | Controls | |||
|---|---|---|---|---|---|---|---|
| Hp (+) | Hp (-) | Hp (+) | Hp (-) | ||||
| Tsang | 2000 | ELISA | 44 | 46 | 37 | 60 | |
| Zhao | 2005 | ELISA | 27 | 19 | 26 | 22 | |
| Pessi | 2005 | ELISA | 115 | 124 | 204 | 192 | |
| Annagur | 2007 | ELISA | 20 | 57 | 6 | 30 | |
| Reibman | 2008 | ELISA | 147 | 171 | 100 | 108 | |
| Hp CagA(+) | Hp (-) | Hp CagA(+) | Hp (-) | ||||
| Zhao | 2005 | ELISA | 10 | 19 | 9 | 22 | |
| Reibman | 2008 | ELISA | 79 | 171 | 65 | 108 | |
Hp: H. pylori
Main results of the pooled data in the meta-analysis
| Group | Hp (+) vs Hp (-) | |||||
|---|---|---|---|---|---|---|
| Number (cases/controls) | OR | 95% CI | P (OR) | I-squared | P (Q-test) | |
| Overall | 770/785 | 1.01 | 0.82-1.24 | 0.919 | 9.5% | 0.352 |
| Ethnicity | ||||||
| Asian | 136/145 | 1.42 | 0.89-2.29 | 0.144 | 0.0% | 0.619 |
| Caucasian | 239/396 | 0.87 | 0.63-1.20 | 0.407 | - | - |
| mixed | 395/244 | 1.00 | 0.72-1.39 | 0.994 | 26.1% | 0.245 |
| Source of control | ||||||
| Population-based | 452/577 | 1.06 | 0.82-1.37 | 0.667 | 26.6% | 0.252 |
| Hospital-based | 318/208 | 0.93 | 0.65-1.32 | 0.678 | - | - |
| Hp CagA status | ||||||
| Hp CagA(+) | 279/204 | 0.82 (a) | 0.56-1.20 | 0.303 | 0.0% | 0.384 |
| Hp CagA(-) | 275/182 | 1.21 (b) | 0.80-1.84 | 0.369 | 0.0% | 0.912 |
Hp: H. Pylori; (a) Hp CagA(+) vs Hp (-); (b) Hp CagA(-) vs Hp (-);
The flow diagram of included/excluded studies.
Quantitative data synthesis
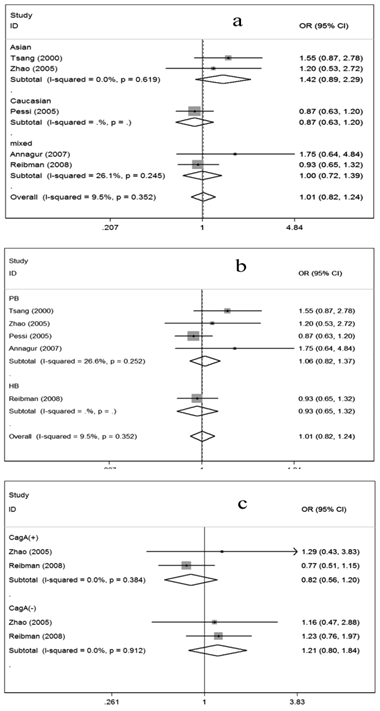
For evaluation of the possible relationship between H. pylori infection and asthma risk, the overall data available for our meta-analysis were obtained from five case-control studies containing 770 cases and 785 controls, of which 353 cases and 373 controls were serologically H. pylori positive while 417 cases and 412 controls were H. pylori negative. As shown in Figure 2, the overall OR was 1.01 (95% CI =0.82-1.24) and the test for overall effect Z value was 0.10 (P>0.05), indicating that individuals who were serologically H. pylori positive may not have increased or decreased asthma risk compared with those who were serologically H. pylori negative.
Considering the possible impact of ethnic variation, source of controls and CagA status on the results, we further conducted subgroup analyses. In the subgroups regarding ethnicity, no associations were shown among Asians, Caucasians and mixed ethnicities, respectively (Figure 2a). Similarly, in the subgroup analysis according to source of controls, no associations could be observed (Figure 2b). When the data were restricted to CagA status, no associations were presented in either the CagA positive subgroup or the CagA negative subgroup (Figure 2c).
Sensitivity analysis
In order to compare the difference and evaluate the sensitivity of the meta-analysis, we reported the results of the random-effect model as follows: the combined OR was 1.02 (95% CI =0.82-1.28), similar to the results obtained from the fixed-effect model. Moreover, one-way sensitivity analysis was also conducted by excluding any single included study [37]. As a consequence, the statistical significance of the results was not altered when any single study was omitted (data not shown), confirming the stability and credibility of the results.
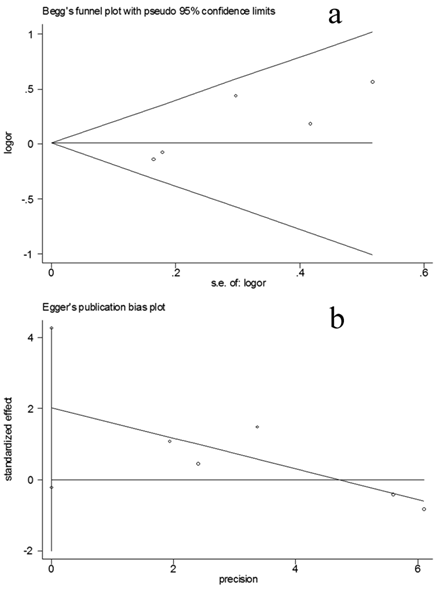
Bias diagnostics
Funnel plot was created for assessment of possible publication bias (Figure 3a). Then, Egger's linear regression test was used to assess the symmetry of the plot (Figure 3b). The data suggest that the funnel plot was symmetrical (t=2.87, P=>0.05), suggesting that the results of the present meta-analyses are relatively stable and the publication bias may have little influence on the results.
Meta-analysis for the association of asthma risk with H. pylori infection. Hp (+) vs Hp (-); (a) Stratified by ethnicity, (b) Stratified by source of controls; (c) Stratified by CagA status; Hp: H. pylori; PB: Population-based; HB: Hospital-based
Publication bias tests for the overall data (Hp positive vs Hp negative). (a): Funnel plot; (b) Egger's linear regression test.
Discussion
In this study, we evaluated the possible relationship between H. pylori infection and asthma risk by performing a quantitative meta-analysis of the published case-control studies. The results failed to suggest a significant association between H. pylori infection and asthma risk. Similar results were shown in the subgroup analyses regarding ethnicity, source of controls and CagA status.
Previously, a number of studies reported that H. pylori infection might play a beneficial role for allergy asthma [10, 14, 38]. Nevertheless, most of the studies were cross-sectional. Compared with case-control studies, cross-sectional design may underestimate the possible risk. Only a proportion of the studies were case-control; however, the results were far from being conclusive. Therefore, we deliberately screened the case-control studies and conducted a pooled analysis assessing the relationship. The crude ORs were calculated according to the raw data obtained from the literature. We did not perform a meta-analysis of the adjusted ORs because adjustment was not comparable among the studies.
Considering the potential effect of the interactions between H. pylori infection and genetic variation on the risk [39], we performed subgroup analyses stratified by ethnicity. In the subgroup with respect to Asians, Caucasians and mixed ethnicities, no associations could be observed. The data indicated little influence of genetic variations on the risk. Then, we conducted subgroup analyses stratified by source of controls and CagA status, respectively, taking into account the potential effects of the confounding factors on the results. Similarly, no associations could be observed in these subgroups, implying that these factors might exert little influence on the risk.
In this present study, the included studies used a stable serological method, ELISA, as a unique approach for detecting the presence of H. pylori infection. Nevertheless, the serology of H. pylori is not a reliable criteria for detecting its effect on asthma. The serologic tests could not give us any information on current H. pylori infection. Therefore, a number of further investigations using multi-approaches such as immunhistochemistry, polymerase chain reaction and smears for exploring the presence of H. pylori are required.
The present study has several limitations. Firstly, the papers identified in our study were limited to those openly published up to Jul 2012; it is possible that some related published or unpublished studies that might meet the inclusion criteria were missed, resulting in any inevitable bias, though the funnel plots and the Egger's tests failed to show any significant publication bias. Secondly, the results may be interpreted with care because of the limited number and small sample sizes of each included studies. Thirdly, subgroup analyses regarding other confounding factors such as smoking status, age and gender have not been conducted in the present study because sufficient information could not be extracted from the primary literature.
In summary, the association of H. pylori infection and asthma risk were assessed by pooling the included data via a systematic meta-analysis. The results failed to suggest a significant association between H. pylori infection and asthma risk. Future well-designed investigations with large sample sizes are required to get a more confidential conclusion.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Finiasz M, Otero C, Bezrodnik L, Fink S. The role of cytokines in atopic asthma. Current medicinal chemistry. 2011;18:1476-87
2. Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG. et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129:735-44 doi:10.1542/peds.2011-2196
3. Blumenthal MN. Genetic, epigenetic, and environmental factors in asthma and allergy. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology. 2012;108:69-73 doi:10.1016/j.anai.2011.12.003
4. Papadopoulos NG, Konstantinou GN. Antimicrobial strategies: an option to treat allergy? Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2007;61:21-8 doi:10.1016/j.biopha.2006.10.004
5. Liu AH. Endotoxin exposure in allergy and asthma: reconciling a paradox. The Journal of allergy and clinical immunology. 2002;109:379-92
6. Sibony M, Jones NL. Recent advances in Helicobacter pylori pathogenesis. Current opinion in gastroenterology. 2012;28:30-5 doi:10.1097/MOG.0b013e32834dda51
7. Kanbay M, Kanbay A, Boyacioglu S. Helicobacter pylori infection as a possible risk factor for respiratory system disease: a review of the literature. Respiratory medicine. 2007;101:203-9 doi:10.1016/j.rmed.2006.04.022
8. Zhuo WL, Zhu B, Xiang ZL, Zhuo XL, Cai L, Chen ZT. Assessment of the relationship between Helicobacter pylori and lung cancer: a meta-analysis. Archives of medical research. 2009;40:406-10 doi:10.1016/j.arcmed.2009.05.002
9. Malfertheiner MV, Kandulski A, Schreiber J, Malfertheiner P. Helicobacter pylori infection and the respiratory system: a systematic review of the literature. Digestion. 2011;84:212-20 doi:10.1159/000329351
10. D'Elios MM, de Bernard M. To treat or not to treat Helicobacter pylori to benefit asthma patients. Expert review of respiratory medicine. 2010;4:147-50 doi:10.1586/ers.10.9
11. Arnold IC, Dehzad N, Reuter S, Martin H, Becher B, Taube C. et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. The Journal of clinical investigation. 2011;121:3088-93 doi:10.1172/JCI45041
12. Strickland DH, Judd S, Thomas JA, Larcombe AN, Sly PD, Holt PG. Boosting airway T-regulatory cells by gastrointestinal stimulation as a strategy for asthma control. Mucosal immunology. 2011;4:43-52 doi:10.1038/mi.2010.43
13. Amedei A, Codolo G, Del Prete G, de Bernard M, D'Elios MM. The effect of Helicobacter pylori on asthma and allergy. Journal of asthma and allergy. 2010;3:139-47 doi:10.2147/JAA.S8971
14. Vakil N. Helicobacter pylori. Can Helicobacter pylori infection prevent allergic asthma? Reviews in gastroenterological disorders. 2009;9:E66-7
15. Raj SM, Choo KE, Noorizan AM, Lee YY, Graham DY. Evidence against Helicobacter pylori being related to childhood asthma. The Journal of infectious diseases. 2009;199:914-5
16. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). 2003;327:557-60 doi:10.1136/bmj.327.7414.557
17. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute. 1959;22:719-48
18. DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7:177-88
19. Munafo MR, Clark TG, Flint J. Assessing publication bias in genetic association studies: evidence from a recent meta-analysis. Psychiatry research. 2004;129:39-44 doi:10.1016/j.psychres.2004.06.011
20. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-34
21. Blaser MJ, Chen Y, Reibman J. Does Helicobacter pylori protect against asthma and allergy? Gut. 2008;57:561-7 doi:10.1136/gut.2007.133462
22. Holster IL, Vila AM, Caudri D, den Hoed CM, Perez-Perez GI, Blaser MJ. et al. The impact of Helicobacter pylori on atopic disorders in childhood. Helicobacter. 2012;17:232-7 doi:10.1111/j.1523-5378.2012.00934.x
23. Zevit N, Balicer RD, Cohen HA, Karsh D, Niv Y, Shamir R. Inverse association between Helicobacter pylori and pediatric asthma in a high-prevalence population. Helicobacter. 2012;17:30-5 doi:10.1111/j.1523-5378.2011.00895.x
24. Fullerton D, Britton JR, Lewis SA, Pavord ID, McKeever TM, Fogarty AW. Helicobacter pylori and lung function, asthma, atopy and allergic disease--a population-based cross-sectional study in adults. International journal of epidemiology. 2009;38:419-26 doi:10.1093/ije/dyn348
25. Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. The Journal of infectious diseases. 2008;198:553-60 doi:10.1086/590158
26. Shiotani A, Miyanishi T, Kamada T, Haruma K. Helicobacter pylori infection and allergic diseases: epidemiological study in Japanese university students. Journal of gastroenterology and hepatology. 2008;23:e29-33 doi:10.1111/j.1440-1746.2007.05107.x
27. Lang L. Childhood acquisition of Helicobacter pylori linked to reduced asthma and allergy risk. Gastroenterology. 2007;133:6. doi:10.1053/j.gastro.2007.05.011
28. Nijevitch AA, Loguinovskaya VV, Tyrtyshnaya LV, Sataev VU, Ogorodnikova IN, Nuriakhmetova AN. Helicobacter pylori infection and reflux esophagitis in children with chronic asthma. Journal of clinical gastroenterology. 2004;38:14-8
29. McCune A, Lane A, Murray L, Harvey I, Nair P, Donovan J. et al. Reduced risk of atopic disorders in adults with Helicobacter pylori infection. European journal of gastroenterology & hepatology. 2003;15:637-40 doi:10.1097/01.meg.0000059127.68845.57
30. Matricardi PM, Rosmini F, Riondino S, Fortini M, Ferrigno L, Rapicetta M. et al. Exposure to foodborne and orofecal microbes versus airborne viruses in relation to atopy and allergic asthma: epidemiological study. BMJ (Clinical research ed). 2000;320:412-7
31. Jaber SM. Helicobacter pylori seropositivity in children with chronic disease in Jeddah, Saudi Arabia. Saudi journal of gastroenterology: official journal of the Saudi Gastroenterology Association. 2006;12:21-6
32. Reibman J, Marmor M, Filner J, Fernandez-Beros ME, Rogers L, Perez-Perez GI. et al. Asthma is inversely associated with Helicobacter pylori status in an urban population. PloS one. 2008;3:e4060. doi:10.1371/journal.pone.0004060
33. Annagur A, Kendirli SG, Yilmaz M, Altintas DU, Inal A. Is there any relationship between asthma and asthma attack in children and atypical bacterial infections; Chlamydia pneumoniae, Mycoplasma pneumoniae and Helicobacter pylori. Journal of tropical pediatrics. 2007;53:313-8 doi:10.1093/tropej/fmm040
34. Jun ZJ, Lei Y, Shimizu Y, Dobashi K, Mori M. Helicobacter pylori seroprevalence in patients with mild asthma. The Tohoku journal of experimental medicine. 2005;207:287-91
35. Pessi T, Virta M, Adjers K, Karjalainen J, Rautelin H, Kosunen TU. et al. Genetic and environmental factors in the immunopathogenesis of atopy: interaction of Helicobacter pylori infection and IL4 genetics. International archives of allergy and immunology. 2005;137:282-8 doi:10.1159/000086421
36. Tsang KW, Lam WK, Chan KN, Hu W, Wu A, Kwok E. et al. Helicobacter pylori sero-prevalence in asthma. Respiratory medicine. 2000;94:756-9 doi:10.1053/rmed.2000.0817
37. Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Techn Bull. 1999;8:15-7
38. Sachs G, Scott DR. Helicobacter pylori: Eradication or Preservation. F1000 medicine reports. 2012;4:7. doi:10.3410/m4-7
39. Snaith A, El-Omar EM. Helicobacter pylori: host genetics and disease outcomes. Expert review of gastroenterology & hepatology. 2008;2:577-85 doi:10.1586/17474124.2.4.577
Author contact
![]() Corresponding author: Yan Wang, MD, Email: wangyanflowercom, Changzheng Wang, Prof. Email: czwangcom. Institute of Respiratory Diseases, Xinqiao Hospital, Third Military Medical University, Chongqing, China
Corresponding author: Yan Wang, MD, Email: wangyanflowercom, Changzheng Wang, Prof. Email: czwangcom. Institute of Respiratory Diseases, Xinqiao Hospital, Third Military Medical University, Chongqing, China

 Global reach, higher impact
Global reach, higher impact