3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2010; 7(6):398-408. doi:10.7150/ijms.7.398 This issue Cite
Research Paper
Optimization of 5-fluorouracil solid-lipid nanoparticles: a preliminary study to treat colon cancer
1. Department of Pharmaceutics, College of Pharmacy, King Saud University, P.O. Box 2457, Riyadh 11451, Saudi Arabia
2. Center of Excellence in Biotechnology Research, King Saud University, P.O. Box 2460, Riyadh 11451, Saudi Arabia
3. College of Pharmacy, Al-Kharj University, Al-Kharj, Saudi Arabia
Received 2010-7-10; Accepted 2010-11-16; Published 2010-11-22
Abstract
Solid lipid nanoparticle (SLNs) formulae were utilized for the release of 5-fluorouracil (5-FU) inside the colonic medium for local treatment of colon cancer. SLNs were prepared by double emulsion-solvent evaporation technique (w/o/w) using triglyceride esters, Dynasan™ 114 or Dynasan™ 118 along with soyalecithin as the lipid parts. Different formulation parameters; including type of Dynasan, soyalicithin:Dynasan ratio, drug:total lipid ratio, and polyvinyl alcohol (PVA) concentration were studied with respect to particle size and drug entrapment efficiency. Results showed that formula 8 (F8) with composition of 20% 5-FU, 27% Dynasan™ 114, and 53% soyalithicin and F14 (20% 5-FU, 27% Dynasan™ 118, and 53% soyalithicin), which were stabilized by 0.5% PVA, as well as F10 with similar composition as F8 but stabilized by 2% PVA were considered the optimum formulae as they combined small particle sizes and relatively high encapsulation efficiencies. F8 had a particle size of 402.5 nm ± 34.5 with a polydispersity value of 0.005 and an encapsulation efficiency of 51%, F10 had a 617.3 nm ± 54.3 particle size with 0.005 polydispersity value and 49.1% encapsulation efficiency, whereas formula F14 showed a particle size of 343 nm ± 29 with 0.005 polydispersity, and an encapsulation efficiency of 59.09%. DSC and FTIR results suggested the existence of the lipids in the solid crystalline state. Incomplete biphasic prolonged release profile of the drug from The three formulae was observed in phosphate buffer pH 6.8 as well as simulated colonic medium containing rat caecal contents. A burst release with magnitudes of 26%, 32% and 28.8% cumulative drug released were noticed in the first hour samples incubated in phosphate buffer pH 6.8 for both F8, F10 and F14, respectively, followed by a slow release profile reaching 50%, 46.3% and 52% after 48 hours.
Keywords: Solid lipid nanoparticles, double emulsion, colon cancer, Dynasan, 5-fluorouracil, polyvinyl alcohol
Introduction
Colorectal cancer is the second leading cause of cancer-related death for men and women worldwide. Each year there are about one million new cases of colorectal cancer. Its incidence has increased over the last 25 years [1]. Colorectal cancer is a disease that is manifested by the formation of adenomatous polyps and malignant cells in the colon [2]. These abnormal cells creating tumors are characterized by unregulated replication and the capability of spreading to other sites [2]. Colon-specific delivery systems would allow for the local delivery of a high concentration of active agents in the colon to improve pharmacotherapy and reduce its potential systemic toxicity and side effects [3]. The early detection, diagnosis, and the use of more effective and less toxic systems would tremendously improve the efficacy of therapy [1-3].
Nanotechnology has become a rapidly growing field with potential applications in health and drug therapy [4-6]. Nanoparticles have extraordinary physical and chemical properties resulting from the nanosize effect [7]. They play an important role in cancer therapy to detect or to deliver the drug to the cancerous cell without attacking the normal cells and have good ability to form a complex with a variety of drugs through chemical bindings [8]. The phenomenon of nanoparticles has been applied in a number of research approaches for the improvement of cancer therapeutics including liposomes [9], polymeric nanoparticles [10], dendrimers [11], and metallic nanoparticles [12]. Generally, lipid-based nanoparticles are considered the least toxic among all the other nanoparticles for in vivo applications, in addition to the significant progress that has been achieved in the delivery of DNA/RNA using lipid-based nano-assemblies [13].
Solid lipid nanparticles (SLNs) have been proposed as an alternative drug carrier system to other novel delivery approaches such as emulsions, liposomes, and polymeric nanoparticles due to various advantages, including feasibility of incorporation of lipophilic and hydrophilic drugs, improved physical stability, low cost, ease of scale-up, and manufacturing [14,15]. In contrast to emulsions and liposomes, the particle matrix of SLNs is composed of solid lipids. The majority of lipids commonly used are triglyceride esters of hydrogenated fatty acids. Hydrogenated cottonseed oil (Lubritab™ or Sterotex™), hydrogenated palm oil (Dynasan™ P60 or Softisan™ 154), hydrogenated castor oil (Cutina™ HR), and hydrogenated soybean oil (Sterotex™ HM, or Lipo™) are typical examples [16].
General features of SLNs are their composition of physiological compounds, possible routes of administration by intravenous, oral and topical, large scale production by high pressure homogenization, and the relatively low costs of excipients [16-18]. A number of studies have recently been published about their production [19], physicochemical characterization of particles [20], and drug incorporation and release [21]. SLNs carrying anticancer drugs such as doxorubicin and paclitaxel had previously been developed and the antiprolifirative effect of SLNs versus conventional drug formulations was also evaluated on HT-29 cells. In vitro cytotoxicity of SLNs carrying anticancer drugs was higher than that of conventional drug formulations [22].
5-FU is an anticancer agent and the most widely used drug in the treatment of malignancies arising from breast, gastrointestinal tract, head, and neck regions of the body for several decades [23]. It is considered the major chemotherapeutic agent with clinical activity against colorectal cancer [24-27]. Localizing 5-FU directly to the colon is expected to reduce systemic side effects allowing more effective and safe therapy with higher tumor diffusivity [28]. 5-FU was used in this study as a model drug. Improvement of tissue distribution and targeting of drugs by using SLNs have been reported for some drugs including anticancers [29].
The aim of this work was to prepare and characterize 5-FU solid lipid nanparticles. A double emulsion-solvent evaporation (w/o/w) method was chosen and was optimized to obtain SLNs with low particle size and a relatively high encapsulation efficiency as well as a consistent release profile in simulated colonic medium.
Materials and Methods
5-FU was obtained from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). Dynasan™ 114 and 118 were acquired from Sasol Germany GmbH (Witten, Germany). Soya lecithin 30% was purchased from AppliChem (Darmstadt, Germany). Polyvinyl alcohol (PVA), M.W. 22,000 was obtained from BDH Laboratories (Poole, England). All other reagents and chemicals were of analytical grade.
Preparation of solid lipid nanoparticles
Weighed amounts of soyalecithin and Dynasan were dissolved in 10 ml of dichloromethane. Certain amounts of 5-FU was dissolved in 4 ml of 2.5% w/v lactose monohydrate in distilled water to avoid particle aggregation after freeze drying of SLNs. Both lipid and aqueous solutions were mixed and emulsified by probe-sonication (Bandelin, Berlin, Germany) for an optimized period of time (3×1 minutes) at 40% voltage efficiency in an ice bath. The formed w/o primary emulsion was immediately poured onto 40 ml aqueous solution of PVA continuously stirred at 1000 rpm over ice bath for 30 minutes. Then, the temperature was increased gradually (15-18 ºC) during stirring and subjected to solvent evaporation for another 30 minutes. Lipid nanoparticles were separated from bulk aqueous phase by centrifugation at 14000 rpm for 30 min (Hettich, MIKRO-120, Tuttlingen, Germany). After subsequent washing with cold distilled water, the residue was dispersed in tris-HCl buffer pH 7 and freeze-dried (Martin Christ Alpha-1-4 LD freeze-drier, Osterode, Germany). Table 1, represents the exact composition of each of the prepared formula. The effect of different formulation parameters, such as type of Dynasan, soyalicithin:Dynasan ratio, drug:total lipid ratio, and the PVA concentration on the particle size and drug entrapment efficiency were investigated.
Composition of each of the prepared formulae
| Formulations | % w/w | % PVA | ||
|---|---|---|---|---|
| Soyalicithin | Dynasan | 5-FU | ||
| F1 | 26 | 52a | 22 | 1 |
| F2 | 52 | 26 | 22 | 1 |
| F3 | 78 | 0 | 22 | 1 |
| F4 | 52 | 27 | 20 | 1 |
| F5 | 26 | 52 | 22 | 0.5 |
| F6 | 52 | 26 | 22 | 0.5 |
| F7 | 39 | 39 | 22 | 0.5 |
| F8 | 53 | 27 | 20 | 0.5 |
| F9 | 26 | 52 | 22 | 2 |
| F10 | 53 | 27 | 20 | 2 |
| F11 | 53 | 27 | 20 | 1 |
| F12 | 53 | 27 | 20 | 3 |
| F13 | 53 | 27b | 20 | 0.5 |
| F14 | 53 | 27 | 20 | 0.5 |
| F15 | 53 | 27 | 20 | 0.5 |
aD114 is Dynasan™ 114
bD118 is Dynasan™ 118
Measurement of particle size
The mean size and polydispersity index of the size distribution for each formula were determined by photon correlation spectroscopy using 90 Plus particle size analyzer, Brookhaven Instruments Corporation (Holtsville, New York, USA). The SLNs dispersions were diluted 1:1000 with distilled water. Analysis was performed at 25 °C with an angle of detection of 90°. Each reported value is the average of three measurements. The polydispersity index measures the size distribution of the nanoparticles population.
Differential scanning calorimetry
The thermal behavior of some selected SLN formulae was investigated by differential scanning calorimetry (DSC) using a Shimadzu DSC-60 (Shimadzu Corporation, Tokyo, Japan). Samples of 4-7 mg were weighed and a heating rate of 10 ºC/min was employed in the range of 25 ºC to 350 ºC.
Fourier transform infrared spectroscopy (FTIR)
The FTIR spectra of samples were recorded on the on a PerkinElmer spectrum BX FTIR (PerkinElmer, Waltham, MA, USA) using the potassium bromide (KBr) disc technique. Samples equivalent to 2 mg of 5-FU were mixed with potassium bromide (about 100 mg) in a clean glass pestle and mortar and were compressed to obtain a pellet. Baseline was corrected and the samples were scanned against a blank KBr pellet background at a wave number ranging from 4000-400 cm-1 with a resolution of 1.0 cm-1.
Determination of % entrapment efficiency and drug loading
The percentage drug entrapment efficiency (%EE) and % drug loading (%DL) of 5-FU in SLNs formulations were determined by centrifugation of the colloidal samples at 14000 rpm at 25 ºC for 30 min. The non-entrapped 5-FU amounts in the supernatant obtained after centrifugation of nanoparticles were determined by UV spectroscopy at 266 nm.
The %EE of 5-FU entrapped within nanoparticles was calculated by dividing the difference between the total amount used (Wtotal 5-FU) and the free amount presented in the aqueous phase of supernatant (Wfree 5-FU) by the total amount used of 5-FU. The %DL was obtained by dividing that difference by the total weight of SLNs according to the following formulae:
Scanning electron microscopic (SEM) analysis
The morphology characteristics of the prepared SLNs were examined under the scanning electron microscope (JSM-6360LV Scanning Microscope; Jeol, Tokyo, Japan). Before microscopy, SLNs produced from F14 were suspended in a phosphate buffer (pH 7) by vortex for 1 minute, and then one drop was spread on a small clean slide cover and left to dry overnight in a desicator. In the next day, they were mounted on carbon tape and sputter-coated using a thin gold palladium layer under an argon atmosphere using a gold sputter module in a high-vacuum evaporator (JFC-1100 fine coat ion sputter; Jeol, Tokyo, Japan). The coated samples were then scanned and photomicrographs were taken at an acceleration voltage of 20 kV.
In vitro release study
Certain weights from each of the selected formula equivalent to 1 mg 5-FU were immersed in 10 ml of phosphate buffer pH 6.8 in biological shaker at 37 °C and 80 rpm speed. Aliquots of 1 ml were withdrawn at certain time intervals and replaced with an equal volume of fresh buffer. After centrifugation, the amount of drug released was determined spectrophotometrically by measuring the absorbance of each aliquot supernatant at 266 nm.
Drug release in medium containing rat caecal contents
The drug release was assessed using a procedure introduced by Yassin et al. (2010) [30]. Briefly, male rats of mixed breeds weighing 200-300 g were used throughout this study. The rats were euthanized while under ether anesthesia and the caecum was exteriorized, legated at the two-ends and was cut-loose. The contents of the formed caecal bags were individually weighed, pooled, and suspended in a chilled phosphate buffer saline (pH 6.8) to give a final dilution of 3% (w/v). Weights equivalent to 1 mg 5-FU from F8, F10 and F14 were incubated in 20 ml of the suspension at 37 °C ± 0.5 and shaken at 80 rpm using a thermostatic shaking water bath. The experiments were performed under nitrogen atmosphere to simulate anaerobic conditions. Aliquots (1 ml) samples were withdrawn, filtered, diluted, and analyzed using HPLC at specified time intervals for 24 h. Replacement of samples were made by the medium stored at the same temperature.
Assay method
A simple and sensitive stability-indicating HPLC method with a UV detection using thymine as an internal standard was adopted [31]. The method was utilized for the assessment of stability of 5-FU in rat caecal content as simulated colon medium under anaerobic conditions. Briefly, the HPLC system consisted of a Waters Model 1515 HPLC pump, a Waters autosampler, Model 717 plus (Waters Inc., Bedford, MA, USA), a Waters 2487 dual absorbance UV detector (Waters Inc., Bedford, MA, USA) governed by a microcomputer running Empower® software (version 1154). The detector wavelength was set at 260 nm. Separation was achieved by isocratic elution with a mobile phase of 40 mM phosphate buffer adjusted to pH 7.0 using 10% w/v potassium hydroxide, delivered at a flow-rate of 1.0 ml/min at ambient temperature through a C18 analytical, µ-Bondapack column (150 mm length × 4. 6 mm i.d., 10 µm particle size).
Statistical analysis
The significance of difference among the different formulae was tested by applying the one way analysis of variance ANOVA test, while Paired t-test was employed to determine the difference between any two formulations using a statistical software package (Statistical Analysis System, SAS Institute, Inc., Cary, NC, USA). Differences between related parameters were considered statistically significant for p-value equal to or less than 0.05.
Results and Discussion
Particle size, entrapment efficiency and drug loading
Table 2 presents the mean particle size, polydispersity and entrapment efficiency for all the prepared formulae. The polydispersity index is a measure of the width of the dispersion of particles. Narrow dispersions comprise polydispersity index values between 0.1 and 0.2. Hence, according to Table 2, most of the dispersions can be labeled as a narrow disperse; except F2 and F3 polydispersity index which was slightly higher. Generally, the particle size showed a wide range of variability ranging from 258 to 2743.7 nm depending on the lipid composition, drug to lipid ratio, and the concentration of the stabilizer (PVA). It is clear that a ratio of 2:1 soyalicithin: Dynasan and a high lipid ratio in the nanoparticles are important factors for getting smaller particle size. The PVA concentration (0.5%) was found to be optimum for both types of Dynasan.
Entrapment efficiency, particle size and polydispersity for each of the prepared formulae
| Formulation | % EE | %DL | Particle size (nm) | Polydispersity |
|---|---|---|---|---|
| F1 | 24.75 | 17.51 | 794.2 ± 113 | 0.046 |
| F2 | 28.70 | 16.75 | 712.9 ± 138 | 0.297 |
| F3 | 6.32 | 20.90 | 258 ± 49 | 0.277 |
| F4 | 45.46 | 12.13 | 606.1 ± 63 | 0.006 |
| F5 | 69.09 | 8.03 | 943.2 ± 97 | 0.041 |
| F6 | 34.92 | 15.51 | 766.3 ± 104 | 0.064 |
| F7 | 32.94 | 15.91 | 924.8 ± 68 | 0.005 |
| F8 | 51.08 | 10.91 | 402.5 ± 34.5 | 0.005 |
| F9 | 35.91 | 15.31 | 1216.7 ± 107 | 0.005 |
| F10 | 49.10 | 11.29 | 617.3 ± 54.3 | 0.005 |
| F11 | 26.17 | 15.59 | 651.6 ± 51.8 | 0.005 |
| F12 | 53.03 | 10.51 | 2743.7 ± 183 | 0.005 |
| F13 | 40.00 | 13.04 | 461.9 ± 52.1 | 0.130 |
| F14 | 59.09 | 9.29 | 343.0 ± 29 | 0.005 |
| F15 | 35.52 | 13.89 | 471.3 ± 38 | 0.005 |
The lipid core material and drug composition were found to affect the extent of 5-FU entrapment in SLNs. Loading efficiency of 5-FU ranging from 6.32-69.09% were also observed at different lipids and drug ratios. As shown in Table 2, formulae F5, F8 and F10 exhibited the highest entrapment efficiencies among all the prepared formulae containing Dynasan 114 with values equal to 69.09%, 51.08% and 49.10%, respectively. The Dynasan 118 containing formulae F14 and F15 had entrapment efficiency values of 59.09% and 35.52%, respectively. Increasing the drug:total lipid ratio from 1:4 (F15) to 1:8 (F14 formula) was found to have a positive effect on both particle size and entrapment efficiency (i.e. smaller particle size and higher entrapment efficiency).
The type of Dynasan showed a little effect on the size and entrapment efficiency; however, comparing F8 with both F13 and F15 revealed that Dynasan 114 was superior to Dynasan 118 when used with the same composition. Briefly, F8 composed of drug to lipid at 1:4 ratio and soyalithicin to Dynasan114 at 2:1 ratio (stabilized by 0.5% PVA) is considered the optimum formula for the Dynasan 114 group with regards to the relatively small particle size (402.5 nm), polydispersity value of (0.005), and high encapsulation efficiency 51%. For the Dynasan 118 group, the best formula was F14 (composed of drug to lipid at 1:8 ratio and soyalithicin to Dynasan118 at 2:1 ratio), which has particle size of 343 nm with 0.005 polydispersity and an encapsulation efficiency of 59.09%.
Particle morphology
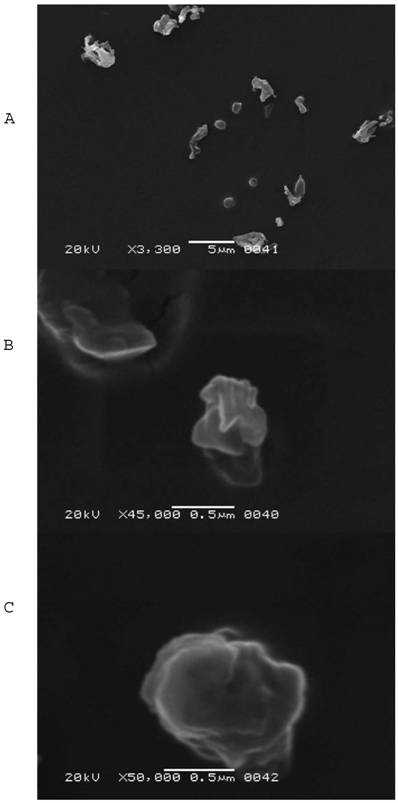
SEM images of SLNs F14 were presented in Fig. 1 (A, B, C). It was clear from image A that 5-FU loaded SLNs were spherical in shape with rough or irregular surfaces with the presence of some particle aggregates. The presence of aggregates might be attributed to a short redispersion time after centrifugation and drying at room temperature. The sizes observed from SEM micrographs were slightly higher than those obtained from particle size analyzer. Micrographs B and C showed irregular surfaces of single particles under high magnifications.
Differential scanning Calorimetry (DSC)
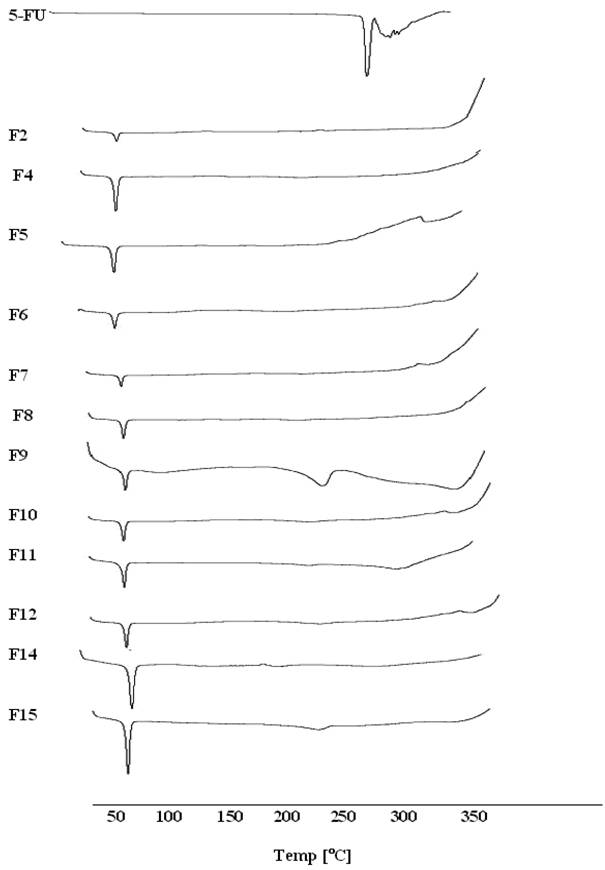
Thermal behavior of the pure drug, Dynasan 114 and 118 compared with the thermograms of different lyophilized SLNs formulae in the range of 25 to 350 ºC is shown in Fig. 2. The thermogram of the pure 5-FU showed a sharp melting endotherm at approximately 282 ºC followed by decomposition, which was in agreement with those reported previously [32, 33]. A slight shift to the melting peaks of 5-FU to 240 °C was only observed in the case of F9 and F15. Same observation was reported with 5-FU in PLGA microspheres [34]. The pure Dynasan 114 thermogram showed a characteristic sharp peak at 58 ºC corresponding to the melting of the lipid. This peak appeared in all thermograms of the prepared SLNs formulae confirming the solid crystalline state of the lipid inside the prepared formulae. No change in the shape of the Dynasan peak was observed in the SLNs formulae [35-36].
Scanning electron microscopy photomicrographs for F14 SLNs: A, a field containing different particle sizes using 3,300 X magnification power, B, a field showing two single particles using 45,000 X magnification power, and C, a field containing single particle using 50,000 X magnification power.
DSC thermograms for some selected SLNs formulae containing 5-FU.
Fourier transform infrared spectroscopy (FTIR)
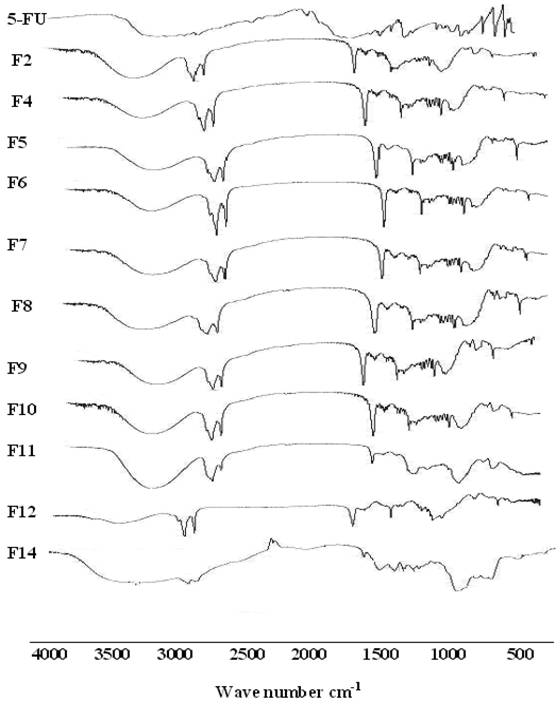
FTIR spectra of 5-FU showed a characteristic peak in the region 3000-2900 cm−1; represents C-H stretching. The 5-FU showed bands in the region 1429-1660 cm−1 corresponding to the C= N and C =C ring stretching vibrations. The bands at about 1348 cm−1 were vibration of the pyrimidine compound. The absorption bands at 1180 cm−1 and 1246 cm−1 were assigned to the C-O and C-N vibrations, respectively. Significant changes were observed in the spectra of formulations as was illustrated in Fig. 3. All the characteristic absorption bands of 5-FU diminished significantly in the finger print region of drug, which revealed that the encapsulated drug inside the lipid core material existed in an amorphous state [37]. However, sharp peaks near 2900 and 2800 and 1750 cm−1 were observed in all formulations due to presence of Dynasan. These changes could be related to those observed by DSC.
FT-IR spectra of some selected SLNs formulae containing 5-FU.
In vitro release
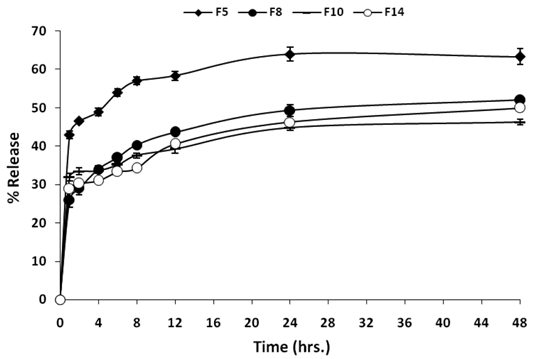
The in vitro drug release profiles (phosphate buffer pH 6.8 at 37 ºC) of entrapped 5-FU from the best four SLNs formulations (F5, F8, F10, and F14), with regard to small particle size and relatively high entrapment efficiency, were presented in Fig. 4. F5 and F10 were included in this part since they both showed relatively high entrapment efficiencies and a submicron particle size. All the formulae showed a burst drug release with values around 30% cumulative drug released. A significant difference in the cumulative % released from F5 was found in comparison with each of the three other formulae at all time points, while the difference among F8, F10, and F14 was insignificant using one way ANOVA test. This may be attributed to the aqueous solubility of 5-FU (12.5 mg/ml) leading to a rapid dissolution of drug molecules present in the surface layer of the particles. Then, the release rate became slow after the first hour sample and remained for 48 hours. The % 5-FU released after 48 hours from all formulations was less than 70% in all formulations. F5 showed the highest magnitude of burst effect; this may be due its low ratio of soyalecithin. This biphasic drug release pattern is very common with SLNs and was reported by many researchers [38, 39]. Recently, Rahman et al. [40] studied compositional variations and the interaction of the solid lipid nanoparticles formulation of risperidone using a response surface methodology. They found that a burst effect in the range of 20-30% appeared according to the variation in the composition. Liu et al. [39] found that insulin release from SLNs prepared by sodium cholate-phosphatidylcholine based mixed micelles followed the same biphasic pattern as it is difficult to encapsulate it into hydrophobic polymers. However, there is a clear difference in the release rate. The slow release of the 5-FU from all formulations suggested a homogeneous entrapment of the drug throughout the systems.
Release profile in rat caecal content
The validated HPLC method used for the determination of 5-FU in rat caecal medium was successful in separation of the drug from major and minor degradation products. It showed a high linearity of the standard calibration curve of 5-FU in the rat caecal content with a R2 value of 0.998 in the concentration range from 0.5 to 5 µg/ml [31].
F8, 10 and F14 were chosen for this study because they showed reasonable slow release profile in phosphate buffer pH 6.8, in addition to their combined small particle sizes and relatively high entrapment efficiencies. Fig. 5, represents the release profile of 5-FU in medium containing rat caecal contents for F8, F10 and F14. Generally, the release profiles from the three formulae were similar with no significant difference (p ≤ 0.05). All of them exhibited a slow release profile with diminishing rate. After three hours incubation, 26.54% ± 4.75, 28.64 ± 7.52 and 31.33% ± 4.27 of the drug were released from F8, F10 and F14, respectively, while only around 8 to 12% were released from the three formulae during the next three consecutive hours (up to six hours). The next three hours witnessed only 4-7% increase in the cumulative amount released. Incomplete drug release was noticed in the three formulae with 51.77%, 49.58% and 56.6% maximum cumulative percentage released for F8, F10 and F14, respectively, after 24 hours incubation in rat caecal suspension. The slow release profile exhibited by the drug may be ascribed by the lower solubility of 5-FU (0.1M ) in neutral phosphate buffers [41]. The high stability of 5-FU in rat caecal content medium was reported in a previous study [31]. Paharia et al. [42] studied the release of 5-FU from Eudragit-coated pectin microspheres in a simulated colonic medium containing rat caecal contents under anaerobic conditions. They reported a release of 70-80% within 8 hours depending on the formulation. Yassin et al. [30] reported that the release profile of 5-FU from chitosan compression-coated tablet incubated in rat caecal contents was complete after 12 hours.
In vitro release of 5-FU from optimized SLNs formulae.
The release profile of 5-FU from two SLNs formulae F8, F10, and F14 in phosphate buffer saline containing 3% rat caecal contents under anaerobic conditions.
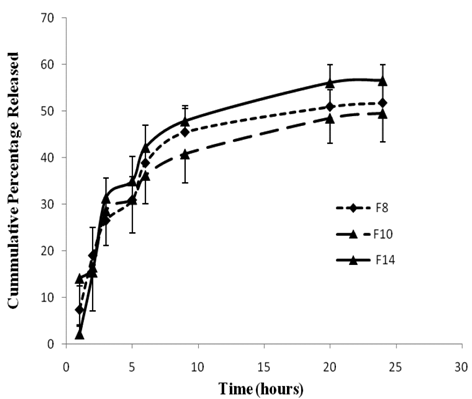
The exhibited slow release of 5-FU from SLNs formulae is not considered a shortcoming since the uptake of SLNs into the tumor tissue is expected by a unique phenomenon of solid tumors called Enhanced Permeation and Retention (EPR) related to their anatomical and pathophysiological differences from normal tissues. Angiogensis results in the incomplete tumor vasculature and leaky vessels with gap sizes of 100 nm to 2 μm depending upon the tumor type [43]. The lack of a well-defined lymphatic system would allow the tumor diffused SLNs to be retained for long period till complete release of the entrapped drug with a possible higher cellular uptake.
Incubation in rat caecal contents is considered a standard method for the assessment of the specificity of colonic micro flora activated systems due to the high similarity to human as they harbour bifidobacterium, bacteroids, and lactobacilli [44-48]. There are about 400 distinct bacteria species resident in the colon [49]. Most of the isolated bacteria are anaerobes and bacteroids [49-50]. Colonic bacteria play a significant role in colonic drug delivery system by producing enzymes and secretatory products that carry out several metabolic reactions such as hydrolysis, reduction, delalkylation, deamination and decarboxylation. The maintenance of anaerobic conditions is very critical for the activity of the colonic microflora. The most commonly used methods for the assessment of colonic delivery systems are rat caecal content and batch fermentation. Both methods maintain anaerobic conditions required for the vitality of the bacteria allowing for better simulation to the in vivo conditions.
Each of F8, F10 or F14 can be incorporated in a colonic site-specific system that allows the release of the formula inside the colon. One suitable system is composed of a hard gelatin capsule, in which the formula will be filled and coated with a pH-dependant polymer such as Eudragit S.
In summary, three SLNs formulae (F8, F10 and F14) have been successfully prepared using a simple double emulsion procedure that offers a better flexibility and least process related stress on the encapsulated drug. These formulae represent a platform for the preparation of SLNs for water soluble anticancer drugs including peptides. The SLNs system has a high potential to improve the uptake of anticancer drugs inside colon tumors. The release profile of the drug in simulated colonic medium showed a prolonged pattern that may allow spreading of the drug inside the colon to cover most of the colonic area wherever the tumors may exist. The incorporation of these formulae inside colon site-specific delivery capsule is currently progressing in our lab and the evaluation of the in vivo performance in colon cancer bearing animal model will be explored in future.
Acknowledgements
The authors acknowledge the generous financial support from the Center of Excellence in Biotechnology Research (grant number CEBR-06), King Saud University, Ministry of Higher Education, Saudi Arabia.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Viñas-Salas J, Biendicho-Palau P, Piñol-Felis C. et al. Calcium inhibits colon carcinogenesis in an experimental model in the rat. Eur J Cancer. 1998;34:1941-1945
2. Davis LE, Krawczeniuk MM. Colorectal Cancer. In: (ed.) Dipiro JT. Pharmacotherapy a Pathophysiologica Approach. New Jersey, USA: Prentice Hall. 1997:2513-2538
3. Rahman Z, Kohli K, Khar R. et al. Characterization of 5-fluorouracil microspheres for colonic delivery. AAPS PharmSciTech. 2006;7:E47-E53
4. Shoo SK, Parveen S, Panda JJ. The present and future of nanotechnology in human health care. Nanotechnology. 2007;3:20-31
5. Park JH, Lee S, Kim JH. et al. Polymeric nanomedicine for cancer therapy. Prog Polym Sci. 2008;33:113-137
6. Sumer B, Gao J. Therapeutic nanomedicine for cancer. Nanomedicine. 2008;3:137-140
7. Kabanov AV, Gendelman HE. Nanomedicine in the diagnosis and therapy of neurodegenerative disorders. Prog Polym Sci. 2007;32:1054-1082
8. Freitas C, Muller RH. Correlation between long-term stability of solid lipid nanoparticles (SLN) and crystallinity of the lipid phase. Eur J Pharm Biopharm. 1999;47:125-132
9. Bellocq NC, Pun SH, Jensen GS. et al. Transferrin-containing cyclodextrin polymer-based particles for tumor-targeted gene delivery. Bioconjug Chem. 2003;14:1122-1132
10. Betancourt T, Brown B, Brannon-Peppas L. Doxorubicin-loaded PLGA nanoparticles by nanoprecipitation: preparation, characterization and in vitro evaluation. Nanomedicine. 2007;2:219-232
11. Kukowska-Latallo JF, Candido KA, Cao Z. et al. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005;65:5317-5324
12. Lowery AR, Gobin AM, Day E. et al. Immunonanoshells for targeted photothermal ablation of tumor cells. Int J Nanomed. 2006;1:149-154
13. Puri A, Loomis K, Smith B. et al. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carrier Syst. 2009;26:523-580
14. Peters K, Muller RH. Nanosuspensions for oral administration of poorly soluble drugs; European Symposium on Formulation of Poorly-Available Drugs for Oral Administration. Paris: APGI. 1983:330-333
15. Ravi Kumar MNV. Nano and microparticles as controlled drug delivery devices. J Pharm Pharm Sci. 2000;3:234-258
16. MacGregor KJ, Embleton JK, Lacy JE. et al. Influence of lipolysis on drug absorption from the gastro-intestinal tract. Adv Drug Deliver Rev. 1997;25:33-46
17. Muller RH, Mehnert K, Gohla S. Solid lipid nanoparticles (SLN)- an alternative colloidal carrier system for controlled drug delivery. Eur J Pharm Biopharm. 1995;1:62-69
18. Muller RH, Weyhers H, zur Mühlen A. et al. Solid lipid nanoparticles (SLN) - ein neuartiger wirkstoff - carrier fur kosmetika und pharmazeutika I. systemeingeschaften, Herstellung und scaling up. Pharm Ind. 1997;7:423-427
19. Westesen K, Siekmann B, Koch MHJ. Investigations on the physical state of lipid nanoparticles synchrotron radiation x-ray diffraction. Int J Pharm. 1997;93:189-199
20. zur Mühlen A, Njehaus E, Mehnert W. Atomic force microscopy studies of solid lipid nanoparticles. Pharm Res. 1996;13:1411-1416
21. zur Mühlen A, Schwarz C, Mehnert W. Solid lipid nanoparticles (SLN) for controlled drug delivery-drug release and release mechanism. Eur J Pharm Biopharm. 1998;45:149-155
22. Serpe L, Catalano MG, Cavalli R. et al. Cytotoxicity of anticancer drugs incorporated in solid lipid nanoparticles on HT-29 colorectal cancer cell line. Eur J Pharm Biopharm. 2004;58:673-680
23. Schmoll HJ, Buchele T, Grothey A. et al. Where do we stand with 5-fluorouracil? Semin Oncol. 1999;26:589-605
24. Calabresi P, Chabner BA. Chemotherapy of neoplastic diseases. In: (ed.) Limbird JG, Perry LE, Raymond BM. Goodman and Gilman's the Pharmacological Basis of Therapeutics. New Delhi, India: McGraw-Hill. 1996:1225-1232
25. Krishnaiah YS, Satyanarayana V, Dinesh Kumar B. et al. In vitro drug release studies on guar gum-based colon targeted oral drug delivery systems of 5-fluorouracil. Eur J Pharm Sci. 2002;16:185-192
26. Phillips TA, Howell A, Grieve RJ. et al. Pharmacokinetics of oral and intravenous 5-fluorouracil in humans. J Pharm Sci. 1980;69:1428-1431
27. Fraile RJ, Baker LN, Buroker TR. et al. Pharmacokinetics of 5-fluorouracil administered orally by rapid intravenous and by slow infusion. Cancer Res. 1980;40:2223-2228
28. Jain SK, Chaurasiya A, Gupta Y. et al. Development and characterization of 5-FU bearing ferritin appended solid lipid nanoparticles for tumour targeting. J Microencapsul. 2008;25:289-297
29. Göppert TM, Müller RH. Adsorption kinetics of plasma proteins on solid lipid nanoparticles for drug targeting. Int J Pharm. 2005;302:172-186
30. Yassin AE, Alsarra IA, Alanazi FK. et al. New targeted-colon delivery system: in vitro and in vivo evaluation using x-ray imaging. J Drug Target. 2010;18:59-66
31. Alanazi FK, Yassin A, El-Badry M. et al. Validated high performance liquid chromatographic technique for determination of 5-fluorouracil: applications to stability studies and simulated colonic media. J Chromatogr Sci. 2009;47:558-563
32. Lee JS, Chae GS, An TK. et al. Preparation of 5-fluorouracil-loaded poly(L-lactide-co-glycolide) and evaluation of in vitro release behavior. Macromol Res. 2003;11:183-188
33. Bunjes H, Unruh T. Characterization of lipid nanoparticles by differential scanning calorimetry, X-ray and neutron scattering. Adv Drug Deliv Rev. 2007;59:379-402
34. Lee JS, Chae GS, An TK. et al. Preparation of 5-fluorouracil-loaded poly(L-lactide-co-glycolide) wafer and evaluation of in vitro release behavior. Macromol Res. 2003;11:183-188
35. Bunjes H, Koch MHJ, Westesen K. Effect of particle size on colloidal solid triglycerides. Langmuir. 2000;16:5234-5241
36. Bunjes H, Koch MHJ, Westesen K. Influence of emulsifiers on the crystallization of solid lipid nanoparticles. J Pharm Sci. 2003;92:1509-1520
37. Illing A, Unruh T. Investigations on the flow behavior of dispersions of solid triglyceride nanoparticles. Int J Pharm. 2004;284:123-131
38. Jain A, Jain SK. In vitro and cell uptake studies for targeting of ligand anchored nanoparticles for colon tumors. Eur J Pharm Sci. 2008;35:404-416
39. Liu J, Gong T, Wang C. et al. Solid lipid nanoparticles loaded with insulin by sodium cholate-phosphatidylcholine-based mixed micelles: preparation and characterization. Int J Pharm. 2007;340:153-162
40. Rahman Z, Zidan S, Khan MA. Non-destructive methods of characterization of risperidone solid lipid nanoparticles. Eur J Pharm Biopharm. 2010;76:127-137
41. Yang YW, Lee JS, Kim I. et al. Synthesis and properties of N-nicotinoyl-2-(5-fluorouracil-1-yl)-D,L-glycine ester as a prodrug of 5-fluorouracil for rectal administration. Eur J Pharm Biopharm. 2007;66:260-267
42. Paharia AK, Yadav G, Rai S. et al. Eudragit-coated pectin microspheres of 5-Fluorouracil for colon targeting. AAPS PharmSciTech. 2007;8:E1-E7
43. Reddy S, Sinha V, Reddy D. Novel oral colon-specific drug delivery systems for pharmacotherapy of peptide and nonpeptide drugs. Drugs Today. 1995;35:537-580
44. Hobbs SK, Monsky WL, Yuan F et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U.S.A. 1998;95:4607-4612
45. Sinha VR, Mittal BR, Bhutani KK. et al. Colonic drug delivery of 5-fluorouracil: an in vitro evaluation. Int J Pharm. 2004;269:101-108
46. Sinha VR, Mittal BR, Kumria R. In vivo evaluation of time and site of disintegration of polysaccharide tablet prepared for colon-specific drug delivery. Int J Pharm. 2005;289:79-85
47. Pandey R, Khuller GK. Solid lipid particle-based inhalable sustained drug delivery system against experimental tuberculosis. Tuberculosis. 2005;85:227-234
48. Pandey R, Sharma S, Khuller GK. Oral solid lipid nanoparticle-based antitubercular chemotherapy. Tuberculosis. 2005;85:415-420
49. Rubinstein A. Microbially controlled drug delivery to the colon. Biopharm Drug Dispos. 1990;11:465-475
50. Salyers AA. Molecular and biochemical approaches to determining what bacteria are doing in vivo. Antonie Van Leeuwenhoek. 1989;55:33-38
Author contact
![]() Corresponding author: Professor Ibrahim A. Alsarra, Phone: +(966)-1-4677504, Fax: +(966)-1-4676363, E-mail: ialsarraedu.sa
Corresponding author: Professor Ibrahim A. Alsarra, Phone: +(966)-1-4677504, Fax: +(966)-1-4676363, E-mail: ialsarraedu.sa

 Global reach, higher impact
Global reach, higher impact