Impact Factor
ISSN: 1449-1907
Int J Med Sci 2011; 8(4):302-308. doi:10.7150/ijms.8.302 This issue Cite
Research Paper
Soluble Endothelial Selectin in Acute Lung Injury Complicated by Severe Pneumonia
1. Department of Cardiology, Pulmonology, and Nephrology, Yamagata University School of Medicine, Yamagata, Japan
2. Division of Clinical Laboratory, Yamagata University Hospital, Yamagata, Japan
Received 2011-3-22; Accepted 2011-5-2; Published 2011-5-11
Abstract
Background: Pneumonia is still one of the most frequent causes of death in the elderly. Complication of acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) by pneumonia makes patients very ill due to severe respiratory failure. Biomarkers that can discriminate the presence of complicating ALI/ARDS are required for early detection. The aim of this research was to investigate whether soluble endothelial selectin (sES) could be a biomarker for ALI.
Methods: Serum sES levels were measured in 27 pneumonia patients, who were enrolled between April 2006 and September 2007. Among these patients, six had ALI or a condition that was clinically comparable to ALI (cALI). All patients who were enrolled were successfully treated and survived.
Results: Circulating sES levels were elevated in pneumonia patients with ALI/cALI, and sES levels decreased following treatment of their pneumonia. Univariate and multivariate logistic regression analyses showed that sES was the only significant factor for identifying complicating ALI/cALI, independently of C-reactive protein (CRP) and lactate dehydrogenase (LDH). By receiver operating characteristic (ROC) curve analysis, the cut-off value for sES was 40.1 ng/mL, with a sensitivity of 0.8 and a specificity of 0.8.
Conclusion: sES may be a useful biomarker for discriminating complicating ALI/cALI in patients with severe pneumonia.
Keywords: Pneumonia, acute lung injury, soluble endothelial selectin
INTRODUCTION
Pneumonia is one of the most common infectious diseases, and is still one of the most frequent causes of death in the elderly (1). In spite of advances in antibiotic therapy, some patients with pneumonia become severely ill due to delays in receiving adequate treatment or due to comorbidities such as cancer and diabetes. In particular, acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) due to severe pneumonia results in respiratory failure, prolongs hospitalization, and sometimes causes death (2,3). The influx of neutrophils into lung tissue is the initial hallmark of ALI/ARDS (2,4). The onset and progression of ALI/ARDS is so acute that it is sometimes difficult to detect the presence of ALI/ARDS, only by the use of portable chest X-ray units in the early phase. Chest computed tomography (CT) is required for early diagnosis based on the ground glass shadow that is typical of ALI/ARDS (5,6). However, performing CT scans on all pneumonia patients is costly, and it is therefore necessary to develop good biomarkers that can discriminate complicating ALI/ARDS, so that a quick decision can be made on whether a CT scan should be performed.
Endothelial selectin (ES) is one of the cell adhesion molecules expressed in the vascular endothelium (7). ES is induced by pro-inflammatory cytokines in thrombosis (8,9), infectious diseases (10), malignant tumors (11) and autoimmune diseases (12), leading to the attachment of leukocytes to endothelial cells and the accumulation of leukocytes in inflamed tissues. A part of the extracellular portion of ES is cleaved and released as a soluble form (13). Circulating levels of soluble ES (sES) were reported to be elevated in patients with sepsis and shock (14-16). In addition, Okajima et al. reported that hypoxia was more prevalent in patients with high sES levels and systemic inflammatory response syndrome (SIRS), compared to patients with normal sES levels and SIRS (17).
Based on this background information, we hypothesized that measurement of circulating sES levels may be useful for the discrimination of complicating ALI/ARDS in severe pneumonia patients. Therefore, we investigated sES levels in patients with community acquired pneumonia, and assessed the association between sES levels and complicating ALI.
METHODS
Study Subjects
We measured serum sES levels in 27 patients who were admitted to Yamagata University Hospital for the treatment of community acquired pneumonia between April 2006 and September 2007. The diagnosis of pneumonia was based on clinical symptoms (cough, sputum production, and fever) and chest X-ray and chest CT findings. Patients with congestive heart failure were excluded from the analysis, to avoid misdiagnosis of ALI. In addition, patients with malignant tumors, thrombotic diseases (deep vein thrombosis and pulmonary artery thrombosis), or active systemic inflammatory diseases such as collagen vascular disease, were excluded, because sES levels are reported to be elevated in these disorders. The study protocol was approved by the institutional review board at Yamagata University, and written informed consent was obtained from all participating patients.
Assessment of Severity of Pneumonia at Admission
The age, dehydration, respiratory failure, orientation disturbance and low blood pressure (A-DROP) scoring system was used to evaluate the severity of pneumonia (18). This is a 6-point scale (0-5) for assessing the clinical severity of community acquired pneumonia that was proposed by the Japanese Respiratory Society. The A-DROP scoring system assesses the following parameters: i) age (male ≥ 70 years, female ≥ 75 years); ii) dehydration [blood urea nitrogen (BUN) ≥ 21 mg/dL]; iii) respiratory failure [(SpO2 ≤ 90% or partial pressure of arterial oxygen (PaO2) ≤ 60 mm Hg]; iv) orientation disturbance (confusion); and v) low blood pressure (systolic blood pressure ≤ 90 mm Hg).
Diagnosis of ALI and Clinical Status Comparable to ALI (cALI)
Generally, data on the fraction of inspired oxygen (FiO2) is required to accurately diagnose whether or not patients have ALI (19). However, as all participants in this study did not receive mechanical ventilation or non-invasive positive pressure ventilation, the accurate FiO2 values were not available in this study. It was previously reported that SpO2/FiO2 (S/F) ratios correlate with PaO2/FiO2 (P/F) ratios, and S/F ratios of 235 and 315 correlate with P/F ratios of 200 and 300, respectively, for diagnosing and following up patients with ALI and ARDS (20). Thus, hypoxic patients were defined as having “ALI/cALI” using the following criteria: 1) bilateral massive ground glass shadow on chest X-ray and/or CT scan; 2) no apparent findings suggesting congestive heart failure, on chest X-ray and ultrasound cardiogram; and 3) requirement of 5 L/min or more of supplemental oxygen therapy using a facial mask, estimated FiO2 ≥0.35 (21), to maintain SpO2 >90%, except for patients with hypoxemia due to their primary chest disease. By this criterion, subjects with estimated S/F <257 had a potential to be classified as ALI/cALI group. The diagnosis of ALI/cALI was made by at least two pulmonary physicians and a cardiologist, according to these criteria. All pneumonia patients received immediate antibiotic therapy. The etiology of pneumonia was aspiration (ALI/cALI group, n = 9; non-ALI/cALI group, n = 4), bacterial infection (ALI/cALI group, n = 11; non-ALI/cALI group, n = 1), and Legionella pneumophilia infection (non-ALI/cALI group, n = 1). Two patients in the ALI/cALI group and two in the non-ALI/cALI group received the neutrophil elastase inhibitor, Sivelestat (Ono Pharmaceutical, Osaka, Japan) (22), but none of the patients in this study received glucocorticosteroid therapy. None of the patients died during the hospital admission, and all were successfully discharged.
Blood Sampling and Measurement of Soluble Endothelial Selectin
Repeat blood samples were obtained at intervals of 3 to 5 days from the time of admission until the patients were recovered from pneumonia, to measure sES levels and other biochemical markers. The time-point of blood sampling was flexibly decided by each doctor as needed. The median number of blood sampling was 2 (1 - 3) in non-ALI/cALI group, and 4 (3.75 - 5) in ALI/cALI group [median (inter quartile range)]. These samples were stored frozen at -20°C until the measurements were made. sES levels were measured by latex photometric immunoassay (LPIA) (Mitsubishi Chemical Medience Corp, Tokyo, Japan) (17). This assay measures serum sES concentrations over a linear range of 5.29 to 300 ng/mL (17). It was reported that the normal range of the plasma sES levels was 4.8 - 29.7 ng/mL (17).
Statistical Analysis
The Mann-Whitney U test for non-parametric data was used to analyze differences between two groups. Multiple comparisons were performed by non-parametric one way analysis of variance (Kruskal-Wallis test) followed by the Student-Newman-Keuls test. Chi-square tests were used to evaluate differences in proportions. These comparisons and the logistic regression analyses were performed using SigmaPlot version 11 computer software (Systat Software, Inc., San Jose, CA, USA) and JMP version 8 software (SAS Institute Inc., Cary, NC, USA). Data in the figures are shown as mean ± SD. Significance was inferred for differences with P < 0.05.
RESULTS
The characteristics of the pneumonia patients enrolled in this study are shown in Table 1. Age, gender, and the number of patients requiring supplemental oxygen did not differ significantly between the non-ALI/cALI and ALI/cALI groups. The length of hospitalization was significantly greater in the ALI/cALI group than in the non-ALI/cALI group (P <0.01). The A-DROP score for severity of community acquired pneumonia was significantly higher in the ALI/cALI group, compared with the non-ALI/cALI group (chi-square test, P <0.05). Among the laboratory results on arrival in hospital, only sES and lactate dehydrogenase (LDH) were significantly higher in the ALI/cALI group than in the non-ALI/cALI group (P <0.05). There was a trend for C-reactive protein (CRP) levels to be higher in the ALI/cALI group than in the non-ALI/cALI group, but the difference did not reach statistical significance (P = 0.06). Among patients with severe pneumonia and a A-DROP score ≥3, there was a trend for sES levels to be higher in the ALI/cALI group than in the non-ALI/cALI group, although the difference was not statistically significant (non-ALI/cALI, 39.7 ± 23.1 ng/mL; ALI/cALI, 56.0 ± 22.2 ng/mL; P = 0.2).
Characteristics of the patients with pneumonia
| non-ALI/cALI (n=21) | ALI/cALI (n=6) | |
|---|---|---|
| Age, years (range) | 77.6 (50 - 93) | 75.0 (51 - 92) |
| Male gender, % | 44.4 | 66.7 |
| Hospitalization, days | 18.8 ± 15.1 | 59.3 ± 41.0** |
| Use of supplemental oxygen, % | 66.6 | 100 |
| A-DROP score # | 2 / 4 / 9 / 6 / 0 / 0 | 0 / 0 / 2 / 3 / 1 / 0* |
| WBC, ×1000/μL # | 11.50± 4.09 | 12.50± 5.46 |
| CRP, mg/dL # | 11.7 ± 6.3 | 18.6 ± 11.0 |
| LDH, IU/L # | 195 ± 64 | 274 ± 104* |
| BUN, mg/dL # | 18.9 ± 7.7 | 18.8 ± 10.0 |
| Na, mEq/L # | 137 ± 2.6 | 134 ± 11.1 |
| Blood glucose, mg/dL # | 156 ± 52.9 | 153 ± 44.0 |
| Hematocrit, % # | 37.0 ± 6.28 | 38.4 ± 5.00 |
| sES, ng/mL # | 33.6 ± 14.8 | 53.0 ± 17.8* |
Data are means ± SD unless indicated otherwise. #Data obtained or evaluated on arrival at the hospital.
* P < 0.05, ** P < 0.01 compared with the non-ALI/cALI group
A-DROP score, a 6-point scale (0-5) for assessing the clinical severity of community acquired pneumonia, proposed by the Japanese Respiratory Society. This scoring system assesses the following parameters: i) age (male ≥ 70 years, female ≥ 75 years); ii) dehydration (BUN ≥ 21 mg/dL); iii) respiratory failure (SpO2 ≤ 90% or PaO2 ≤ 60 mm Hg); iv) orientation disturbance (confusion); and v) low blood pressure (systolic blood pressure ≤ 90 mm Hg).
ALI, acute lung injury; BUN, blood urea nitrogen; cALI, clinical status comparable to ALI; CRP, C-reactive protein; LDH, lactate dehydrogenase; sES, soluble endothelial selectin; WBC, white blood cell count
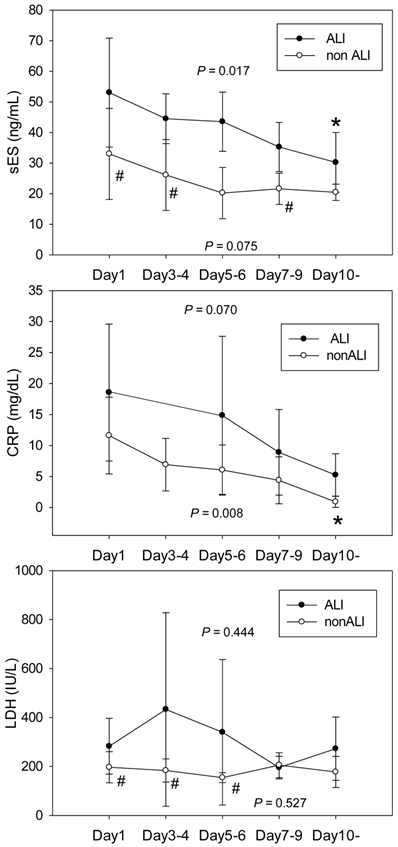
The time courses for sES, CRP and LDH according to complicating ALI/cALI are shown in Figure 1. sES levels were higher in pneumonia patients with ALI/cALI than in those patients without ALI/cALI. sES levels decreased after commencement of treatment in the ALI/cALI group (P = 0.017). However, in the non-ALI/cALI group sES levels did not differ significantly at each time point (P = 0.075). CRP levels in pneumonia patients with ALI/cALI tended to be higher than those in patients without ALI/cALI, although the difference was not statistically significant. CRP levels decreased after the commencement of treatment in the non-ALI/cALI group (P = 0.008). However, in the ALI/cALI group, the differences in CRP levels at each time point did not reach statistical significance (P = 0.07). LDH levels on day 1, days 3-4, and days 5-6 were higher in pneumonia patients with ALI/cALI than in those without ALI/cALI. However, the differences in LDH levels at each time point were not statistically significant either in the ALI/cALI group or in the non-ALI/cALI group (P = 0.444 and P = 0.527, respectively).
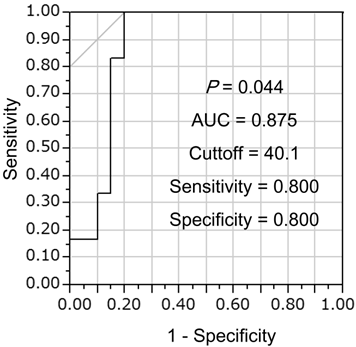
Univariate logistic regression analysis showed that sES was a significant factor for identifying complicating ALI/cALI (Table 2), whereas age, gender, white blood cell count (WBC), CRP, LDH, BUN, Na, blood glucose, and hematocrit were not significant factors. Furthermore, multiple logistic regression analysis demonstrated that sES was an independent factor for identifying the presence of ALI/cALI (Table 3). From analysis of the receiver operating characteristic (ROC) curve, the cut-off value for sES was 40.1 ng/mL, for discrimination of complicating ALI/cALI in pneumonia patients, with a sensitivity of 0.8 and a specificity of 0.8 (Figure 2).
Univariate logistic regression analysis for factors identifying complicating ALI/cALI in patients with pneumonia
| Variable | Coefficient | SD | P value |
|---|---|---|---|
| Age, years | 0.022 | 0.038 | 0.570 |
| Male gender | 0.144 | 0.489 | 0.769 |
| sES, ng/mL | -0.072 | 0.036 | 0.044 |
| WBC, per 1000/μL | -0.059 | 0.103 | 0.565 |
| CRP, mg/dL | -0.113 | 0.067 | 0.091 |
| LDH, IU/L | -0.012 | 0.007 | 0.073 |
| BUN, mg/dL | -0.044 | 0.049 | 0.368 |
| Na, mEq/L | 0.104 | 0.08 | 0.194 |
| Blood glucose, mg/dL | 0.0003 | 0.01 | 0.977 |
| Hematocrit, % | -0.035 | 0.078 | 0.658 |
ALI, acute lung injury; BUN, blood urea nitrogen; cALI, clinical status comparable to ALI; CRP, C-reactive protein; LDH, lactate dehydrogenase; sES, soluble endothelial selectin; WBC, white blood cell count
Time courses of soluble endothelial selectin, C-reactive protein and lactate dehydrogenase after commencement of treatment for pneumonia, according to complicating acute lung injury. The time courses of soluble endothelial selectin (sES, A), C-reactive protein (CRP, B) and lactate dehydrogenase (LDH, C) are shown according to complicating acute lung injury (ALI)/clinical status comparable to ALI (cALI). sES levels were higher in pneumonia patients with ALI/cALI than in those without ALI/cALI. sES levels decreased after commencement of treatment in the ALI/cALI group (P = 0.017). However, sES levels in the non-ALI/cALI group were not significantly different at each time point (P = 0.075). CRP levels in pneumonia patients with ALI/cALI tended to be higher than those in patients without ALI/cALI, although the difference was not statistically significant. CRP levels decreased after treatment in the non-ALI/cALI group (P = 0.008). However, in the ALI/cALI group, the differences in CRP levels at each time point did not reach statistical significance (P = 0.07). LDH levels on Day 1, Days 3-4, and Days 5-6 were higher in pneumonia patients with ALI/cALI than in those without ALI/cALI. However, the differences in LDH levels at each time point were not statistically significant either in the ALI/cALI group or in the non-ALI/cALI group (P = 0.444 and P = 0.527, respectively). * P < 0.05 compared with Day1; # P < 0.05 compared with the ALI/cALI group
Determination of the soluble endothelial selectin (sES) cut-off value for discrimination of complicating ALI/cALI in pneumonia patients. Receiver operating characteristic (ROC) curve analysis was performed to determine the sES cut-off value for discrimination of complicating ALI/cALI in pneumonia patients. The area under the curve (AUC) was 0.875, and the cut-off value was 40.1 ng/mL, with a sensitivity of 0.8 and a specificity of 0.8.
Multiple logistic regression analysis for factors identifying complicating ALI/cALI in patients with pneumonia
| Variable | OR | 95% CI | P value | |
|---|---|---|---|---|
| sES, per 1 ng/mL increase | 1.099 | 1.012 | 1.260 | 0.021 |
| CRP, per 1 mg/dL increase | 1.029 | 0.829 | 1.293 | 0.795 |
| LDH, per 1 IU/L increase | 1.017 | 0.999 | 1.046 | 0.052 |
ALI, acute lung injury; cALI, clinical status comparable to ALI; CI, confidence interval; CRP, C-reactive protein; LDH, lactate dehydrogenase; sES, soluble endothelial selectin; OR, odds ratio
DISCUSSION
This study demonstrated that circulating sES levels were elevated in pneumonia patients with ALI/cALI, and that sES levels decreased following treatment for pneumonia. Although LDH levels were higher in pneumonia patients with ALI/cALI than in those without ALI/cALI, the time course of changes in LDH did not accord with improvement in disease status. Univariate and multivariate logistic regression analyses revealed that sES was the only significant factor for identifying complicating ALI/cALI, independently of CRP and LDH. Among patients with severe pneumonia and an A-DROP score ≥3, sES levels tended to be higher in the ALI/cALI group than in the non-ALI/cALI group, although the difference was not statistically significant. Therefore, it may be possible to predict complicating ALI/cALI in pneumonia patients from laboratory measurements showing elevated sES levels.
Pneumonia is an infectious disease, in which pathogenic bacteria or viruses infect the lower respiratory tract and proliferate, leading to focal inflammation of the lungs (4). In patients with severe pneumonia, infiltrating neutrophils produce pro-inflammatory cytokines and chemokines, resulting in the induction of cellular adhesion molecules on circulating leukocytes and pulmonary endothelial cells (23,24). Inflamed alveolar cells, in particular alveolar macrophages, produce chemoattractant proteins such as interleukin-8 (IL-8), which attract circulating leukocytes into the lung (25). In ALI/ARDS, the inflamed lesion spreads over the original pneumonic lung segment, leading to the accumulation of leukocytes in a large pulmonary area. sES is selectively expressed in vascular endothelial cells, and plays important roles in the accumulation of leukocytes during pneumonia (26). Thus, elevation of sES in pneumonia patients is thought to indicate the presence of major pulmonary parenchymal inflammation. As the data from the present study demonstrates, it is possible that sES becomes a biomarker for ALI/ARDS in patients with severe pneumonia.
To date, WBC and CRP have been used as biomarkers for the degree of inflammation, with LDH as a biomarker for tissue damage. We demonstrated that circulating sES levels were significantly associated with complicating ALI, whereas WBC, CRP and LDH were not, indicating the usefulness of sES for evaluating the presence of ALI in severe pneumonia. In particular, measurement of sES may be recommended in patients with severe pneumonia and a high A-DROP score.
The limitations of present study are: 1) since the study was performed at a single institute, the number of patients with ALI/cALI was small; 2) patients with very severe pneumonia, who could not provide written informed consent due to severe respiratory failure comparable to a clinical status of ARDS, were not enrolled in this study. All patients, even the patients with severe pneumonia, were successfully treated and survived; hence, sES was not measured in very severe patients in the present study. Therefore, it is necessary to perform large clinical investigations at multiple medical institutions to confirm the usefulness of sES for the discrimination of complicating ALI.
A system for rapid measurement of sES has already been established (17). In addition, the specific neutrophil elastase inhibitor, sivelestat, is in clinical use for SIRS patients (27). Neutrophil elastase induces IL-8 in alveolar epithelial and bronchial cells through activation of signaling cascades induced by deformation of cell shape (28-30). Sivelestat has been reported to inhibit the production of cytokines from lung epithelial cells not only by inhibiting elastolytic activity but also by modulating signaling cascades involved in the production of cytokines (31). Early administration of sivelestat in pneumonia patients with high sES levels may improve the outcome of ALI/ARDS treatment by attenuating the influx of neutrophils into the lung parenchyma.
In conclusion, measurement of sES in patients with severe pneumonia may be useful for the discrimination of complicating ALI/cALI. The clinical application of this potentially useful biomarker may improve the accuracy and rapidity of diagnosis, and the outcome of treatment in patients with ALI.
Acknowledgements
We thank Taiko Aita and Eiji Tsuchida for their excellent technical assistance.
Funding
This study was supported by a Grant-in-aid from the Global COE program of the Japan Society for the Promotion of Science, and grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (18590835, 18790530, 19590880, and 20590892).
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global burden of disease study. Lancet. 1997;349:1269-76
2. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334-49
3. Matthay MA, Zimmerman GA, Esmon C. et al. Future research directions in acute lung injury: Summary of a national heart, lung, and blood institute working group. Am J Respir Crit Care Med. 2003;167:1027-35
4. Balamayooran G, Batra S, Fessler MB. et al. Mechanisms of neutrophil accumulation in the lungs against bacteria. Am J Respir Cell Mol Biol. 2010;43:5-16
5. Endo S, Shibata S, Sato N. et al. A prospective cohort study of ali/ards in the tohoku district of japan (second report). J Anesth. 2010;24:351-8
6. Mortelliti MP, Manning HL. Acute respiratory distress syndrome. Am Fam Physician. 2002;65:1823-30
7. Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB J. 1994;8:504-12
8. Korkmaz S, Ileri M, Hisar I. et al. Increased levels of soluble adhesion molecules, e-selectin and p-selectin, in patients with infective endocarditis and embolic events. Eur Heart J. 2001;22:874-8
9. Roldan V, Marin F, Lip GY. et al. Soluble e-selectin in cardiovascular disease and its risk factors. A review of the literature. Thromb Haemost. 2003;90:1007-20
10. Darveau RP, Cunningham MD, Bailey T. et al. Ability of bacteria associated with chronic inflammatory disease to stimulate e-selectin expression and promote neutrophil adhesion. Infect Immun. 1995;63:1311-7
11. Benekli M, Gullu IH, Tekuzman G. et al. Circulating intercellular adhesion molecule-1 and e-selectin levels in gastric cancer. Br J Cancer. 1998;78:267-71
12. McMurray RW. Adhesion molecules in autoimmune disease. Semin Arthritis Rheum. 1996;25:215-33
13. Gearing AJ, Hemingway I, Pigott R. et al. Soluble forms of vascular adhesion molecules, e-selectin, ICAM-1, and VCAM-1: Pathological significance. Ann N Y Acad Sci. 1992;667:324-31
14. Kayal S, Jais JP, Aguini N. et al. Elevated circulating e-selectin, intercellular adhesion molecule 1, and von willebrand factor in patients with severe infection. Am J Respir Crit Care Med. 1998;157:776-84
15. Newman W, Beall LD, Carson CW. et al. Soluble e-selectin is found in supernatants of activated endothelial cells and is elevated in the serum of patients with septic shock. J Immunol. 1993;150:644-54
16. Reinhart K, Bayer O, Brunkhorst F. et al. Markers of endothelial damage in organ dysfunction and sepsis. Crit Care Med. 2002;30:S302-12
17. Okajima K, Harada N, Sakurai G. et al. Rapid assay for plasma soluble e-selectin predicts the development of acute respiratory distress syndrome in patients with systemic inflammatory response syndrome. Transl Res. 2006;148:295-300
18. Shindo Y, Sato S, Maruyama E. et al. Comparison of severity scoring systems A-DROP and CURB-65 for community-acquired pneumonia. Respirology. 2008;13:731-5
19. Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: A clinical review. Lancet. 2007;369:1553-64
20. Rice TW, Wheeler AP, Bernard GR. et al. Comparison of the SpO2/FiO2 ratio and the PaO2/FiO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132:410-7
21. Kallstrom TJ. Aarc clinical practice guideline: Oxygen therapy for adults in the acute care facility--2002 revision & update. Respir Care. 2002;47:717-20
22. Kawabata K, Suzuki M, Sugitani M. et al. Ono-5046, a novel inhibitor of human neutrophil elastase. Biochem Biophys Res Commun. 1991;177:814-20
23. Albelda SM. Endothelial and epithelial cell adhesion molecules. Am J Respir Cell Mol Biol. 1991;4:195-203
24. Standiford TJ, Kunkel SL, Greenberger MJ. et al. Expression and regulation of chemokines in bacterial pneumonia. J Leukoc Biol. 1996;59:24-8
25. Maus U, Rosseau S, Knies U. et al. Expression of pro-inflammatory cytokines by flow-sorted alveolar macrophages in severe pneumonia. Eur Respir J. 1998;11:534-41
26. Glynn P, Coakley R, Kilgallen I. et al. Neutrophil CD11b and soluble ICAM-1 and e-selectin in community acquired pneumonia. Eur Respir J. 1999;13:1380-5
27. Okayama N, Kakihana Y, Setoguchi D. et al. Clinical effects of a neutrophil elastase inhibitor, sivelestat, in patients with acute respiratory distress syndrome. J Anesth. 2006;20:6-10
28. Abe S, Nakamura H, Inoue S. et al. Interleukin-8 gene repression by clarithromycin is mediated by the activator protein-1 binding site in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2000;22:51-60
29. Nakamura H, Yoshimura K, Jaffe HA. et al. Interleukin-8 gene expression in human bronchial epithelial cells. J Biol Chem. 1991;266:19611-7
30. Shibata Y, Nakamura H, Kato S. et al. Cellular detachment and deformation induce IL-8 gene expression in human bronchial epithelial cells. J Immunol. 1996;156:772-7
31. Misumi T, Tanaka T, Mikawa K. et al. Effects of sivelestat, a new elastase inhibitor, on IL-8 and MCP-1 production from stimulated human alveolar epithelial type II cells. J Anesth. 2006;20:159-65
Author contact
![]() Corresponding author: Dr. Yoko Shibata, 2-2-2 Iida-Nishi, Yamagata City, Yamagata 990-9585, Japan. Telephone: +81-23-628-5302, FAX: +81-23-628-5305, Email: shibataid.yamagata-u.ac.jp
Corresponding author: Dr. Yoko Shibata, 2-2-2 Iida-Nishi, Yamagata City, Yamagata 990-9585, Japan. Telephone: +81-23-628-5302, FAX: +81-23-628-5305, Email: shibataid.yamagata-u.ac.jp

 Global reach, higher impact
Global reach, higher impact