3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2011; 8(7):514-522. doi:10.7150/ijms.8.514 This issue Cite
Research Paper
Impairment of Pulmonary Function is an Independent Risk Factor for Atrial Fibrillation: The Takahata Study
1. Department of Cardiology, Pulmonology, and Nephrology, Yamagata University School of Medicine, Yamagata, Japan
2. Department of Gastroenterology, Yamagata University School of Medicine, Yamagata, Japan
3. Department of Neurology, Hematology, Metabolism, Endocrinology, and Diabetology, Yamagata University School of Medicine, Yamagata, Japan
4. Department of Neurosurgery, Yamagata University School of Medicine, Yamagata, Japan
Received 2011-6-13; Accepted 2011-8-19; Published 2011-8-29
Abstract
Background: Chronic pulmonary disorders, such as chronic obstructive pulmonary disease (COPD) and fibrosing lung diseases, and atrial fibrillation (AF), are prevalent in elderly people. The impact of cardiac co-morbidities in the elderly, where pulmonary function is impaired, cannot be ignored as they influence mortality. The relationship between the prevalence of AF and pulmonary function is unclear. The aim of this study was to evaluate this relationship in participants in a health check.
Methods: Subjects aged 40 or older (n = 2,917) who participated in a community-based annual health check in Takahata, Japan, from 2004 through to 2005, were enrolled in the study. We performed blood pressure measurements, blood sampling, electrocardiograms, and spirometry on these subjects.
Results: The mean FEV1 % predicted and FVC % predicted in AF subjects was significantly lower than in non-AF subjects. The prevalence of AF was higher in those subjects with airflow limitation or lung restriction than in those without. Furthermore, AF prevalence was higher in those subjects with severe airflow obstruction (FEV1 %predicted < 50) than in those who had mild or moderate airflow obstruction (FEV1 %predicted ≥ 50), although there was no difference between the prevalence of AF in subjects with 70≤ FVC %predicted <80 lung restriction and those with FVC %predicted <70. Multiple logistic regression analysis revealed that FEV1 %predicted and FVC %predicted are independent risk factors for AF (independent of age, gender, left ventricular hypertrophy, and serum levels of B-type natriuretic peptide).
Conclusion: Impaired pulmonary function is an independent risk factor for AF in the Japanese general population.
Keywords: atrial fibrillation, pulmonary function, general population
Introduction
Long-term cigarette smoking causes respiratory airway injury and results in pulmonary diseases such as chronic obstructive pulmonary disease (COPD) and lung cancer (1). COPD is characterized by airflow limitation that is not fully reversible; post-bronchodilation forced expiratory volume in 1 second (FEV1)/ forced vital capacity (FVC) < 70% (2). In Japan, it is estimated that 10% of the general population have COPD (3), most of whom are undiagnosed because patients with mild or moderate COPD show less clinical symptoms, such as dyspnea, cough, and sputum. Recently, the clinical phenotype of COPD was suggested to be associated with chronic inflammation, not only in the lung, but in the whole body, resulting in various co-morbidities such as unexplained cachexia, osteoporosis, and diabetes mellitus (4). As we previously demonstrated, differences in genetic backgrounds in COPD patients are involved in this exaggerated inflammatory response (5-7).
Coronary artery disease is one of the most well-known co-morbidities seen in COPD patients (8), and is a risk factor that influences mortality associated with this disease. In addition, serum levels of B-type natriuretic peptide (BNP), a marker of congestive heart failure (9), are reportedly, significantly elevated in COPD patients (10), and COPD patients frequently have cardiac disease (8). Consistent with this, left ventricular filling is reportedly impaired in patients with pulmonary emphysema (11).
Atrial fibrillation (AF) is one of the most common arrhythmias. The incidence of AF is higher in the older generation (12,13), and its prevalence accordingly increases with a shift to the aging population (14). As with COPD, the development of AF is thought to be involved in systemic inflammation (15), and known risk factors are aging (16,17), cardiac disease (18), hypertension (19), obesity (20), smoking (21,22), and hypoxia (23). A couple of cohort studies on the relationship between AF and COPD are available (24-26), however, their conflicting results make any conclusions difficult.
Long-term cigarette smoking significantly reduces the vital capacity of smokers' lungs (27), and cigarette smoking is a risk factor for fibrosing lung diseases, such as idiopathic pulmonary fibrosis. In some fibrosing lung diseases, impairment of pulmonary vasculatures frequently causes pulmonary hypertension (28). This hemodynamic alteration may induce arrhythmias, such as AF.
We assessed the findings of electrocardiograms and spirometry in Japanese subjects who participated in a community-based annual health check-up. We evaluated the association of AF and impaired pulmonary function in this population.
Methods
Study population
This study formed part of the Molecular Epidemiological Study utilizing the Regional Characteristics of 21st Century Centers of Excellence (COE) Program and the Global COE Program in Japan. Details of the study methodology have been described elsewhere (29). This study was approved by the institutional ethical committee and all participants gave written informed consent.
This study utilized a community-based annual health check, in which all inhabitants of Takahata town (total population 26,026) in northern Japan, who were aged 40 years or older, were invited to participate. This region has a resident population of 15,222 adults, aged 40 or older (7,109 males and 8,113 females). Since employees in public institutes and in many companies are examined annually in health check programs organized by the local health centers, they usually do not participate in the health check held by their local government, such as Takahata town. The local government-organized health check covers farmers, employees of small companies, self-employed persons, housewives and retirees. Four thousand two hundred and eighty-two adults aged 40 years or older participated in this health check program from June 2004 through November 2005, and 1,380 males and 1,735 females (a total of 3,165 subjects) agreed to be enrolled in the study. Two hundred and forty-eight subjects were excluded from the analysis due to spirometry data that did not meet the Japanese Respiratory Society (JRS) criteria (30). Data for a total of 2,917 subjects (1,325 males, 1,592 females) were entered into the final statistical analyses.
Subjects used a self-reporting questionnaire to document their medical histories, smoking habits, current medications, and clinical symptoms. In the questionnaire, 2.2% of subjects reported a history of respiratory disease, although the details were not known. Brinkman index [inhaled cigarettes /day × duration of smoking (years)] was used as an estimate of the amount of inhaled cigarettes in the subject's life.
Measurements
Systolic and diastolic blood pressures were measured with a mercury manometer, and placed on the right arm of seated subjects who had rested in a sitting position for at least 5 min before measurement. This was carried out twice, and the mean value was used for statistical analysis. Fasting blood samples were collected from the antecubital vein with the subject in a sitting position, and samples were transferred immediately to chilled tubes. Immediately following serum isolation, laboratory examinations were performed. Of the 2,917 participants, data on serum BNP levels were available in 2,906 subjects. The remaining serum was stored at -80°C. High sensitivity C-reactive protein (HS CRP) levels were measured in 2010 in the stored serum samples with sufficient volume (1,206 serum samples).
Spirometric values (FVC; FEV1; forced expiratory volume in 6 seconds, FEV6; forced expiratory flow between 25% and 75% FVC, FEF25-75; maximum expiratory flow at 75% FVC, V75; V50 and V25) were measured using standard techniques, with subjects performing FVC maneuvers on a CHESTAC-25 part II EX instrument (Chest Corp., Tokyo, Japan) under the JRS guidelines (30). Bronchodilator was not administered prior to measurements. The highest value from at least three FVC maneuvers by each subject was used for analysis. The results were assessed by visual inspection of flow-volume curves by two pulmonary physicians. Subjects with poor data (according to JRS criteria) were excluded (30). Predictive equations were as described previously (27,31). Subjects whose FEV1/FVC was < 70% were identified as having airflow limitation, and those with FVC %predicted <80% were described as having lung restriction. According to GOLD criteria, the subjects with airflow limitation and FEV1 %predicted ≥80 were identified as having mild airflow limitation, 50≤ FEV1 %predicted <80 were described as moderate, and FEV1 %predicted <50 were described as having severe airflow limitation. In addition, subjects with lung restriction were divided into two groups; 70≤ FVC %predicted <80 and FVC %predicted <70. FVC %predicted < 70 comprised the bottom 2.5 percentile of all the subjects.
A standard 12-lead electrocardiogram (ECG) was recorded after subjects had rested sufficiently. All ECGs were read by cardiologists and classified according to the Minnesota Code revised edition in 1982. Left ventricular hypertrophy (LVH) was considered present if, 1) the summation of R amplitude in lead V5 or V6, and S amplitude in lead V1, was 4.0 mV or over, and 2) R amplitude in lead V5 or V6 was 2.6 mV or over (32).
Chest X-ray findings and the final diagnosis of each subject were unavailable in the database of this study.
Statistical analysis
The Mann-Whitney U test for non-parametric data was used to analyze differences between two groups. Fisher's exact test was used to evaluate differences in proportions. These comparisons and the logistic regression analyses were performed using JMP version 8 software (SAS Institute Inc., Cary, NC, USA). Data in the figures are shown as mean ± SD. Significance was inferred for differences with P < 0.05.
Results
Characteristics of the subjects are summarized in Table 1.
The characteristics of study subjects with and without atrial fibrillation (AF), as determined by electrocardiogram
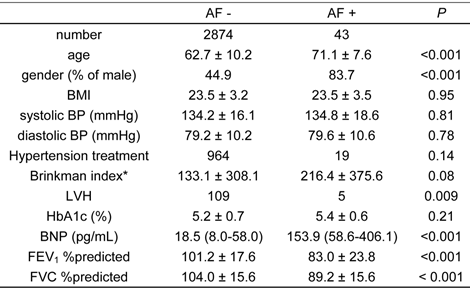
*: Brinkman index is inhaled cigarettes /day × duration of smoking (years).
Age, BMI, BPs, Brinkman index, HbA1c, FEV1 %predicted and FVC %predicted are expressed as mean ± S.D. BNP is expressed as a median (interquartile range).
AF, atrial fibrillation; BMI, body mass index; BP, blood pressure; LVH, left ventricular hypertrophy; HbA1c, hemoglobin A1c; BNP, B-type natriuretic peptide; FEV1 %predicted, percent predicted forced expiratory volume in 1 second; FVC %predicted, percent predicted forced vital capacity
Distribution of atrial fibrillation (AF) relative to age group (generation), gender, serum B-type natriuretic peptide (BNP) level, co-morbidity of left ventricular hypertrophy (LVH), degree of airflow limitation (AFL), and lung restriction (LR).
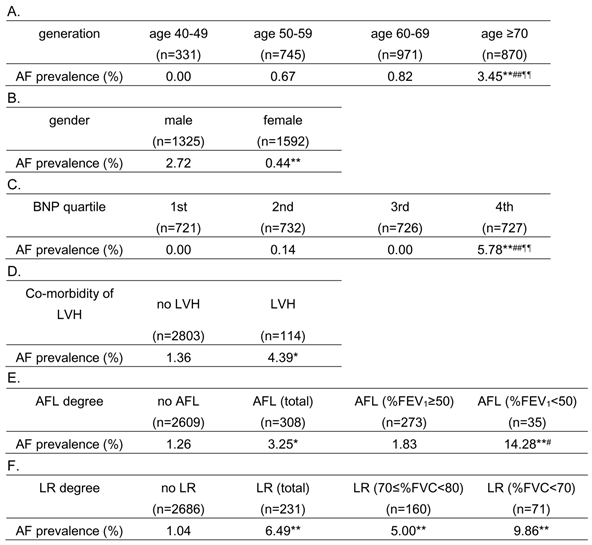
Median (interquartile range) value of BNP was 18.75 (10.2 - 34.3) pg/mL.
* P < 0.05 vs. “no LVH” and “no AFL”, ** P < 0.001 vs. “age 40-49”, “male”, “1st BNP quartile”, “no AFL” and “no LR”, # P < 0.05 vs. “AFL (%FEV1 ≥ 50)”, ## P < 0.001 vs. “age 50-59” and “2nd BNP quartile”, ¶¶ P < 0.001 vs. “age 60-69” and “3rd BNP quartile” in Fisher's exact test
%FEV1, percent predicted forced expiratory volume in 1 second; %FVC, percent predicted forced vital capacity
The prevalence of AF was 1.5%. Subjects with AF were significantly older than subjects without AF, and AF was also more prevalent in males than females. Body mass index (BMI), blood pressure (BP), Brinkman index, and hemoglobin A1c (HbA1c), did not significantly differ between subjects with and without AF. Left ventricular hypertrophy was more prevalent in the AF group, and serum levels of B-type natriuretic peptide (BNP) were significantly higher in subjects with AF than those without. Interestingly, FEV1 %predicted and FVC %predicted were significantly lower in those subjects with AF than those without. As previously demonstrated, the prevalence of airflow limitation in the Takahata study was 10.6% (27). The prevalence of lung restriction in this study population was 9.89% in males, and 6.28% in females. The relative risk of habitual cigarette smoking for the prevalence of lung restriction was 1.876 (95% confidence interval: 1.284 - 2.740) in males, and 1.334 (95% confidence interval: 0.731 - 2.433) in females.
As shown in Table 2, the prevalence of AF was significantly higher in the older subjects (Table 2A), males (Table 2B), subjects in the highest BNP-quartile group (Table 2C), and those with LVH (Table 2D). In addition, AF was more prevalent in subjects with airflow limitation, compared to those without (Table 2E). Furthermore, subjects with severe airflow limitation (FEV1 %predicted < 50) had a higher prevalence of AF than subjects with no airflow limitation and subjects with mild/moderate airflow limitation (FEV1 %predicted ≥ 50), although AF prevalence in the subjects with mild/moderate airflow limitation did not differ from those without airflow limitation. In addition, AF was more prevalent in subjects with lung restriction, compared to those without (Table 2F). Both subjects with 70≤ FVC %predicted <80 lung restriction and those with FVC %predicted <70 restriction had a higher prevalence of AF than subjects without lung restriction. However, the difference in AF prevalence was not statistically significant between subjects with 70≤ FVC %predicted <80 lung restriction and those with FVC %predicted <70 restriction (Table 2F).
As shown in Table 3A, FEV1 %predicted was significantly associated with age and serum BNP levels while FVC %predicted was significantly associated with BNP levels, but not age. Also, these pulmonary function parameters were significantly decreased in males and subjects with LVH (Table 3B). Thus, age, gender, co-morbidity of LVH, and BNP levels might be confounders for the relationship between AF and pulmonary dysfunction.
To investigate whether the degree of airflow limitation (FEV1 %predicted) and lung restriction (FVC %predicted) are independent risk factors for AF from these significant factors, we performed univariate and multivariate logistic regression analyses. Because FEV1 %predicted and FVC %predicted had a very strong correlation (Pearson product-moment correlation coefficient; r =0.85, P <0.00001), these two factors were separately assessed. As shown in Table 4, all variables were independent risk factors for the presence of AF. Importantly, airflow limitation (FEV1 %predicted) and lung restriction (FVC %predicted) were shown to be significant risk factors for AF by multivariate logistic regression analysis.
Table 5 summarizes pulmonary function of the subjects relative to presence of AF. All spirometric values (FEV6 %predicted, FEV1/FVC, FEV1/FEV6, FEF25-75 %predicted, V75 %predicted, V50 %predicted and V25 %predicted) were significantly lower in subjects with AF than in those without.
To investigate the involvement of systemic inflammation in the mechanisms of AF development in the subjects with reduced pulmonary function, HS CRP levels were measured in some subjects, as described in Methods. As shown in Table 6A, HS CRP levels were significantly elevated in those subjects with airflow limitation, compared to those without. Although not statistically significant, HS CRP levels tended to be higher in subjects with lung restriction and with AF, compared to those without (Table 6B and 6C).
Association of pulmonary function with age and serum B-type natriuretic peptide (BNP) levels, and pulmonary function relative to gender and co-morbidity of left ventricular hypertrophy (LVH) findings by electrocardiogram.
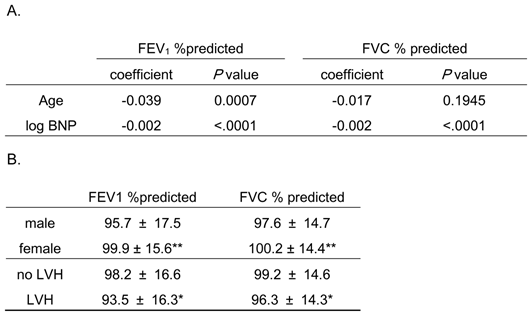
FEV1 %predicted and FVC %predicted are expressed as mean ± S.D.
* P < 0.05 vs. “no LVH”, ** P < 0.001 vs. “male”
Because the distribution of BNP was highly skewed toward the larger values, the logarithm-transformed BNP was used in this analysis.
FEV1 %predicted, percent predicted forced expiratory volume in 1 second; FVC %predicted, percent predicted forced vital capacity
Univariate and multivariate logistic regression analysis for the presence of atrial fibrillation in this study
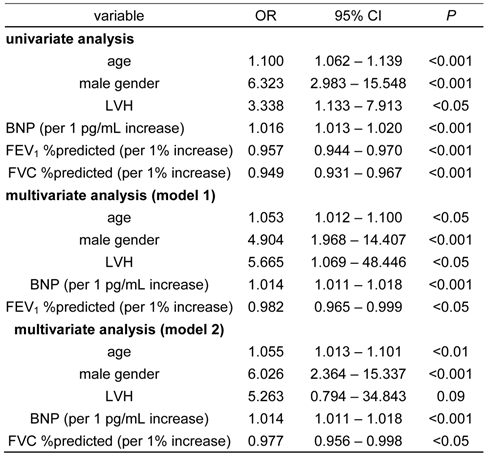
LVH, left ventricular hypertrophy; BNP, B-type natriuretic peptide; FEV1 %predicted, Percent predicted forced expiratory volume in 1 second; FVC %predicted, percent predicted forced vital capacity; OR, odds ratio; 95%CI, 95% confidence interval
Comparison of spirometric measurements on subjects with and without atrial fibrillation (AF), as determined by electrocardiogram.
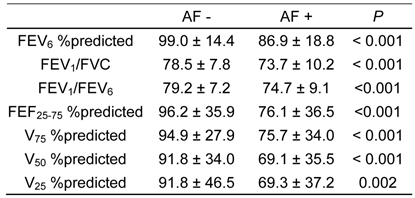
Data are expressed as mean ± S.D.
FEVx, forced expiratory volume in X second; FEF25-75, forced expiratory flow between 25% and 75% FVC; Vx, maximum expiratory flow at X% FVC
Comparison of high sensitivity C-reactive protein (HS CRP) values in subjects with and without airflow limitation (AFL), lung restriction (LR) or atrial fibrillation (AF)
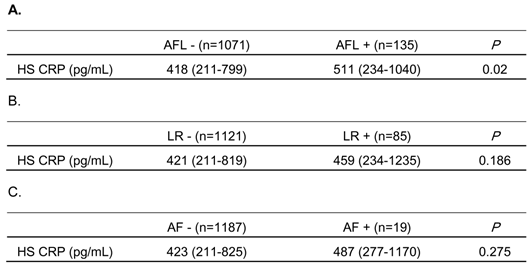
HS CRP levels were measured in 1,206 stored serum samples.
The subjects whose FEV1/FVC was less than 0.7, or whose FVC %predicted was less than 0.8 were identified as having AFL, or LR, respectively.
Data are expressed as median (interquartile range).
Discussion
In this study, mean FEV1 %predicted and FVC %predicted in AF subjects were significantly lower than in non-AF subjects. AF prevalence was higher in those subjects with airflow limitation or lung restriction than those without. Furthermore, the prevalence of AF was higher in those subjects who had severe airflow obstruction (FEV1 %predicted < 50) compared to those with mild or moderate airflow obstruction (FEV1 %predicted ≥ 50). Multiple logistic regression analysis revealed that both FEV1 %predicted, representing the degree of airflow limitation in COPD patients, and FVC %predicted, representing the lung size of the subjects, were independent (from age, male gender, the co-morbidity of LVH, and BNP levels) risk factors for AF.
To date, there are a few epidemiological studies on the relationship between the incidence of AF and pulmonary function (24-26). In the Framingham Heart Study, 4,731 subjects were followed for 38 years, and they concluded that the incidence of AF did not correlate with FEV1 %predicted (24). In the Renfrew/Paisley Study, 15,406 subjects were followed for 4 years and, although they showed that FEV1 %predicted was not an independent risk factor for the incidence of AF, the subjects with AF had lower FEV1 %predicted than those subjects without (26). In the Copenhagen City Heart Study, 13,430 subjects were followed for 5 years, and they demonstrated that the odds ratio for the incidence of AF in subjects whose FEV1 %predicted was 60% to 80% was significantly higher than subjects whose FEV1 %predicted was 80% and over (25). However, in those subjects whose FEV1% predicted was below 60% the incidence of AF was not significantly higher than the reference group (25). They demonstrated that the relative risk of hospital admission for AF was significantly associated with the degree of FEV1 %predicted (25). Taken together, it was suggested that there was a relationship between AF and the impairment of pulmonary function, but this relationship was not conclusive. Although our study was a cross-sectional one, we showed the significant association of the prevalence of AF with impairment of pulmonary function. Most importantly we demonstrated that the parameters indicating the degree of airflow limitation and lung restriction, FEV1 %predicted and FVC %predicted, were risk factors for the prevalence of AF and were independent of various other AF risk factors, especially cardiac dysfunction, as indicated by BNP levels. In contrast with the Copenhagen City Heart Study, there were many more subjects with AF in those subjects with severe airflow limitation. This was even more impressive given that the prevalence of AF in the severe airflow limitation group was even higher than the prevalence of AF in the group with abnormal cardiac findings, such as LVH (14.28% vs. 4.39%).
Coronary artery disease is a frequent co-morbidity in patients with COPD. Many of our subjects with airflow limitation were suggested to have COPD, as this disease is one of the major causes of airflow limitation in Japan. However, since the relationship between AF and reduced pulmonary function was independent of serum BNP levels, which is a well-known marker of cardiac damage, it was not likely that cardiac damage induced by chronic respiratory disease was a primary factor in AF development. The mechanisms involved in the relationship between AF and reduced pulmonary function are suggested to be: 1) hypoxia, 2) elevation of pulmonary pressure, and 3) chronic inflammation.
First, subjects with severely-impaired pulmonary function may have hypoxia. Hypoxia reportedly induces sympathetic drive, resulting in the incidence of AF (33). This may be one of the mechanisms whereby AF is induced in subjects with airflow limitation. Unfortunately, PaO2, measured by arterial blood gas analysis, and oxygen saturation, measured by pulse oximetry, were not assessed in this health check program, as it was not hospital-based clinical research. However, as shown in Table 2E and 2F, the finding that the prevalence of AF was much higher in the group with severely impaired pulmonary function, supports this hypothesis.
Second, subjects with reduced pulmonary function may have elevated pulmonary artery pressure. In patients with COPD, pulmonary hypertension is sometimes induced by a loss of pulmonary vasculature and hypoxic vascular contraction. In some fibrosing lung diseases, impairment of pulmonary vasculatures frequently causes pulmonary hypertension (28). Ectopic beats that initiate AF are reported to often originate in the pulmonary vein (34). Kang et al. demonstrated the elevation of pulmonary artery pressure and reduced pulmonary function in AF patients (35). This alteration of hemodynamics may induce arrhythmias, such as AF.
Third, chronic systemic inflammation is reported to be involved in the pathogenesis of some chronic respiratory disease, such as COPD and bronchial asthma (4,36). Circulating pro-inflammatory cytokines, such as interleukin-6 and tumor necrosis factor-alpha, are elevated in such patients (36). Recently, the involvement of systemic inflammation was also suggested in the pathogenesis of AF (15). Anatomically, the pulmonary circulation flows directly into the left atrium through the pulmonary vein where the trigger for AF is initiated. Thus, chronic inflammation originating from the lung may trigger AF. In this study, we measured HS CRP (an indicator of systemic inflammation) in some of the subjects. We showed no significant difference in HS CRP levels in AF and non-AF subjects. However, only 1,206 serum samples out of a total of 2,917 subjects were available for this assay. Furthermore, there were only 19 samples from subjects with AF. Therefore, a larger sample number may be required to prove the involvement of chronic systemic inflammation in the mechanism of AF development in subjects with reduced pulmonary function.
The details of airflow limitation and lung restriction in this study population were not specified because, 1) the self-reporting question on the history of respiratory disease only required a yes/no answer, 2) there was no information on chest X-ray findings or, 3) the final diagnosis. COPD and bronchial asthma are the most likely cause of airflow limitation in this study population (3,37). The prevalence of fibrosing lung diseases is not as high as COPD and bronchial asthma. Even the prevalence of idiopathic pulmonary fibrosis, one of the most common fibrosing lung diseases, is estimated to be only 3.44 per 100,000 people in Japan (38). As we previously demonstrated, FVC %predicted in the general Japanese population was inversely associated with the pack-years of cigarette smoking (27). Thus, although the precise etiology of lung restriction is unclear, the habit of cigarette smoking may play an important role in reduced FVC values.
Another possible limitation in this study was sampling bias. Twenty-eight percent of adults aged 40 years or older came to receive this health examination, organized by Takahata town, and 74% of these were enrolled in this study. Workers in the public institutes and many companies did not participate in this research due to reasons described in the Methods. We cannot deny the possibility that the difference in jobs or life-styles between participants and non-participants may affect the results of this research.
In conclusion, we demonstrated that impaired pulmonary function is an independent risk factor for AF in the general Japanese population. There were, however, some study limitations, such as sampling bias and lack of data for blood oxygen, pulmonary artery pressure, and details of respiratory diseases in the subjects. Physicians should pay attention to the status of pulmonary function in patients with AF. Early intervention for hidden chronic lung disorders, such as COPD, may improve the prognosis of AF patients.
Acknowledgements
We thank Taiko Aita and Eiji Tsuchida for their excellent technical assistance.
Funding: This study was supported by a Grant-in-aid from the Global COE program of the Japan Society for the Promotion of Science, and grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (19590880, 20590892, and 23390220).
Ethics approval: This study was approved by the institutional ethics committee and all participants gave written informed consent.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Yao H, Rahman I. Current concepts on the role of inflammation in COPD and lung cancer. Curr Opin Pharmacol. 2009;9:375-83
2. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. Global initiative for chronic obstructive lung disease. http://www.goldcopd.org/
3. Fukuchi Y, Nishimura M, Ichinose M. et al. COPD in Japan: The Nippon COPD Epidemiology Study. Respirology. 2004;9:458-65
4. Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet. 2007;370:797-9
5. Igarashi A, Shibata Y, Yamauchi K. et al. Gly80ser polymorphism of phospholipase A2-IID is associated with cytokine inducibility in A549 cells. Respiration. 2009;78:312-21
6. Takabatake N, Sata M, Abe S. et al. Impaired systemic cell-mediated immunity and increased susceptibility to acute respiratory tract infections in patients with COPD. Respir Med. 2005;99:485-92
7. Takabatake N, Shibata Y, Abe S. et al. A single nucleotide polymorphism in the CCL1 gene predicts acute exacerbations in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174:875-85
8. Falk JA, Kadiev S, Criner GJ. et al. Cardiac disease in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:543-8
9. Valli N, Gobinet A, Bordenave L. Review of 10 years of the clinical use of brain natriuretic peptide in cardiology. J Lab Clin Med. 1999;134:437-44
10. Inoue Y, Kawayama T, Iwanaga T. et al. High plasma brain natriuretic peptide levels in stable COPD without pulmonary hypertension or cor pulmonale. Intern Med. 2009;48:503-12
11. Barr RG, Bluemke DA, Ahmed FS. et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362:217-27
12. Andrawes WF, Bussy C, Belmin J. Prevention of cardiovascular events in elderly people. Drugs Aging. 2005;22:859-76
13. Aronow WS. Heart disease and aging. Med Clin North Am. 2006;90:849-62
14. Inoue H, Fujiki A, Origasa H. et al. Prevalence of atrial fibrillation in the general population of Japan: An analysis based on periodic health examination. Int J Cardiol. 2009;137:102-7
15. Chung MK, Martin DO, Sprecher D. et al. C-reactive protein elevation in patients with atrial arrhythmias: Inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886-91
16. Amar D, Zhang H, Leung DH. et al. Older age is the strongest predictor of postoperative atrial fibrillation. Anesthesiology. 2002;96:352-6
17. You RX, McNeil JJ, Farish SJ. et al. The influence of age on atrial fibrillation as a risk factor for stroke. Clin Exp Neurol. 1991;28:37-42
18. Naccarelli GV, Hynes BJ, Wolbrette DL. et al. Atrial fibrillation in heart failure: Prognostic significance and management. J Cardiovasc Electrophysiol. 2003;14:S281-6
19. Alpert JS, Petersen P, Godtfredsen J. Atrial fibrillation: Natural history, complications, and management. Annu Rev Med. 1988;39:41-52
20. Tsang TS, Barnes ME, Miyasaka Y. et al. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: A longitudinal cohort study of 21 years. Eur Heart J. 2008;29:2227-33
21. Heeringa J, Kors JA, Hofman A. et al. Cigarette smoking and risk of atrial fibrillation: The Rotterdam study. Am Heart J. 2008;156:1163-9
22. Tuan TC, Chang SL, Tai CT. et al. Impairment of the atrial substrates by chronic cigarette smoking in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2008;19:259-65
23. Gramley F, Lorenzen J, Jedamzik B. et al. Atrial fibrillation is associated with cardiac hypoxia. Cardiovasc Pathol. 2010;19:102-11
24. Benjamin EJ, Levy D, Vaziri SM. et al. Independent risk factors for atrial fibrillation in a population-based cohort. The framingham heart study. JAMA. 1994;271:840-4
25. Buch P, Friberg J, Scharling H. et al. Reduced lung function and risk of atrial fibrillation in the Copenhagen City Heart Study. Eur Respir J. 2003;21:1012-6
26. Stewart S, Hart CL, Hole DJ. et al. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley Study. Heart. 2001;86:516-21
27. Osaka D, Shibata Y, Abe S. et al. Relationship between habit of cigarette smoking and airflow limitation in healthy Japanese individuals: The Takahata Study. Intern Med. 2010;49:1489-99
28. Ryu JH, Krowka MJ, Pellikka PA. et al. Pulmonary hypertension in patients with interstitial lung diseases. Mayo Clin Proc. 2007;82:342-50
29. Konta T, Hao Z, Abiko H. et al. Prevalence and risk factor analysis of microalbuminuria in Japanese general population: The Takahata Study. Kidney Int. 2006;70:751-6
30. The Committee of Pulmonary Physiology. JRS: Guidelines for pulmonary function tests: Spirometry, flow-volume curve, diffusion capacity of the lung. Tokyo. 2004
31. Kishi H, Shibata Y, Osaka D. et al. FEV6 and FEV1/FEV6 in Japanese participants of the community-based annual health check: The Takahata Study. Intern Med. 2011;50:87-93
32. Hsieh BP, Pham MX, Froelicher VF. Prognostic value of electrocardiographic criteria for left ventricular hypertrophy. Am Heart J. 2005;150:161-7
33. Mehra R, Benjamin EJ, Shahar E. et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The sleep heart health study. Am J Respir Crit Care Med. 2006;173:910-6
34. Haissaguerre M, Jais P, Shah DC. et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659-66
35. Kang H, Bae BS, Kim JH. et al. The relationship between chronic atrial fibrillation and reduced pulmonary function in cases of preserved left ventricular systolic function. Korean Circ J. 2009;39:372-7
36. Higashimoto Y, Yamagata Y, Taya S. et al. Systemic inflammation in chronic obstructive pulmonary disease and asthma: Similarities and differences. Respirology. 2008;13:128-33
37. Fukutomi Y, Nakamura H, Kobayashi F. et al. Nationwide cross-sectional population-based study on the prevalences of asthma and asthma symptoms among Japanese adults. Int Arch Allergy Immunol. 2010;153:280-7
38. Ohno S, Nakaya T, Bando M. et al. [Nationwide epidemiological survey of patients with idiopathic interstitial pneumonias using clinical personal records]. Nihon Kokyuki Gakkai Zasshi. 2007;45:759-65
Author contact
![]() Corresponding author: Dr. Yoko Shibata, 2-2-2 Iida-Nishi, Yamagata City, Yamagata 990-9585, Japan. Telephone: +81-23-628-5302, FAX: +81-23-628-5305, Email: shibataid.yamagata-u.ac.jp
Corresponding author: Dr. Yoko Shibata, 2-2-2 Iida-Nishi, Yamagata City, Yamagata 990-9585, Japan. Telephone: +81-23-628-5302, FAX: +81-23-628-5305, Email: shibataid.yamagata-u.ac.jp

 Global reach, higher impact
Global reach, higher impact