Impact Factor
ISSN: 1449-1907
Int J Med Sci 2012; 9(7):527-538. doi:10.7150/ijms.4446 This issue Cite
Review
A Review of the Human Clinical Studies Involving Citrus aurantium (Bitter Orange) Extract and its Primary Protoalkaloid p-Synephrine
1. Dean Emeritus, Creighton University Medical Center, Omaha, NE 68078, USA;
2. Departments of Biochemistry, Medicine and Pathology, Georgetown University Medical Center, Washington, DC 22039, USA;
3. Faculty of Pharmacy, Jordan University of Science and Technology, Irbid 22110, Jordan.
Received 2012-4-5; Accepted 2012-7-9; Published 2012-8-29
Abstract
This review summarizes the published as well as unpublished human studies involving Citrus aurantium (bitter orange) extract and its primary protoalkaloid p-synephrine, providing information and an assessment of the safety and efficacy of these widely used products. The results of over 20 studies involving a total of approximately 360 subjects that consumed p-synephrine alone or in combination with other ingredients are reviewed and critiqued. Over 50 % of the subjects involved in these studies were overweight/obese, and approximately two-thirds of these overweight/obese subjects consumed caffeine (132-528 mg/day) in conjunction with p-synephrine (10-53 mg/day). Bitter orange/p-synephrine containing products were consumed for up to 12 weeks. Approximately 44 % of the subjects consumed a bitter orange/p-synephrine only product, while the remainder consumed a complex product that contained multiple ingredients in addition to p-synephrine. In general, bitter orange extract alone (p-synephrine) or in combination with other herbal ingredients did not produce significant adverse events as an increase in heart rate or blood pressure, or alter electrocardiographic data, serum chemistry, blood cell counts or urinalysis. p-Synephrine alone as well as in combination products were shown to increase resting metabolic rate and energy expenditure, and modest increases in weight loss were observed with bitter orange extract/p-synephrine-containing products when given for six to 12 weeks. Longer term studies are needed to further assess the efficacy of these products and affirm their safety under these conditions.
Keywords: Citrus aurantium, bitter orange, p-synephrine, human subjects, obesity/overweight, safety, efficacy, caffeine.
Introduction
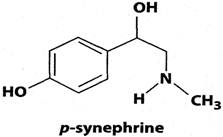
Citrus aurantium extract and its primary protoalkaloidal constituent p-synephrine (Figure 1) are extensively used in weight management products and as thermogenic agents. They are also used in sports performance products to enhance stamina. C. aurantium extract is widely known as bitter orange extract, a product that is derived from the immature (green) fruits of the Seville orange. It is also known as “Chih-shi” or “Zhi shi” in traditional Chinese medicine [1]. Bitter orange extract is used in weight management products due to its purported effects on metabolic processes, including an increase in basal metabolic rate and lipolysis as well as mild appetite suppression [2].
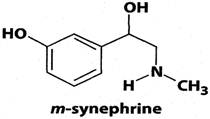
Uncertainty has existed concerning the safety of bitter orange extract and p-synephrine. In general, both the lay public and scientific communities have failed to differentiate between p-synephrine which is a phenylethylamine derivative that has the hydroxy group in the para position on the benzene ring and the synthetic m-synephrine (phenylephrine) which has a hydroxyl group in the meta position on the benzene ring) (Figure 2). m-Synephrine exhibits cardiovascular effects but is not a constituent of bitter orange [3-5]. Properties possessed by m-synephrine are inappropriately attributed to bitter orange extract and p-synephrine, and clinical case study reports and reviews involving bitter orange extract frequently make inappropriate references to m-synpephrine (see for example [6-9]).
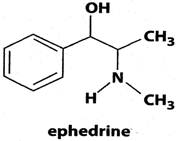
A limited number of studies have been conducted with p-synephrine and bitter orange extracts without the addition of various other ingredients and herbal products. The issue of safety and efficacy is further clouded and complicated by the structural similarity of p-synephrine to ephedrine (Figure 3) and other biogenic amines, in spite of the fact that the pharmacokinetics of the these compounds and their receptor binding specificities are vastly different due to significant structural and stereochemical differences [10, 11]. As a consequence, markedly different pharmacological properties should be anticipated.
Other issues have also obfuscated the picture with respect to the safety and efficacy of bitter orange extract. Some of the extracts that are used are either non-standardized or poorly standardized, making it difficult if not impossible to establish reproducibility. In some cases, lack of knowledge of the chemical composition of the extracts being used precludes useful comparisons. Products containing bitter orange extract in most cases contain a variety of other herbal extracts, many of which are caffeine containing.
A final issue that has added to the overall confusion regarding bitter orange has been the release of erroneous adverse events information by governmental agencies. Statements implying that large numbers of adverse events and even deaths due to the use of products containing bitter orange extract have clearly been shown to be incorrect and misleading [12], but are still widely reported.
Several reviews have addressed the safety of bitter orange extract based on animal, in vitro and receptor binding studies as well as some human studies [2, 10, 13]. This review addresses data associated with published human studies, clinical case reports, and unpublished clinical studies. Several recent studies have been conducted with p-synephrine/bitter orange extract alone and will be reviewed. Information with respect to unpublished studies is derived from final research reports available on the internet, presentations at national meetings of professional organizations, and presentations and research reports from the investigators directly involved. References are cited based on the origin of the information.
Chemical structure of p-synephrine.
Chemical structure of m-synephrine.
Chemical structure of ephedrine
Human Studies
A limited number of well-designed and controlled human studies have been conducted with bitter orange extracts assessing efficacy and safety. The majority of studies have been conducted using products that contain not only an extract of C. aurantium, but other ingredients such as caffeine, green tea, ginkgo, ginseng, guarana, and yerba mate'. The incorporation of bitter orange extract into products containing other potentially active ingredients makes comparative analyses difficult. However, several human safety and efficacy studies have been conducted on bitter orange extract (p-synephrine) alone. The 16 studies published in refereed journals involving bitter orange extract/p-synephrine are reviewed in chronological sequence, and are summarized in Table 1, while 7 studies unpublished to date are summarized in Table 2.
Colker et al. [15] conducted the first study on the effects of a bitter orange extract-containing product on body fat loss, lipid levels, safety and mood in 20 overweight adult subjects (Table 1). The product which was consumed on a daily basis contained 975 mg C. aurantium extract (6% synephrine alkaloids), 528 mg caffeine, and 900 mg St. John's wort. The total daily intake of phenylethylamine-related protoalkaloids was approximately 58.5 mg. All subjects in the study followed an 1800 kcal/day American Heart Association Step One diet, and performed a circuit training exercise program three days per week.
Summary of published human studies involving bitter orange extract and p-synephrine
| Product | Treated Subjects | overwt/ obese | Duration | p-Synephrine dose (mg) | Caffeine dose (mg) | End Point | Adverse events | Reference |
|---|---|---|---|---|---|---|---|---|
| Complex | 9 | 9 | 42 days | 58.5 | 528 | Wt. loss, ↑BMR | No | Colker et al., 199915 |
| Bitter orange juice | 12 | -- | 1 day | 27 | --- | CV | No | Penzak et al., 20016 |
| Complex | 15 | 15 | 56 days | 10 | 400 | Wt. loss Multiple | No | Kalman et al., 200021 |
| Complex | 13 | 13 | 14 days | 10 | 400 | CV | No | Kalman et al., 200222 |
| Bitter orange extract | 12 | -- | 28 days | 30.6 | -- | Cyt.P450 enzymes | No | Gurley et al., 200424 |
| Complex | 18 | 18 | 56 days | 36 | 132 | No wt. loss ↑RMR | No | Zenk et al., 200526 |
| Bitter orange extract | 18 | -- | 8 hrs | 27 | --- | CV | No | Min et al., 200527 |
| Complex | 10 | -- | 6 hrs | 5.5 | 239 | CV | ↑HR, ↑BP | Haller et al., 200528 |
| Bitter orange extract | 10 | -- | 6 hrs | 46.9 | --- | CV | ↑HR | Haller et al., 200528 |
| Bitter orange extract | 15 | -- | 6 hrs | 54 | --- | CV | ↑HR, ↑BP | Bui et al., 200629 |
| Complex | 10 | 10 | 7 hrs | 12 | 150 | ↑ RMR, ↑ Kcal use | No | Sale et al., 200630 |
| Bitter orange extract | 22 | 22 | 8 hrs | 30 | --- | ↑RQ | No | Gougeon et al., 200631 |
| Complex | 10 | -- | 3 hrs | 21.6 | 450 | ↑RQ, ↑RMR | ↑BP | Hoffman et al., 200732 |
| Complex | 10 | -- | 2 hrs | 21 | 304 | CV | ↑↑DBP | Haller et al., 200834 |
| Complex | 23 | 23 | 25 hrs | 52 | 704 | ↑ kcal use, ↓RER | No | Seifert et al., 201135 |
| Bitter orange extract | 40 | -- | 2 hrs | 50 | --- | ↑RMR | No | Stohs et al., 201136 |
BMR=basal metabolic rate; RMR=resting metabolic rate; CV=cardiovascular; RQ=respiratory quotient; RER=respiratory exchange ratio.
Summary of unpublished human studies involving bitter orange extract and p-synephrine.
| Product | Treated subjects | overwt/ obese | Duration | p-Synephrine dose (mg) | Caffeine dose (mg) | End Point | Adverse events | Reference |
|---|---|---|---|---|---|---|---|---|
| Complex | 22 | 22 | 70 days | 21.5 | 15 | Wt. loss | No | Kendall-Reed, 200017 |
| Complex | 6 | 3 | 6 hrs | 12 | 239 | ↑RMR | No | Kalman et al., 200218 |
| Complex | 10 | 5 | 5 hrs | 12 | 239 | ↑RMR | No | Kalman et al., 200219 |
| Complex extract | 16 | 16 | 14 days | 24 | 300 | Multiple enzymes | No | Kalman et al., 200320 |
| Complex | 28 | 28 | 56days | ? | 10 | Multiple | No | Zenk & Kuskowski, 200525 |
| Bitter orange extract | 15 | ? | 42days | 80 | --- | CV | No | Talbott et al., 200733 |
| Bitter orange | 16 | -- | 15 days | 49 | --- | Multiple | No | Shara et al., 201237 |
BMR=basal metabolic rate; RMR=resting metabolic rate; CV=cardiovascular; RQ=respiratory quotient; RER=respiratory exchange ratio.
After six weeks, the treated group lost small but significant amounts of body weight (1.4 kg) and body fat (2.9%). No significant changes in blood pressure, heart rate, electrocardiographic findings, serum chemistry or urinalysis were noted and no significant changes were observed in the results of the Profile of Mood States Questionnaire for fatigue or vigor. The treated group also experienced a significant increase in basal metabolic rate as compared to the placebo group.
The amount of caffeine consumed daily in the product (528 mg) is equivalent to approximately four cups of coffee or over five cups of tea, and is a well-known thermogenic agent [16]. It is not clear whether the weight loss and increase in basal metabolic rate were due to the caffeine, the bitter orange extract, exercise, caloric restriction or a combination thereof. This combination of ingredients and protocol appeared to be effective and safe for promoting modest fat and body weight loss in healthy, overweight adults, although the number of subjects in the study was small [15].
Kendall-Reed [17] conducted a 10 week unpublished study on a system (Ultra Slim Down®) that consisted of two products (Table 2). The final report of the study is available on line. One product contained 125 mg hydroxycitric acid (CitrimaxTM), 125 mg bitter orange extract (Advantra Z®) and 50 mg kola nut extract, while the second product contained 344 mg chitosan. Thirty-two overweight subjects were divided into three groups and either given the two products (one capsule of each in conjunction with each meal), a diet and exercise program, or the products in conjunction with the diet and exercise program. At the end of the study no adverse side effects were observed or reported. The group consuming the product-only lost an average of 4.63 kg, the group on the diet and exercise regime lost 3.45 kg, and the group taking the product plus diet and exercise lost 6.59 kg. In summary, consumption of the products alone was more effective than diet and exercise, while consuming the products in combination with diet and exercise was most effective. No adverse effects were reported. This study was not published and subjected to peer review.
Possible cardiovascular effects of Seville (sour) orange juice in normotensive adults were assessed by Penzak et al. [6] (Table 1). The synephrine concentration in the orange juice was approximately 57 mcg/ml, while octopamine was not detected. Twelve subjects consumed 8 ounces of orange juice (approximately 13 mg p-synephrine) and water in a crossover design followed by repeat ingestion 8 hours later. Hemodynamic parameters including heart rate and blood pressure did not significantly differ between control and treated groups. In spite of the lack of evidence, the authors concluded that individuals with tachyarrhythmias, severe hypertension and narrow angle glaucoma as well as monoamine oxidase inhibitor receptors should avoid Seville orange juice. The warning was based on the erroneous assumption that the form of synephrine present in the orange juice was m-synephrine [6].
Kalman and associates have conducted three unpublished studies using a commercial weight loss product (Xenadrine EFX®) (Table 2). The product contained a proprietary blend of extracts from C. aurantium (bitter orange), yerba mate, grape seed, green tea and ginger root in addition to several vitamins and amino acids. The product contained 6 mg p-synephrine, 150 mg caffeine and 150 mg catechin polyphenols in capsule form. The results of these studies were presented at various scientific meetings, but were never published in a scientific journal and subjected to peer review. In each of these studies it is not possible to determine the role of p-synephrine in the observed effects.
A double blind cross-over study involving 6 healthy human subjects who received two capsules of Xenadrine EFX® (12 mg p-synephrine) (Kalman et al.[18]). A significant increase (2.41%) in resting metabolic rate was observed one hour after ingestion of the product relative to the placebo control. No effects on heart rate or blood pressure were observed, and no subjective complaints or adverse events were reported.
Kalman et al. [19] conducted a second double blind cross-over study involving 10 healthy human subjects. Basal metabolic rates were determined at baseline, four hours after a standardized meal at which time two capsules of Xenadrine EFX® or the placebo were ingested, and hourly for the next five hours. At the two and three hour time points after ingestion of the product relative to the control, 13.4 % and 8.9% increases, respectively, were observed in the basal metabolic rate. No significant differences in heart rate or blood pressure were observed in response to the product relative to baseline and control values.
Kalman et al. [20] reported the results of a 14-day clinical trial using Xenadrine EFX® in combination with exercise involving 16 healthy subjects who were either overweight or obese. Two capsules of the product were consumed twice daily, containing a total of 24 mg p-synephrine/day. No significant effects of the product were noted as compared to the placebo group with respect to blood pressure, heart rate, electrocardiographic data, fasting blood glucose, renal function, hepatic function or complete blood count with differentials over the 14 days of the study. The treated group experienced a significant reduction in fatigue levels, while sleep quality was negatively impacted. At the end of the study, the treated group experienced a reduction in diastolic blood pressure as compared to the placebo group (-8.0 vs ±4.2 mm Hg). The authors concluded that the product was safe over the course of the study [20]. No weight loss was observed over the two weeks of the study.
The weight loss effects of a product (Xenadrine™) containing 20 mg ephedrine, 5 mg p-synephrine, 200 mg caffeine and 15 mg salicin given twice daily were examined by Kalman et al.[21] involving 30 healthy but overweight subjects in a placebo-controlled double-blinded protocol (Table 1). Half of the subjects were randomly assigned to either the treatment or control group. All subjects performed a cross-training exercise program three days per week with a 22 kcal/kg per day diet. After 8 weeks the experimental group had lost a significantly greater amount of weight than the control group (3.14 kg vs 2.05 kg), and no significant changes in systolic or diastolic blood pressure, heart rate, serial electrocardiograms, serum chemistry or calorie intake were observed between the two groups. The daily intake of p-synephrine (10 mg) was small, and its relative contribution to the overall weight loss cannot be determined.
Kalman et al. [22] also examined the cardiovascular effects of Xenadrine RFA-1™ that contained 20 mg ephedrine, 5 mg p-synephrine and 200 mg caffeine in two capsules in 27 healthy, overweight individuals (Table 1). The study was a 14 day, placebo-controlled double-blinded crossover protocol where subjects received two capsules of the product or placebo for the first seven days and four capsules per day for the next seven days. Analyses were conducted at baseline, and days seven and 14. No significant differences were observed at any time point between treated and placebo control with respect to systolic and diastolic blood pressures, heart rate, or heart valve function and left ventricular ejection fraction as determined by serial echocardiograms or Doppler echocardiograms. The maximum amount of p-synephrine (10 mg per day) was small compared to the maximum amounts of ephedrine (40 mg per day) and caffeine (400 mg per day).
Kalman et al. [23] published a subsequent commentary on this study [22] where they reassessed the incidence of adverse effects. A statistically larger proportion of subjects taking the product reported minor adverse effects as dry mouth, increased activity, and sleep disorders, but there were no serious adverse events and no significant difference with regard to cardiovascular measurements (heart rate, blood pressures, serial echocardiograms and Doppler echocardiograms) between the treated and placebo groups.
Gurley et al. [24] conducted a study in 12 human subjects who were given a bitter orange extract for 28 days (Table 1). The daily consumption of p-synephrine was 30.6 mg. The authors concluded that a supplement containing C. aurantium extract did not appear to significantly modulate cytochrome P450 enzyme activities in human subjects, and therefore posed minimal risk for cytochrome P450-mediated, herb-drug interactions. The bitter orange extract had no significant effect on CYP1A2, CYP2D6, CYP2E1 or CYP3A4, the major drug-metabolizing cytochrome enzymes. No adverse effects were observed. The authors did not assess the possible effects on body weight or blood chemistry.
Zenk and Kuskowski [25] conducted a randomized double-blind 8 week study to evaluate the effect of a proprietary weight management product (Lean Source™) on body composition of overweight men and women. The study was not published (Table 2), but a copy of the final report is available on line. Of the 65 adults enrolled, 54 completed the study with 6 dropouts from the placebo group and 5 subjects from the product treatment group. The product contained extracts of bitter orange, guarana and green tea as well as 7-oxo-dehydroandrostenedione (DHEA), conjugated linoleic acid and chromium picolinate. The daily consumption of bitter orange extract was 200 mg but the p-synephrine content was not noted.
At the completion of the 8 week study, the treated group lost an average of 2.9 kg body weight, while the placebo group lost 1.5 kg body weight. No significant differences occurred between the treatment and placebo groups with respect to systolic and diastolic blood pressures, heart rate or temperature. Furthermore, there were no significant differences in serum chemistry profiles and complete blood counts between the two groups. There was also no difference in the reported incidence of adverse events between the two groups and no serious adverse events were reported [25]. It is not possible to determine the role of p-synephrine and the bitter orange extract in the observed effects.
A study involving the use of another commercial weight loss product (Lean System 7™) on various parameters was assessed by Zenk et al. [26] (Table 1). This was a randomized, double-blind placebo-controlled study involving healthy, overweight adults. A total of 35 subjects completed the 8 week study. Each adult received three capsules of the weight-loss product twice daily or a placebo in conjunction with a calorie-restricted diet and an exercise program. The product contained 6 mg p-synephrine/capsule (36 mg/day). The product also contained 3-acetyl-7-oxo-dehydroepiandrosterone (17 mg), Coleus forskohlli extract (50 mg extract, 10 mg forskolin), yerba mate extract (167 mg), guarana extract (233 mg extract, 51 mg caffeine), piperine (1.67 mg from Piper nigrum) and dandelion leaf and root powder (83 mg). The most significant finding of the study was a 7.2% increase in resting metabolic rate in the treated subjects relative to the control group. No significant differences were noted between the treated and the placebo-controlled groups with respect to body weight, body fat, or lean tissue [26]. No changes in heart rate or blood pressure were observed and no serious adverse events were reported. The relative role of each of the ingredients cannot be determined.
Min et al. [27] assessed the QTc-prolonging and hemodynamic effects of a single dose of a bitter orange extract (Nature's Way) containing 27 mg p-synephrine (Table 1). This randomized, placebo-controlled, double blind and crossover study involved 18 healthy subjects. The rate-corrected QT (QTc) interval and blood pressure were measured before dosing and at 1, 3, 5 and 8 hrs after dosing. The bitter orange extract did not significantly alter the QTc interval or the systolic or diastolic blood pressures at any time point.
Haller et al. [28] have examined potential cardiovascular changes associated with a single oral dose of a bitter orange extract (Advantra Z®) (46.9 mg p-synephrine) and a multiple component dietary supplement (Xenedrine EFX®) which contained 5.5 mg p-synephrine, caffeine and other ingredients (Table 1). The protocol consisted of a randomized, double blind, placebo-controlled crossover design involving 10 subjects with a one-week washout between treatments. The results showed that the dietary supplement but not the p-synephrine-containing bitter orange extract increased both systolic and diastolic blood pressures at two hours post-treatment, while heart rate increased at six hours by 16.7 beats/min with the complex dietary supplement and 11.4 beats/min with the bitter orange (p-synephrine) extract. The authors concluded that the pressure effects were not likely caused by the C. aurantium alone since no blood pressure effect was observed with an 8-fold higher dose of p-synephrine. The authors also concluded that the increase in blood pressure may be attributable to caffeine and other stimulants in the dietary supplement. The increase in heart rate reported for p-synephrine at 6 hours is not consistent with the pharmacokinetics of p-synephrine or a number of other studies, and is discussed in detail below. The amounts of the ingredients in the products were verified by analytical analysis.
Bui et al. [29] conducted a randomized, double-blind, placebo-controlled crossover study involving 15 healthy subjects who received a single dose of 900 mg bitter orange extract (Nature's Way) standardized to 6 % synephrine (54 mg p-synephrine) or the placebo (Table 1). Heart rate and blood pressure were measured every hour for six hours. These investigators reported small but clinically insignificant increases in heart rate, and systolic and diastolic blood pressures for up to five hours. The difference in the results between this study and the study of Min et al. [24] which involved the same product and may be related to the dose which was twice as large in this study by Bui et al. [29]. However, these effects on heart rate and blood pressure were small and have not been observed in other studies.
Sale et al. [30] conducted a double blind study on the physiological and metabolic effects of Xenadrine EFX® in 20 overweight individuals at rest and during treadmill walking (Table 1). As noted above, this product contained bitter orange, guarana and green tea extracts. Subjects received either the placebo or the product and were followed for seven hours or exercised on a treadmill for 60 min. The product had no effect on ATP utilization under resting or exercise conditions relative to control. However, a 30 % increase in carbohydrate oxidation was observed. Fatty acid oxidation to ATP decreased while plasma levels of fatty acids increased in response to the product. The product had no effect on resting heart rate or blood pressure.
Gougeon et al. [31] investigated the thermic effect of food in conjunction with the phenylethylamine protoalkaloids extracted from C. aurantium in 30 healthy but mostly overweight (average BMI≈27) male and female subjects (Table 1). No other ingredients were present in the product. The thermic effect of food on a 1.7 MJ, 30 gram protein meal was determined intermittently for 300 min by indirect calorimetry. Five capsules of the C. aurantium extract provided 26 mg p-synephrine, and 4 mg or less each of other phenethylamines (Advantra Z®). The thermic effect of food was determined on an initial 30 subjects. A subset of 11 men and 11 women were additionally studied after ingestion of the bitter orange extract in conjunction with the protein meal, while a another subset of 12 women and 8 men were studied a third time following ingestion of the C. aurantium-containing capsules alone.
This study [31] demonstrated that the thermic effect of food was 20% lower in women than men following a meal. An increase in the thermic effect of food was seen only in women, increasing by 29%, when the bitter orange extract was used in conjunction with the protein meal. The thermic effect of the bitter orange extract was greater in men than women in the absence of a meal. A significant increase in the respiratory quotient occurred in both sexes in response to the bitter orange extract alone. No significant changes occurred in systolic and diastolic blood pressures or pulse rates when compared with base line values following exposure to the bitter orange extract.
Hoffman et al. [32] examined the thermogenic effect of a coffee which contained added C. aurantium extract, Garcinia cambogia and chromium (JavaFitTM) over a three hour period of time in a randomized, double blind study which involved 10 healthy, physically active subjects (Table 1). The enriched coffee product contained 450 mg caffeine, 21.6 mg p-synephrine, 600 mg hydroxycitric acid and 225 mcg chromium polynicotinate. Significant increases were observed in responders with respect to resting metabolic rate and respiratory exchange ratio. No significant differences were observed in average heart rate or diastolic blood pressure while a 3 mm Hg increase was observed in the systolic blood pressure. The modest effect on blood pressure is not surprising based on the amount of caffeine in the product.
Talbott et al. [33] conducted a six week study involving 30 healthy subjects, half of whom consumed 80 mg p-synephrine/day in the form of the patented C. aurantium extract product Advantra Z® while the other half of the subjects received the placebo (Table 2). Heart rate and blood pressures were determined at the start of the study and after six weeks. No significant differences were observed between the treated and placebo control groups at the conclusion of the study with respect to these cardiovascular parameters. No effects on body weight were reported. This study represents the highest dose of p-synephrine for the longest period of time that has been reported. The study was presented at a scientific meeting but never published.
Haller et al. [34] examined the effects of a performance-enhancing dietary supplement under resting and exercise conditions involving 10 subjects. The product (Ripped Fuel Extreme CutTM) contained 21 mg p-synephrine and 304 mg caffeine, as well as other ingredients including herbal extracts of green tea, ginger root, cocoa seed, willow bark, and wasabi. The product or placebo was taken one hour prior to 30 min of moderately intense exercise. No significant treatment-related differences in systolic blood pressure, heart rate or body temperature were observed. Product-related increases in diastolic blood pressure (8.7 mmHg) and blood glucose levels were observed. Exercise was perceived as being less strenuous after consumption of the product. Due to the poly-alkaloidal and protoalkaloidal nature of this product, the factor or factors responsible for the effects on blood glucose and diastolic blood pressure cannot be determined.
Seifert et al. [35] conducted a study on the effects of an herbal blend on energy expenditure in mildly obese subjects (Table 1). The product contained 13 mg p-synephrine (as Advantra Z®), 176 mg caffeine (as guarana), and 55.5 mg of a green tea extract per capsule. The study involved 14 females and 9 males in a placebo-controlled, crossover design. Subjects ingested one capsule with each of three meals on day one of treatment, and one more capsule on the morning of the second day. Data were collected 60 min after the last administration of the product. The results demonstrated that from pre-test on day one to post-test on day two, caloric expenditure increased by approximately 8% following ingestion of the product. Oxygen uptake increased from 230 to 250 ml/minute following treatment. No differences were observed in heart rate or blood pressure following treatment. This was an acute study which did not provide information on long-term effects, but did demonstrate an increase in energy expenditure.
Stohs et al. [36] examined the effect of 50 mg p-synephrine (Advantra Z®, 60 % p-synephrine) alone or in combination with selected flavonoids in 40 human subjects on resting metabolic rate (Table 1). The study was a randomized, placebo-controlled, double blind design with the vehicle for the p-synephrine being one ounce of tomato juice. The amount of p-synephrine in the product was verified by independent analysis. Measurements were taken at baseline prior to consuming the product and at 75 min. At this time point, a 6.9 % increase in resting metabolic rate was observed in response to the p-synephrine relative to the placebo-control group. No significant effects were observed with respect to blood pressure or heart rate, nor were there any significant differences in responses to a 10 item self-report questionnaire which addressed such issues as nervousness, tension, anxiety, hunger, energy, headache, general discomfort, and sleepiness. The results of this study using p-synephrine/bitter orange alone support the previous thermic calorie expenditure studies [18-20, 26, 30-32, 35], indicating that p-synephrine increases the metabolic rate. Longer term safety and efficacy studies involving p-synephrine alone are warranted.
In an as yet unpublished randomized, placebo-controlled, double blind crossover study that was presented at a scientific meeting, 16 healthy subjects consumed a capsule containing 49 mg p-synephrine (Advantra Z®, 30 % p-synephrine) or the placebo daily for 15 days [37] (Table 2). The amount of p-synephrine in the capsules was determined by high pressure liquid chromatographic analysis. Electrocardiograms, blood pressures, heart rates, blood chemistries and blood cell counts with differentials were determined at baseline, 30 min, 60 min, 90 min, 2 hours, 4 hours, 6 hours and 8 hours, as well as after 5, 10 and 15 days. Blood samples were drawn after 2 hours after the first dose as well as at 5, 10 and 15 days to measure p-synephrine levels to ensure compliance. p-Synephrine had no significant effect on heart rate, blood pressure, blood chemistries, or blood cell counts, and caused no cardiovascular abnormalities. This is the most detailed investigation on the safety of bitter orange/p-synephrine alone.
Bloomer et al. [38, 39] have reported on the effects of a multi-component product (Meltdown®) that contains neither p-synephrine nor bitter orange extract but does contain methyl-synephrine HCl and several other synthetic phenethylamine derivatives as well as yohimbine. Methyl-synephrine is purported to occur in nature, does not occur in bitter orange extract, and is of synthetic origin in this product and other products that have been marketed. For the sake of completeness, the results of these studies will be summarized, but these results will not be incorporated into the general discussion of bitter orange extract and p-synephrine provided below.
Both studies examined the effects of the product on metabolic rate, and plasma free fatty acids, glycerol, norepinephrine and epinephrine levels. The initial study [38] measured changes over a 90 min time frame in 10 healthy male subjects. The second study [39] measured the same parameters over a six hour time frame in 10 healthy male and 10 healthy female subjects. Increases in each of the parameters were observed, with a 13.5 % increase in caloric expenditure over the six hours. It is also important to note that significant increases in heart rate as well as systolic and diastolic blood pressure occurred in both studies in response to the consumption of the product. The authors note that the product may be useful in healthy, normotensive, closely monitored individuals. However, it is not a product that should be recommended to the general public. Furthermore, methyl-synephrine should not be confused or equated with p-synephrine/bitter orange, does not occur in significant amounts in nature if at all, and exhibits cardiovascular effects.
In a study similar to those reported by Bloomer et al. [38, 39], Hoffman et al. [40] conducted a double blinded, placebo-controlled cross-over study involving the methyl-synephrine-containing product Meltdown®. Over a three hour time period following ingestion of the product significant increases in resting oxygen uptake and caloric expenditure were occurred. However, increases in heart rate, systolic blood pressure, tension and confusion were also observed, confirming the highly undesirable properties of this synthetic product.
Clinical Case Reports
Stohs [41] has reviewed and assessed the 22 FDA adverse event reports (AERs) from April 2004 through October 2009 associated with bitter orange (C. aurantium)-containing products, as well as 10 clinical case reports published during this time interval regarding the possible involvement of bitter orange-containing weight management products with cardiovascular incidents and other adverse events. Bitter orange extract and/or p-synephrine were implicated as the possible causative agent in the case reports by all of the authors. In all reported AERs and case cases, the products involved were poly-herbal, poly-alkaloidal and poly-protoalkaloidal.
Adverse events that have been purported in conjunction with the published clinical case reports included: acute lateral-wall myocardial infarction, exercise-induced syncope associated with QT prolongation, ischemic stroke, ischemic colitis, vasospasm and stroke, variant angina, coronary vasospasm and thrombosis, exercise induced rhabdomyolysis, ST segment myocardial infarction, and ventricular fibrillation [41]. In one case report it was suggested that a bitter orange-containing dietary supplement may have masked bradycardia and hypotension while exacerbating weight loss in an individual with anorexia nervosa, although no evidence was provided that an adverse event had actually occurred.
Although the products consumed were all multi-ingredient, in each case reference was specifically made to C. aurantium, bitter orange or p-synephrine as the most likely causative agent. Unfortunately, a wide range of confounding factors also existed among the published case reports including: heart murmur, pre-existing heart disease, hypertriglyceridemia, obesity, a history of smoking, gastroesophageal disease, physical inactivity, sickle cell trait, dehydration, pneumonia, possible use of anabolic steroids and/or performance enhancing drugs, high caffeine intake, and high alcohol consumption. Furthermore, products were not always being taken as recommended, and it was not always clear if the subjects were using other unreported dietary supplements and/or drugs [41]. A more probable culprit for at least some of these effects may have been the high caffeine intake associated with the products in question.
Another factor to be considered is the occurrence of up to 25-40 mg p-synephrine per quarter liter of various Citrus juices [42, 43] which are widely consumed without the report of adverse events. Millions of individuals ingest p-synephrine and bitter orange-containing food products as orange juices and marmalades as well as dietary supplements on a daily basis. Therefore, although these case reports should raise the level of awareness with regard to the use of complex weight management products, it is not possible to extrapolate the cause of these adverse effects to the p-synephrine which may have been present in the products. No evidence showing a direct link between bitter orange extract and the adverse events is provided [41]. Furthermore, it should be remembered that “case reports are incomplete, uncontrolled, retrospective, lack operational criteria for identifying when an adverse event has actually occurred, and resemble nothing so much as hearsay evidence, a type of evidence that is prohibited in all courts of industrialized societies” [44].
Discussion
A total of 23 published and unpublished studies involving a total of approximately 450 total human subjects were reviewed. Of these total subjects, approximately 360 consumed bitter orange/p-synephrine containing products, with 247 subjects involved in published studies and 113 subjects involved in studies that have not been published to date. The authors located information regarding the unpublished studies through presentations at scientific meetings and availability of research reports on the internet, as well as information from the investigators involved in the studies.
In the 23 studies, over 50 % of the subjects were overweight/obese, and about two-thirds of these overweight/obese subjects who consumed p-synephrine (10-53 mg/day) did so in combination with caffeine. No increase in heart rate or blood pressure was reported for any of the over-weight/obese subjects, with various studies lasting for two to 12 weeks.
Seven of the studies were not published in peer reviewed journals [17-20, 25, 33, 37] (Table 2). However, six of these studies were presented at national meetings [18-20, 25, 33, 37], and one of these six studies is in the process of being submitted for consideration for publication [37]. As noted in the references, information including presentations and final reports are available on the internet regarding these unpublished studies. The results associated with these unpublished studies (Table 2) in general are consistent with the results of the published studies (Table 1).
Of the total subjects associated with all of the studies that consumed bitter orange extract/p-synephrine, 149 were male, 211 were female, and the gender of the remainder was not reported. Of the p-synephrine consuming subjects, about 35 % of the subjects consumed products containing caffeine in combination with p-synephrine. The remaining subjects consumed only a p-synephrine/bitter orange extract-containing product or a p-synephrine containing multi-ingredient product devoid of caffeine, but may have consumed unknown amounts of caffeine through ingestion of coffee, tea or cola beverages.
Five published studies [6, 24, 27, 31, 36] and two unpublished studies [33, 37] reported no cardiovascular effects when using p-synephrine (bitter orange) only containing products. The published studies involved a total of 104 subjects with a total of 31 subjects in the two unpublished studies. However, in one of these studies [24] consisting of 12 subjects it is not clear that effects on heart rate and blood pressure were specifically examined, with the authors simply reporting that no adverse effects were observed. Five published studies [15, 21, 22, 26, 30, 35] using p-synephrine in combination with other ingredients reported no cardiovascular effects. A total of 88 subjects were involved in these studies.
Small cardiovascular effects were reported by Bui et al. [29] (15 subjects) using a 50 mg dose of p-synephrine which was not replicated by others [33, 36, 37] (total of 71 subjects) using a similar dose. Small cardiovascular effects were reported for three studies that involved subjects consuming p-synephrine plus caffeine [28, 32, 34]. Haller et al. reported an increase in heart rate [28] and diastolic blood pressure [34] (10 subjects). Hoffman et al. [32] observed a small increase (3 mm Hg) in the systolic blood pressure of seven subjects considered to be responders with no significant increase relative to all subjects.
Upon careful review, the study of Haller et al. [28] involving a small number (10) of subjects has a number of issues in terms of design and conclusions. p-Synephrine (Advantra Z®) alone had no effect on systolic or diastolic blood pressure. The authors reported an increase in heart rate six hours after treatment. The half-life of p-synephrine is two to three hours [28, 34, 45]. As a consequence, an increase in heart rate after two to three half-lives when the p-synephrine blood levels will have dropped to one-fourth to one-eighth the peak blood levels would not be expected. Furthermore, a major complicating factor is that all subjects consumed a meal three hours after ingesting the p-synephrine (46.9 mg) or a product containing p-synephrine (5.5 mg), octopamine (5.7 mg) and caffeine (239 mg). The cardiovascular and thermic effects of food are well known [31], and an increase in heart rate in the control group was also observed. Thus, it is not plausible to attribute the increase in heart rate to p-synephrine, or an increase in heart rate and blood pressure to a product that contained very little p-synephrine (5.5 mg) in combination with caffeine under these experimental conditions. This study did show that the commercial product Xenadrine EFX® which contained only 5.5 mg p-synephrine produced a significant increase in heart rate and blood pressure at two hours. This product was reported to also contain 5.7 mg octopamine. C. aurantium extracts are either devoid of octopamine or contain only trace amounts [3], thus the product being used [28] appears to have been adulterated.
Hansen et al. [46] examined the cardiovascular effects of p-synephrine at 10 mg/kg and 50 mg/kg orally for 28 days given to rats in the form of 95% p-synephrine or as a 6% p-synephrine containing bitter orange extract. Caffeine (25 mg/kg) was added to some of these doses. Using the standard metabolic equivalency factor of 6 based on body surface area in converting doses in rats to doses in humans [47], a 50 mg/kg dose of p-synephrine and a 25 mg/kg dose of caffeine given to rats translate into 667 mg p-synephrine and 333 mg caffeine per day for an 80 kg (176 lb) human. These doses represent over 13 times the usual daily dose for p-synephrine and the equivalent of the caffeine in about three cups of coffee given together as a single bolus dose to these animals.
Animals treated with the 95% p-synephrine alone demonstrated no significant effects on heart rate or blood pressure, even at the high 50 mg/kg dose. When caffeine was added, increases in heart rate and blood pressure were observed [46]. These studies indicate that in rats at very high doses of p-synephrine the combination with caffeine may result in cardiovascular effects. However, due to the highly inequivalent dosing between this study in rats and typical dosing in humans, the results of this study in rats cannot be directly extrapolated to humans. A dose of 3.0-3.75 mg/kg p-synephrine given to rats would have been more representative of a typical human dose.
Various studies indicate that the lipolytic activity of p-synephrine is due to binding to β-3 adrenergic receptors in adipose tissues [10]. These same β-3 adrenergic receptors are also associated with cardiovascular tissues, and their activation results in a down-regulation of cardiovascular stimulation [48, 49]. Thus, p-synephrine stimulation of β-3 adrenoreceptors in the cardiovascular system does not result in an increase in blood pressure or heart rate but may exhibit a modulating rather than a stimulatory effect. This cardiovascular receptor response may explain why an increase in heart rate or blood pressure is not seen in most cases when p-synephrine is used alone or in combination with caffeine in dietary supplements, in spite of the fact that caffeine alone may produce modest increases in these parameters under some conditions [50, 51].
Approximately half of the clinical studies involved the use of commercial products. In only one case [28] was the actual amount of p-synephrine and other protoalkaloids determined, while in the remaining studies involving commercial products the reported amounts of p-synephrine and caffeine were simply based on label claim. The amount of p-synephrine was independently determined in two studies in which bitter orange extract was used as a single ingredient product [36, 37]. Various studies have shown that there are not always good correlations between the label claim of marketed products or the product data sheet and the amount of p-synephrine shown to be present by independent analysis [52-56]. Therefore, the actual amount of p-synephrine consumed in the majority of the studies was not verified.
Finally, nine studies involving the administration of bitter orange extract alone or in combination with other constituents have demonstrated an increase in metabolic rate without an increase in heart rate or blood pressure [18-20, 26, 30-32, 35, 36]. These results suggest that bitter orange extract and p-synephrine may be beneficial in weight management. However, a need exists for longer term studies involving bitter orange extract/p-synephrine alone to assess the effects on body weight and verify the safety data.
Summary and Conclusions
The results involving both published and unpublished clinical studies indicate that p-synephrine alone or in combination with caffeine does not appear to produce significant adverse cardiovascular effects or pose a risk to human health at doses commonly ingested orally. No adverse effects have been directly attributable to bitter orange extract or p-synephrine. p-Synephrine/bitter orange extract alone as well as in combination with other ingredients results in significant increases in resting metabolic rate, and when taken for periods of time up to 12 weeks may result in modest weight loss.
The results indicate that bitter orange extract and p-synephrine increase metabolism and energy expenditure. The data accumulated to date do not support hypothesized concerns regarding potential adverse effects of p-synephrine particularly with respect to the cardiovascular system due to a paucity of binding to α-, β-1 and β-2 adrenergic receptors while exhibiting modest binding to β-3 adrenergic receptors. However, a need exists for additional well controlled, long term human efficacy and safety studies involving p-synephrine/bitter orange extract.
Acknowledgements
All authors have served as consultants for Nutratech, Inc., a company that markets bitter orange extracts. Nutratech Inc. provided some of the unpublished research reports.
Competing Interests
See Acknowledgements. Authors declare they have no other competing interest.
References
1. Chen JK, Chen TT. Zhi Shi (Fructus Aurantii Immaturus). Chinese Medical Herbology and Pharmacology. City of Industry, CA USA: Art of Medicine Press. 2004:485-487
2. Stohs SJ, Shara M. A review of the safety and efficacy of Citrus aurantium in weight management. In: (ed.) Bagchi D, Preuss HG. Obesity: Epidemiology, Pathophysiology, and Prevention. Boca Raton, FL, USA: CRC Press. 2007:371-382
3. Pellati F, Benvenuti S. Chromatographic and electrophoretic methods for the analysis of phenethylamine alkaloids in Citrus aurantium. J Chromatog A. 2007;1171:71-88
4. Sander LC, Putzbach K, Nelson BC. et al. Certification of standard reference materials containing bitter orange. Analyt Bioanalyt Chem. 2008;391:2023-2034
5. Evans RL, Pho AN, Roman MC, Betz JM. Certification of standard reference materials containing bitter orange. Analyt Bioanalyt Chem. 2008;391:2023-2034
6. Penzak SR, Jann MW, Cold JA. et al. Seville (sour) orange juice: synephrine content and cardiovascular effects in normotensive adults. J Clin Pharmacol. 2001;41:1059-1063
7. Bent S, Padula A, Neuhaus J. Safety and efficacy of Citrus aurantium for weight loss. Amer J Cardiol. 2004;94:1359-1361
8. Nasir JM, Durning SJ. et al. Exercise-induced syncope associated with QT prolongation and ephedra-free Xenadrine. Mayo Clinic Proceed. 2004;79:1059-1062
9. Stephensen TA, Sarlay JrR. Ventricular fibrillation associated with use of a synephrine containing dietary supplement. Military Med. 2009;174:1313-1319
10. Stohs SJ, Preuss HG, Shara M. A review of the receptor-binding properties of p-synephrine as related to its pharmacological effects. Oxid Med Cell Long. 2011 doi:10.1155/2011/482873
11. Stohs SJ, Preuss HG. Stereochemical and pharmacological differences between naturally occurring p-synephrine and synthetic p-synephrine. J Funct Foods. 2011 doi:10.1016/j.jff.2011.09.004
12. McGuffin M. Media spins numbers on bitter orange AERs based on erroneous information from FDA. HerbalGram. 2006;69:52-55
13. Stohs SJ, Preuss HG. The Safety of bitter orange (Citrus aurantium) and its primary protoalkaloid p-synephrine. HerbalGram. 2011;89:34-39
14. Stohs SJ, Preuss HG, Shara M. The safety of Citrus aurantium (bitter orange) and its primary protoalkaloid p-synephrine. Phytother Res. 2011 doi: 10.1002/ptr.3490
15. Colker CM, Kalman DS, Torina GC, Perlis T, Street C. Effects of Citrus aurantium extract, caffeine, and St. John's wort on body fat loss, lipid levels, and mood states in overweight healthy adults. Curr Therap Res. 1999;60:145-153
16. Dulloo AG, Duret C, Rohrer D. et al. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Amer J ClinNutr. 1999;70:1040-1045
17. Kendall-Reed P. Study on the effectiveness of Ultra Slim Down® for the reduction of body weight. Unpublished report. 2000. http://www.nutratechinc.com/advz/advz.php?p=2
18. Kalman DS, Oxford S, Schwartz HI, Krieger DR. A double blind clinical evaluation of the metabolic effects of Xenadrine EFXTM compared to two ephedra-containing products in normal healthy volunteers. Presented at the annual meeting of the American College of Nutrition, Abstract No. 101. J Amer Coll Nutr. 2002;21:488
19. Kalman DS, Rubin S, Martinez T, Schwartz HI. Efficacy of a commercial green tea extract/caffeine based product to increase basal metabolism in healthy adults. Presented at a meeting of NIH-National Institute of Diabetes and Digestive and Kidney Diseases. Unpublished report. 2002. http://www.nutratechinc.com/advz/advz.php?p=2
20. Kalman DS, Rubin S, Schwartz HI. An acute clinical trial to evaluate the safety and efficacy of a popular commercial weight loss supplement when used with exercise. Presented at Federation of American Societies of Experimental Biology. Unpublished report. 2003. http://www.nutratechinc.com/advz/Studies2011/Efficacy/E11%20Kalman%202003.pdf
21. Kalman DS, Colker CM, Shi Q, Swain MA. Effects of a weight-loss aid in healthy overweight adults: double-blind, placebo-controlled clinical study. Curr Ther Res. 2000;61:199-205
22. Kalman DS, Incledon T, Gaunaurd I. et al. An acute clinical trial evaluating the cardiovascular effects of an herbal ephedra-caffeine weight loss product in healthy overweight adults. Int J Obes. 2002;26:1363-1366
23. Kalman DS. Comment on: An acute clinical trial evaluating the cardiovascular effects of an herbal ephedra-caffeine weight loss product in healthy overweight adults. Int J Obes Relat metab Disord. 2004;28:1355-1356
24. Gurley BJ, Gardner SF, Hubbard MA. et al. In vivo assessment of botanical supplementation on human cytochrome P450 phenotypes: Citrus aurantium, Echinacea purpurea, milk thistle, and saw palmetto. Clin Pharmacol Therap. 2004;76:428-440
25. Zenk JL, Kuskowski MA. A prospective, randomized, double blind study to evaluate the effect of Lean Source™ on body composition in overweight adult men and women. Unpublished report. 2005. http://www.nutratechinc.com/advz/advz.php?p=2
26. Zenk JL, Leikam SA, Kassen LJ, Kuskowski MA. Effect of Lean System 7 on metabolic rate and body composition. Nutrition. 2005;21:179-185
27. Min B, Cios D, Kluger J, White CM. Absence of QTc interval- prolonging or hemodynamic effects of a single dose of bitter orange extract in healthy subjects. Pharmacother. 2005;25:1719-1724
28. Haller CA, Benowitz N, Peyton J III. Hemodynamic effects of ephedra-free weight loss supplements in humans. Amer J Med. 2005;118:998-1003
29. Bui LT, Nguyen DT, Ambrose PJ. Blood pressure and heart rate affects following a single dose of bitter orange. Ann Pharmacodyn. 2006;40:53-57
30. Sale C, Harris RC, Delves S, Corbett J. Metabolic and physiological effects of ingesting extracts of bitter orange, green tea and guarana at rest and during treadmill walking in overweight males. Int J Obesity. 2006;30:764-773
31. Gougeon R, Harrigan K, Tremblay JF. et al. Increase in the thermic effect of food in women by adrenergic amines extracted from Citrus aurantium. Obesity Res. 2006;13:1187-1194
32. Hoffman JR, Kang J, Ratamess A. et al. Thermogenic effect from nutritionally enriched coffee consumption. J Int Soc Sports Nutr. 2006;3:35-41
33. Citrus aurantium extract has no effect on blood pressure or heart rate in healthy adults. Unpublished report 2007 Talbott SM, Christopulos AM, Richards E. www.nutratechinc.com/adz/UploadFile/Talbott%2020907.pdf
34. Haller CA, Duan M, Peyton J III, Benowitz N. Human pharmacology of a performance-enhancing dietary supplement under resting and exercise conditions. Brit J Clin Pharmacol. 2008;65:833-840
35. Seifert JG, Nelson A, Devonish J. et al. Effect of acute administration of an herbal preparation on blood pressure and heart rate in humans. International J Med Sci. 2011;8:192-197
36. Stohs SJ, Preuss HG, Keith SC. et al. Effects of p-synephrine alone and in combination with selected bioflavonoids on resting metabolism, blood pressure, heart rate and self-reported mood changes. Int J Med Sci. 2011;8:295-301
37. Shara M, Stohs SJ. Safety evaluation of bitter orange extract (p-synephrine) in healthy volunteers. Presented at the annual meeting of the American College of Nutrition. Abstract No. 16. J Amer Coll Nutr. 2012;30:358
38. Bloomer RJ, Canale RE, Blankenship MM. et al. Effects of the dietary supplement Meltdown on catecholamine secretion, markers of lipolysis, and metabolic rate in men and women: a randomized, placebo controlled, cross-over study. Lipids Health Disease. 2009;8:32-40
39. Bloomer R, Fisher-Wellman KH, Hammond KG. et al. Dietary supplement increases plasma norepinephrine, lipolysis, and metabolic rate in resistance trained men. J Int Soc Sports Nutr. 2009;6:6-12
40. Hoffman JR, Kang J, Ratamess NA. et al. Thermogenic effect of an acute ingestion of a weight loss supplement. J Int Soc Sports Nutr. 2009;6:1-5
41. Stohs SJ. Assessment of the adverse event reports associated with Citrus aurantium (bitter orange) from April 2004 to October 2009. J Funct Foods. 2010;2:235-238
42. Dragull K, Breksa AP, Cain B. Synephrine content of juice from Satsuma mandarins (Citrus unshiu Marcovitch). J Agric Food Chem. 2008;56:8874-8878
43. Uckoo RM, Jayaprakasha GK, Nelson DS, Pati BS. Rapid simultaneous determinations of amines and organic acids in citrus using high-performance liquid chromatography. Talanta. 2010;83:948-954
44. Karch SB. Peer review and the process of publishing of adverse drug event reports. J Forensic Legal Med. 2007;14:79-84
45. Hengtmann JH, Aulepp H. Pharmacokinetics and metabolism of synephrinc. Arzneimittel Forschung. 1978;28:2326-2331
46. Hansen DK, George NI, White GE. et al. Physiological effects following administration of Citrus aurantium for 28 days in rats. Toxicol Appl Pharmacol. 2012 doi: 10.1016/j.tap.2012.04.006
47. Reagan-Shaw S, Nihal M, Ahmed N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659-661
48. Rozec B, Gauther C. β3-Adrenoreceptors in the cardiovascular system: Putative roles in human pathologies. Pharmacol Therap. 2006;111:652-673
49. Moens AL, Yang R, Watts V, Barouch LA. Beta 3-adrenoreceptor regulation of nitric oxide in the cardiovascular system. J Molec Cell Cardiol. 2010;48:1088-1095
50. Yang A, Palmer AA, DeWit H. Genetics of caffeine consumption and responses to caffeine. Psychopharmacol. 2010;211:245-257
51. Riksen NP, Smits P, Rongen GA. The cardiovascular effects of methylxanthines. Handbook Exper Pharmacol. 2011;200:413-437
52. Avula B, Upparapalli SK, Navarrete A, Khan IA. Simultaneous quantification of adrenergic amines and flavonoids in C. aurantium, various Citrus species, and dietary supplements by liquid chromatography. J AOAC Int. 2005;88:1592-1606
53. Slezak T, Francis PS, Anastos N, Barnett NW. Determination of synephrine in weight-loss products using high performance liquid chromatography with acidic potassium permanganate chemiluminescence detection. Anal Chim Acta. 2007;593:98-102
54. Evans LE, Siitonen PH. Determination of caffeine and sympathomimetic alkaloids in weight loss supplements by high-performance liquid chromatography. J Chromatog Sci. 2008;46:61-67.53
55. Mercolini L, Mandrioli R. et al. Fast CE analysis of adrenergic amines in different parts of Citrus aurantium fruit and dietary supplements. J Sep Sci. 2010;33:1-8
56. Percy DW, Adcock JL, Conlan XA. et al. Determination of Citrus aurantium protoalkaloids using HPLC with potassium permanganate chemiluminescence detection. Talanta. 2010;80:2191-2195
Author contact
![]() Corresponding author: Harry G. Preuss, M.D., preusshgedu, phone: 1-202-687-1441.
Corresponding author: Harry G. Preuss, M.D., preusshgedu, phone: 1-202-687-1441.

 Global reach, higher impact
Global reach, higher impact