Impact Factor
ISSN: 1449-1907
Int J Med Sci 2010; 7(4):213-223. doi:10.7150/ijms.7.213 This issue Cite
Research Paper
Extension of the PNA world by functionalized PNA monomers eligible candidates for inverse Diels Alder Click Chemistry
1. German Cancer Research Center, Dept. of Imaging and Radiooncology, INF 280, D-69120 Heidelberg, Germany
2. German Cancer Research Center, Division of Biophysics of Macromolecules, INF 580, D-69120 Heidelberg, Germany
3. German Cancer Research Center, Central Peptide Synthesis Unit, INF 580, D-69120 Heidelberg, Germany
4. Mannheim University of Applied Sciences, Department of Biotechnology, Paul-Wittsack-Straße 10, D-68163 Mannheim, Germany
Received 2010-3-10; Accepted 2010-6-22; Published 2010-6-27
Abstract
Progress in genome research led to new perspectives in diagnostic applications and to new promising therapies. On account of their specificity and sensitivity, nucleic acids (DNA/RNA) increasingly are in the focus of the scientific interest. While nucleic acids were a target of therapeutic interventions up to now, they could serve as excellent tools in the future, being highly sequence-specific in molecular diagnostics. Examples for imaging modalities are the representation of metabolic processes (Molecular Imaging) and customized therapeutic approaches (“Targeted Therapy”). In the individualized medicine nucleic acids could play a key role; this requires new properties of the nucleic acids, such as stability. Due to evolutionary reasons natural nucleic acids are substrates for nucleases and therefore suitable only to a limited extent as a drug. To use DNA as an excellent drug, modifications are required leading e.g. to a peptide nucleic acid (PNA). Here we show that an easy substitution of nucleobases by functional molecules with different reactivity like the Reppe anhydride and pentenoic acid derivatives is feasible. These derivatives allow an independent multi-ligation of functionalized compounds, e.g. pharmacologically active ones together with imaging components, leading to local concentrations sufficient for therapy and diagnostics at the same time. The high chemical stability and ease of synthesis could enhance nucleic chemistry applications and qualify PNA as a favourite for delivery. This system is not restricted to medicament material, but appropriate for the development of new and highly efficient drugs for a sustainable pharmacy.
Keywords: Click Chemistry, Diels Alder Reactioninvers (DARinv), Peptide Nucleic Acid (PNA), PNA building block functionalization
Introduction
Open questions in the world of nucleic acids are areas for improvement of the hypotheses concerning the origin of the life and the crucial genetic building blocks. The search for simpler precursor molecules leads to the peptide nucleic acid world (PNA).[1-3] At present PNA finds increasing interest in the scientific community. This manuscript does not intend to answer questions concerning the greater plausibility of PNA world as compared to the RNA/DNA world, but shows that PNA is an excellent biochemical tool in the ligation chemistry. Qualified ligation reactions, like the Huisgens's 1,3-dipolar cycloaddition [4], the Staudinger ligation refined by Bertozzi using a chemical reaction of phoshines with azides [5] and the established thio-ester-method [6] fulfil almost criteria of the term “Click Chemistry” introduced by Sharpless and which can be considered as a chemical philosophy.[7] As described by Finn and Fokin: the ''Click'' moniker is meant to signify that by use of these ligation methods. Joining molecular pieces is as easy as ''clicking'' together the two pieces of a buckle.[8] Some attributes of this philosophy are applicable to the broad spectrum of the general Diels Alder Reaction (DAR). Their potential and the synthesis' mechanisms as well as its characteristic physico-chemical traits are well documented and traced back to 1948.[9-11] In contrast, the DAR with inverse-electron-demand (DARinv) was described almost 10 years later.[12-15] Its chemical properties (rapid reaction rate, complete chemical reaction, lack of reverse reaction, chemical reaction in aqueous solution, under room temperature, no need for a catalyst) predetermines the DARinv as a suitable Click Chemistry-technology in cellular systems for intravital ligation of components. With respect to reaching high local concentrations of diagnostics in cells for molecular imaging and specific therapeutically active molecules, PNAs are powerful tools providing a multi-faced range of biochemical applications.[16-18] Similar to DNA derivatives like phosphothioates, phosphoramidates, 2'-O-alkyl-DNAs, morpholino and bicyclically locked nucleic acid derivatives (LNA), PNA mimics the DNA and RNA compositions and matches with nucleic acids under Watson-Crick hydrogen-bond formation. [19-24] Whereas the DNA derivatives still harbour the nucleic acid skeletal structure and possess the original stereochemical features resulting in a different affinity and specificity behaviour, the PNA is a substantially derivatized molecule. In PNA the phospho-ribose backbone is substituted with N-(2-amino-ethyl)-glycine units connected to an ethylene-diamine linker. Only the distance of the nucleobases remains conserved and corresponds to the nucleic acids' nucleobases interspace. The physico-chemical properties specific to PNA are based on its typical molecular structure: PNA mimics DNA through a pseudo-peptide backbone.[25] PNA is neither a nucleic acid nor a peptide and therefore not a substrate for nucleases and peptidases.[26] Furthermore the lack of asymmetric centers results in a higher affinity.[27] In this context, the PNA represents a new class of efficient tools for molecular diagnosis, chromosomal investigations, molecular genetics and cytogenetics, antisense and antigenic agents, and for transfer of genetic material into target cells as reversible coupling molecules.[28]
The main drawback however is based in other PNA specific properties: The lack of electrical charge and therefore much higher hydrophobicity leads to insolubility and self-aggregation of chains of more than 14mers in water, which results in a poor cellular uptake into cells and restricts the applications.[29]
To circumvent these drawbacks and to improve the local intracellular PNA concentrations manifold different approaches were considered: like: transfection technologies, virally-[30] and non-virally [31-33] mediated uptake procedures, lipofection [34], liposome[35], electroporation and ultrasound[36] mediated methods, gene gun etc.[37] We preferred the coupling of such a PNA cargo to carrier molecules which is possible with variable chemistry: (I) either in a cleavable form by a reversible disulfide bridge bond or (II) by non-cleavable covalent bonds and a (III) by hydrogen bridge formation. The main problems in coupling these molecules turned out to be the slow reaction rates and the incomplete chemical ligation reactions, as well as their reverse reactions, which all were improved in this publication. A further restriction lies in the insufficient amounts of active substances at the reaction site. Our approach circumvents this by synthesis of PNA polymers through PNA pentamers. Both, the proper and rapid DARinv mediated ligation and the easy design of PNA polymers can meet demands on modern drugs and diagnostic molecules.
Chemical Procedures
Monomer Synthesis
Functionalization of PNA backbone building blocks
The synthesis of functionalized PNA for the DARinv was carried out as depicted in the steps described here. To circumvent the above mentioned problems the development of suitable reactants is essential. The generally accepted syntheses of the desired PNA building blocks are shown in the following schemata and are documented in detail by the Thomson group.[38] The synthesis begins with the synthesis of 5 a Reppe anhydride PNA derivative based on the educts cyclooctatetraene (COT) 1 and maleic acid anhydride 2 as described by Reppe [39] is shown in scheme 1 (Figure 1).
(Scheme 1) illustrates the steps for synthesis of a nucleobase-substituent, with tetracyclo-[5.4.21,7.O2,6.O8,11]3,5-dioxo-4-aza-9,12-tridecadiene (TcT) as an example (documented as “Reppe anhydride”). The chemical reaction is described in detail by Reppe.[39] The reaction product of 3 with glycine is 4 whose carboxyl group was transferred immediately with thionyl chloride to the corresponding acid chloride 5 for further processing as described in scheme 3 (Figure 3).
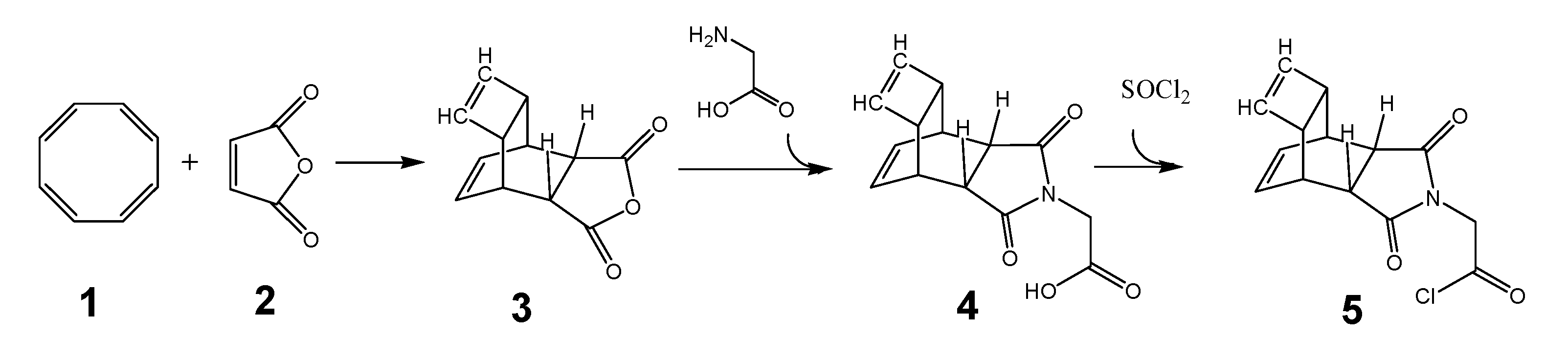
Synthesis of the “Reppe anhydride”-PNA building block
We started with the chemical synthesis of the “Reppe anhydride”-PNA building block (tetracyclo-[5.4.21,7.O2,6.O8,11]3,5-dioxo-4-aza-9,12-tridecadiene) 3 as illustrated in scheme 1/Figure 1.
Synthesis of the PNA building block backbone
In the next step, the synthesis of the PNA backbone monomer using the fluorenylmethoxycarbonyl (Fmoc) protection occurred as described by Atherton and Sheppard.[40]
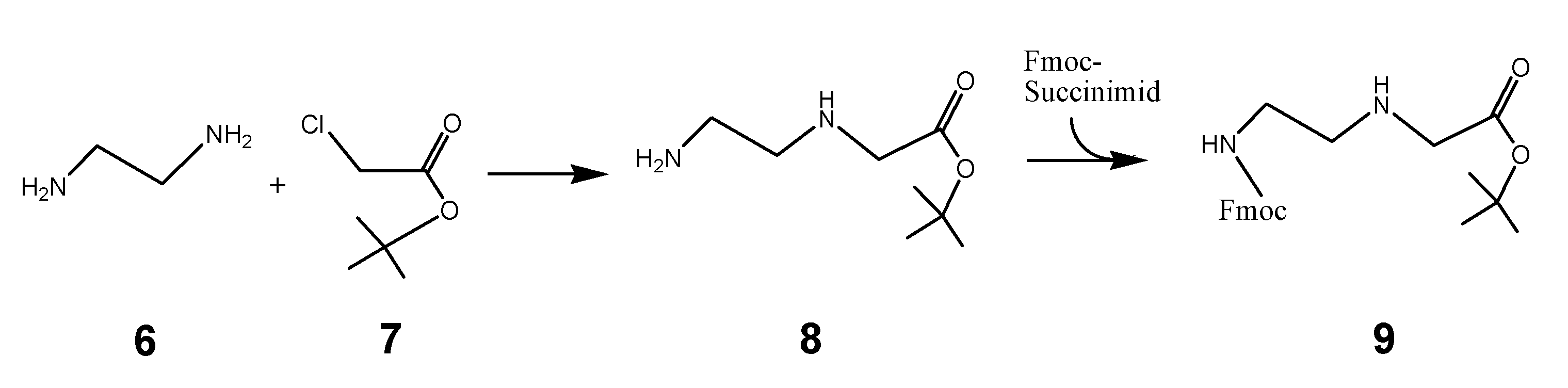
Synthesis of the Fmoc-C2-glycine-tert-butyl ester
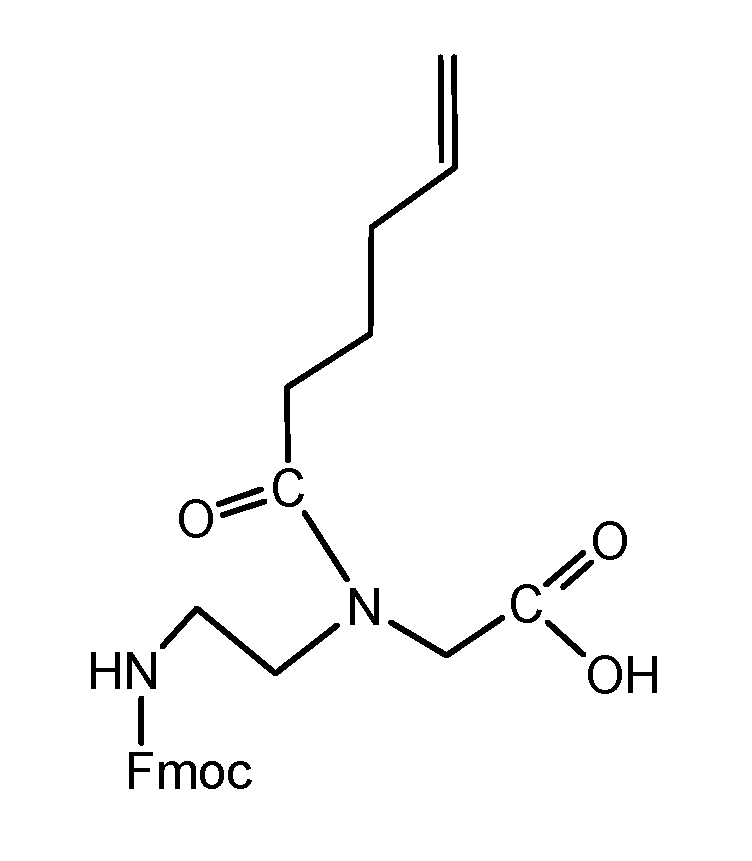
The synthesis of the peptide nucleic acid backbone requires the introduction of protecting groups as shown in the Fmoc-C2-glycine-tert-butyl ester derivative 9 (scheme 2/Figure 2). A reaction product which was converted to the final product 9 is the tert-butyl protected 3-[(2-aminoethyl)amino]glycine 8. Details of the synthesis protocol are shown in the footnote.1
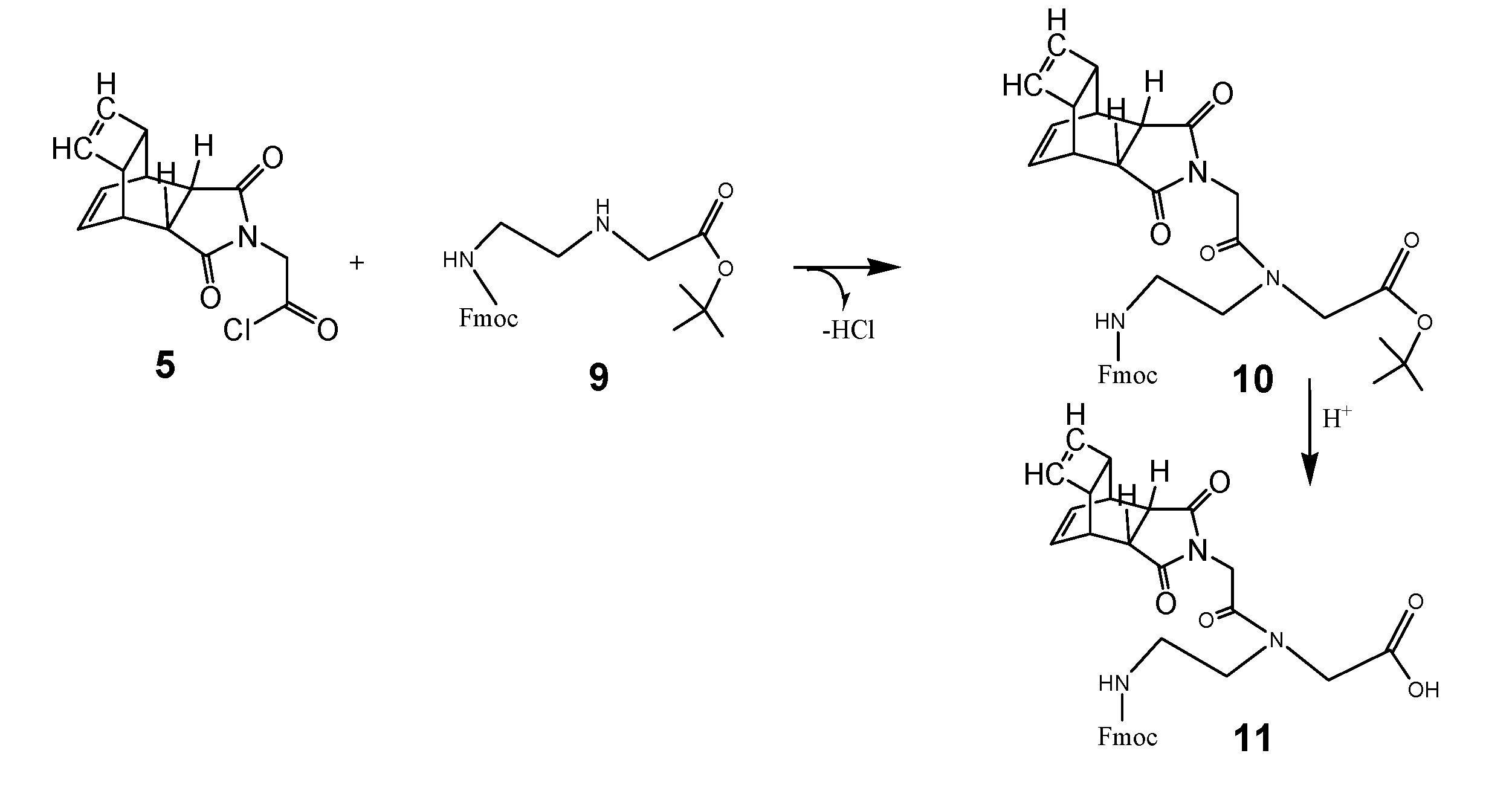
Coupling of the Fmoc-C2-glycine-tert-butyl ester with the Reppe anhydride
The next scheme illustrates the chemical reaction steps to the complete PNA monomer functionalized with the Reppe anhydride called RE-PNA 11 was then ready for use in the solide phase PNA synthesis. The instructions for synthesis are documented in the footnote.2 All steps of the chemical reactions are illustrated in scheme 3/Figure 3.
(Scheme 2) reports the chemical reaction of the PNA back bone module Fmoc protected-[(2-aminoethyl)glycine] tert-butyl ester unit 9, which was received by the reaction of ethylenediamine 6 and chloride acetic acid-tert-butyl ester 7. The reaction product 8 reacts with Fmoc-succinimide to 9.
(Scheme 3) 5 reacts with 9 to the tert-butyl ester of the peptide nucleic acid monomer (Fmoc protected) 10 as a reaction product. After hydrolysis of the tert-butyl ester, catalyzed by acid, the peptide nucleic acid monomer (Fmoc protected) was functionalized with the Reppe anhydride 11 referred to as RE-PNA in the text. According the scheme 3 the synthesis consists of two procedures carried out as described in detail in the footnote 2.
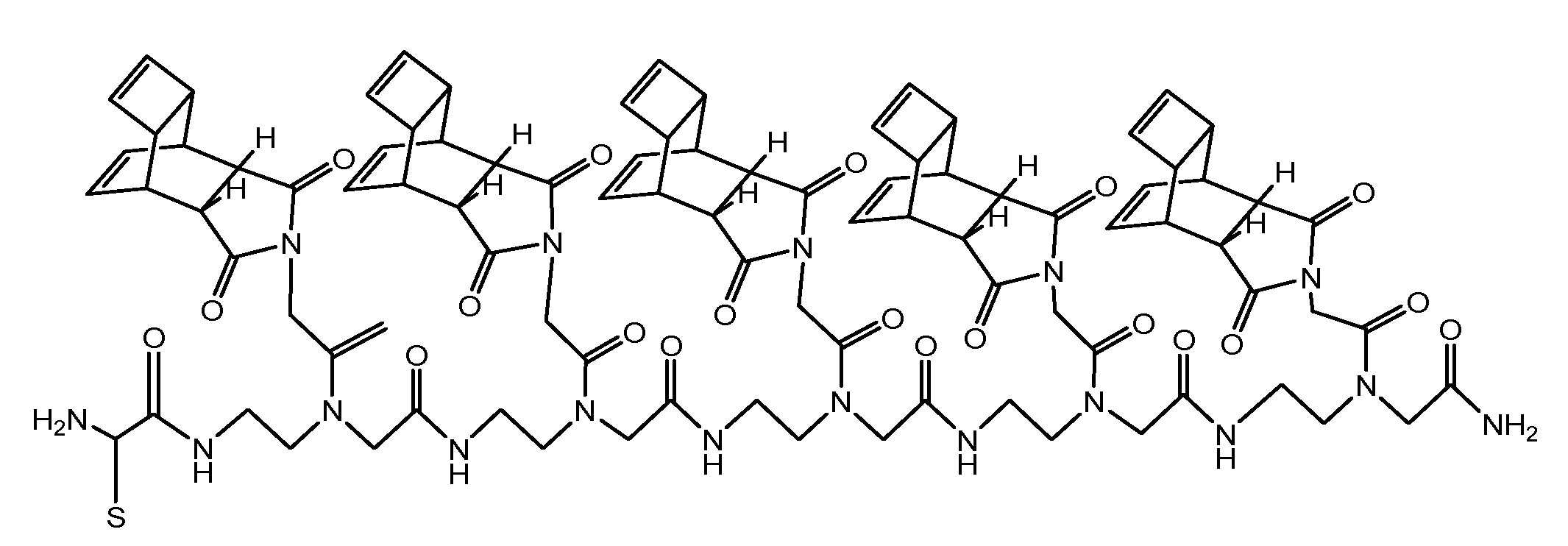
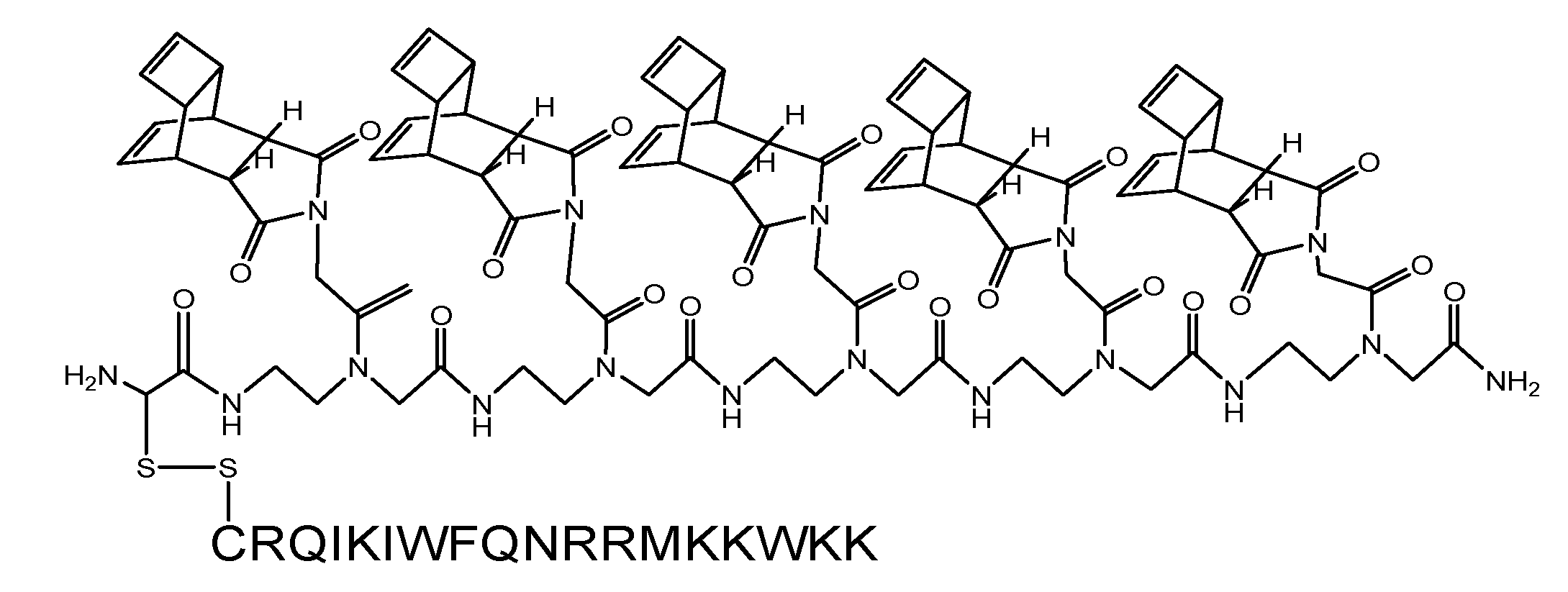
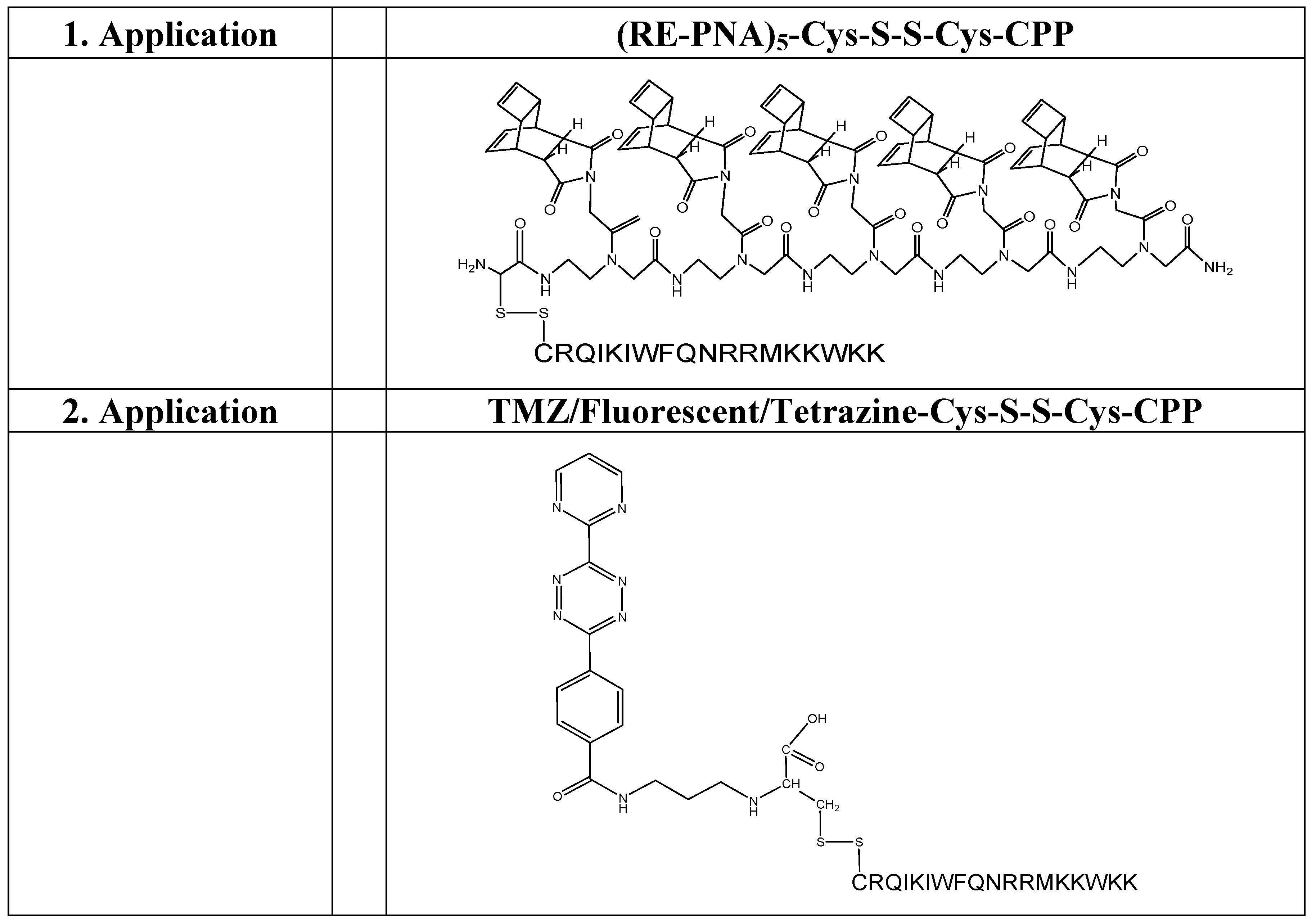
(Scheme 4): exemplifies the chemical structure of the pentamer consisting of the “Reppe anhydride” [(RE-PNA)5Cys]. The amino terminus of the PNA backbone possesses a cysteine which acts as a Redox coupling site.
(Scheme 5): shows the pentamer of the “Reppe anhydride” [(RE-PNA)5Cys] connected by the cysteine mediated disulfide formation with the CPP-Cys (displayed in amino acid single letter code). The ligation procedure of the two components by disulfide-bridge formation is documented [43].
Synthesis of the 4-pentenoic acid PNA monomer
To enhance the application spectrum of the “Click” chemistry we used as an additional example the pentenyl-PNA building block, a further PNA derivative, whose PNA monomer is functionalized with a 4-pentenoic acid. Scheme 6 (Figure 6) demonstrates our synthesis procedures of functional molecules for DAR (X), exemplarily monomers of the 4-pentenyl-PNA (scheme 7/Figure 7).
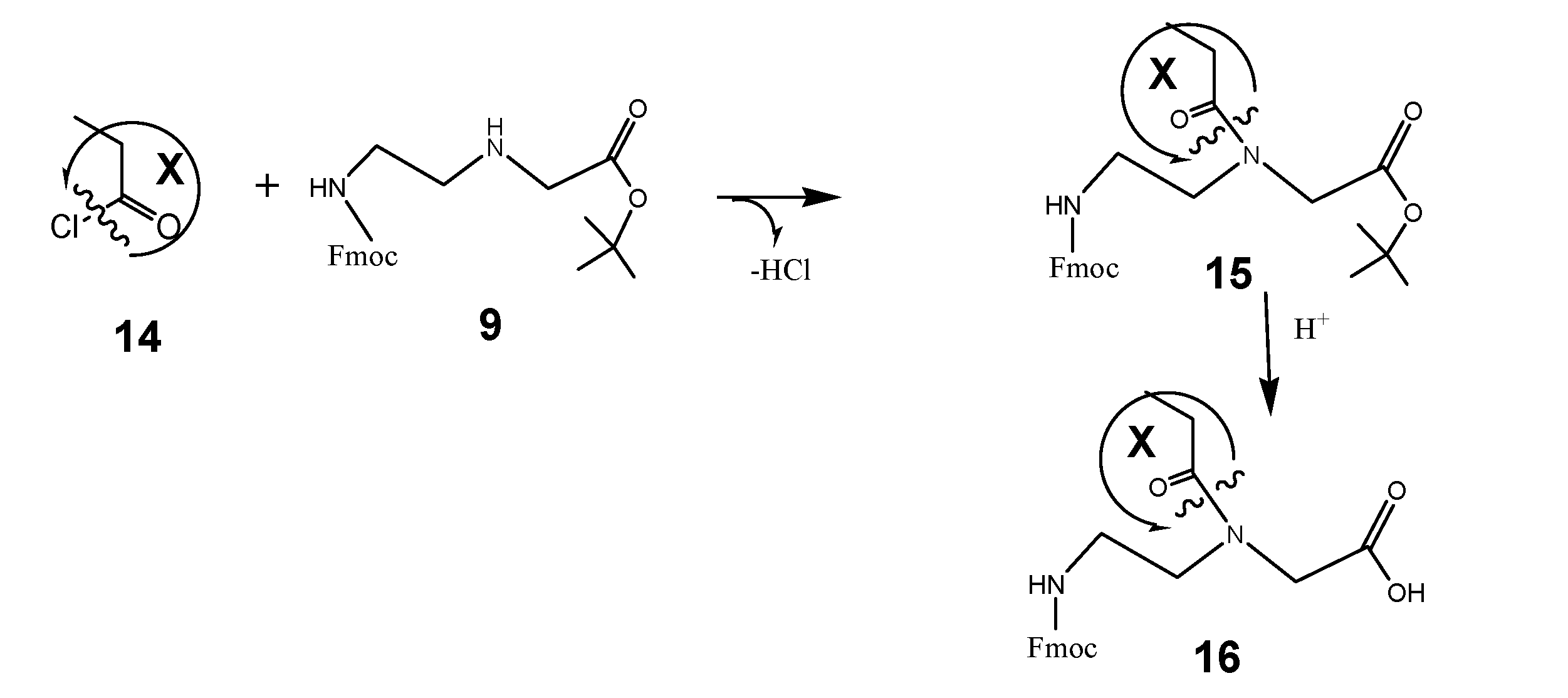
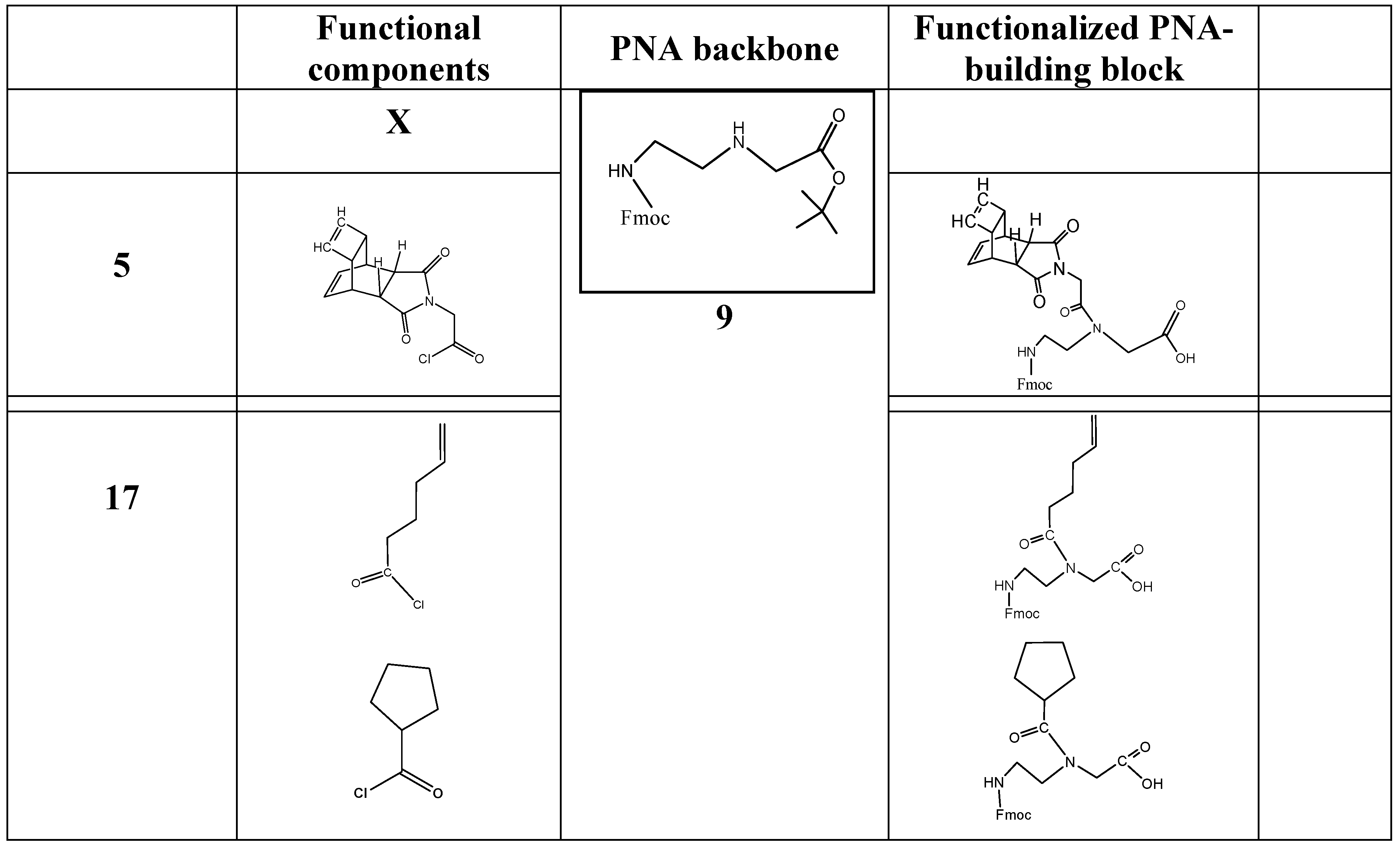
Based on the synthesis protocols as described under schemata 1 to 3, the scheme 1 acts as a “hard” and fast rule for the synthesis of functional molecules suitable for the design of functionalized building blocks of PNA or other nucleic acid derivatives. Here the component 14 is comparable to number 5 in the scheme 3 and can be substituted by a broad spectrum of functional molecules according the reasons of research. Examples of functional molecules are listed in table 1.
(Scheme 6) exemplifies a commonly applicable instruction for synthesis of molecules suitable for functionalization of PNA: Fmoc-C2-glycine-tert-butyl ester 9 reacts with the carbonic acid chloride of the DAR component X 14 to the corresponding tert-butyl ester of the peptide nucleic acid monomer (Fmoc protected) 15. After hydrolysis of the tert-butyl ester catalyzed by acid, the peptide nucleic acid monomer (Fmoc protected) functionalized with the DAR component X 16 is received.
Synthesis of the 4-pentenoic acid PNA building block
The synthesis of the PNA building block functionalized with the 4-pentenoic acid is shown as an example for a myriad of applications. The synthesis is described in the footnote3 and represents a general prescription for the functionalization of PNA.
(Scheme 7) shows the PNA building block Fmoc protected and functionalized with 4-pentenoic acid 17 acting as a dienophile component. According to the scheme 6 the synthesis consists of two procedures and was carried out as described in the footnote4.
This device works in a variety of ligation areas which will be described in the following.
Synthesis of PNA polymers
Using the solid phase synthesis we obtained functional modular PNA oligomers for coupling different active agents and imaging molecules together, or just one of those in parallel to reach local concentrations unachievable until now.
Solid phase synthesis of the “Reppe anhydride”-PNA pentamer (RE-PNA)5
In order to demonstrate the high efficiency of the DARinv-based “Click” chemistry and to realize experiments with high local concentrations of active agents in cells, a pentamer of “Reppe anhydride” (RE-PNA)5 was synthesized exemplarily (scheme 4/Figure 4). The instructions are documented in the footnote4.
For chemical reaction by the solid phase peptide synthesis (SPPS) we produced a pentamer of the RE-PNA where an additional cysteine was attached to the amino terminus [(RE-PNA)5Cys = (TcT)5-Cys] which in turn will be coupled later to the cysteine of a cell membrane transport facilitating peptide (CPP), which permits an efficient cellular uptake imperative for biochemical studies.
This (RE-PNA)5Cys acts as a cargo and represents the corresponding reaction partner, (a dienophile compound) for the intracellular DARinv to functionalize active substances harbouring diene reaction groups. The following features predispose the “Reppe anhydride” molecule for use in the DARinv chemistry.[39] To “hit two birds with one stone”, this Reppe anhydride PNA monomer differs from the commonly used PNA monomers whose nucleobase is substituted by the “Reppe anhydride”, harboring two independent but time dependant dienophiles with valuable different reactivity.
With the two dienophiles different diene componends can be connected to the molecule important for experiments in cells (scheme 4/Figure 4) after combination with facilitating features for the passage across biological membranes.
As illustrated in scheme 4/Figure 4, a cysteine is attached at the amino terminus of the PNA backbone for redox coupling with the cysteine of a cell penetrating peptide (CPP). This formulation of the conjugate is exemplified in scheme 5/Figure 5.
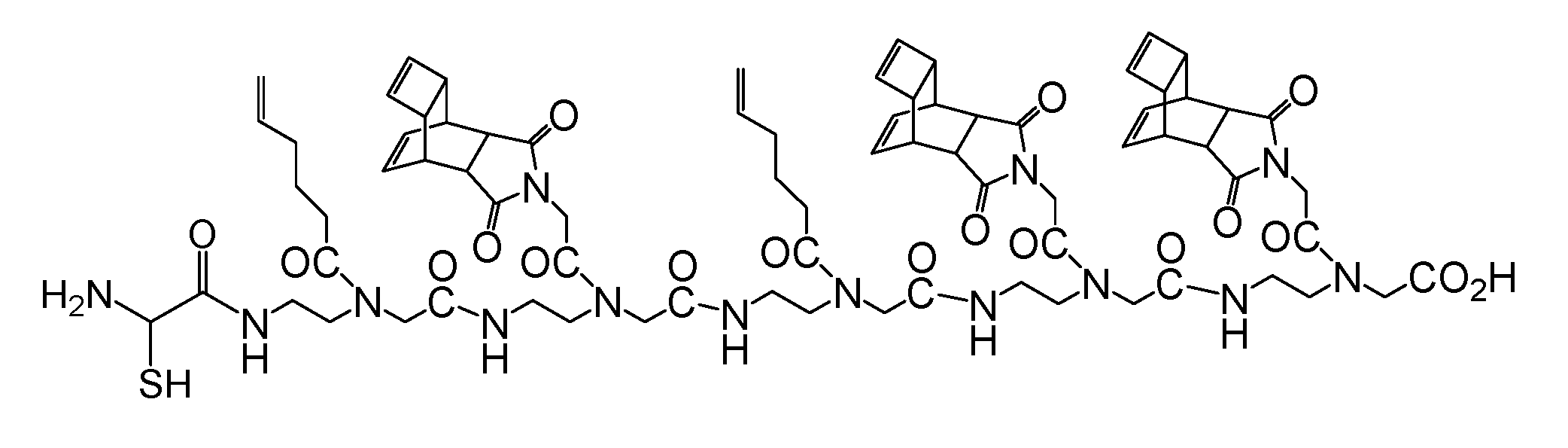
The controlled different reactivity of the pentenyl group compared to the dienophile groups in the Reppe anhydride allows the synthesis of PNA oligomers consisting of two or more different dienophiles suitable for two or more independent Diels Alder Reactions with inverse-electron-demand (DARinv) as shown exemplarily in scheme 8/Figure 8.
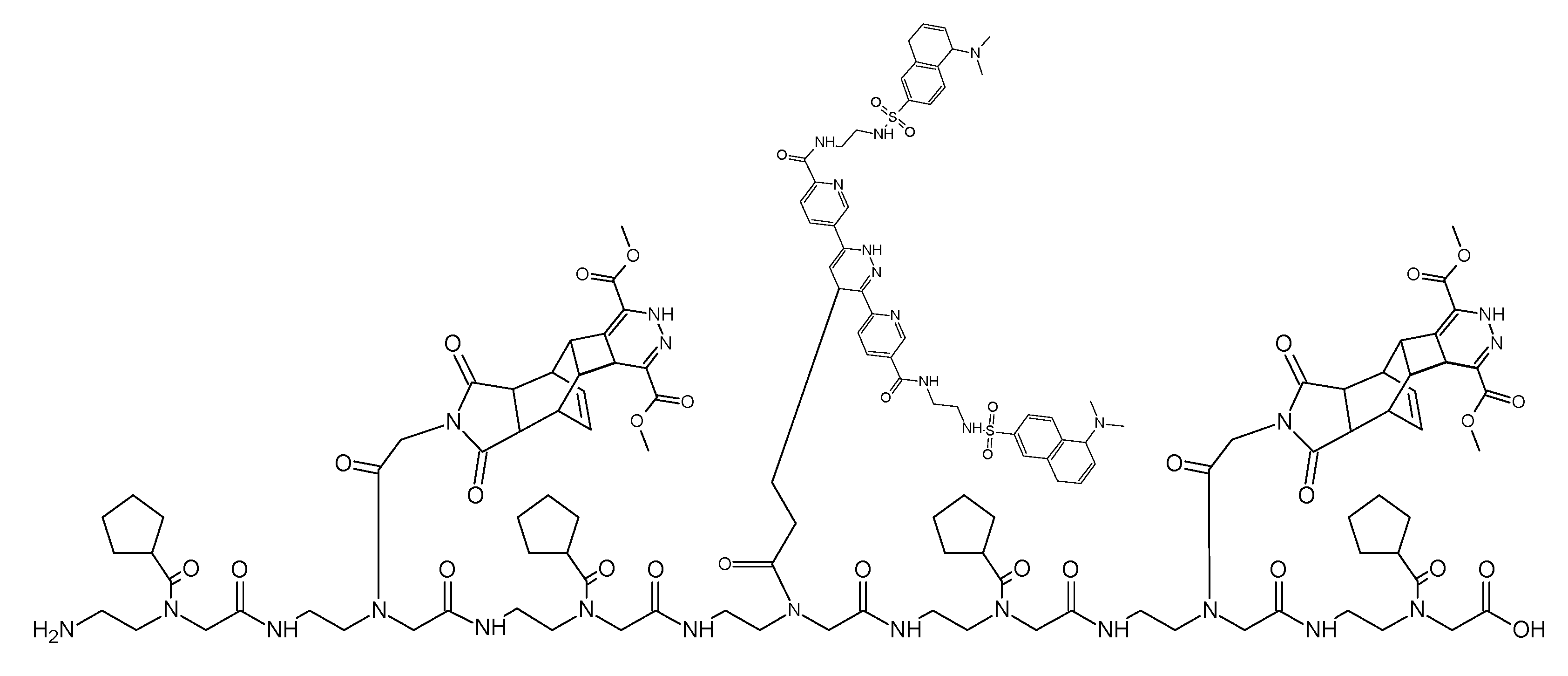
(Scheme 8): the scheme exemplifies the PNA pentamer consisting of three Reppe anhydride 5 and two pentenoic acid 17 building blocks; additionally a cysteine for disulfide coupling was included at the amino terminus.
DARinv ligation of the PNA pentamer tetrazine derivatized
Scheme 9 (Figure 9) depicts the molecule after complete ligation by the DARinv focussed on the reaction site. Details of the chemical reaction are documented by Wiessler[44].
Ligation of (RE-PNA)5 with dimethyl-1,2,4,5-tetrazine-3,6-dicarboxylate
The first dienophile, being highly reactive, allows the ligation of functional molecules like carrier molecules on one side of the molecule. The second dienophile on the other side with lower reactivity is available for further functionalization under different reaction conditions e.g. as a coupling site for fluorescent markers.
Ligation of (RE-PNA)5 with dimethyl-1,2,4,5-tetrazine-3,6-dicarboxylate dansyl chloride connected via an ethylene diamine linker
This DARinv mediated ligation describes the reaction product of the complete ligation of the Reppe anhydride pentamer (RE-PNA)5 with the dimethyl-1,2,4,5-tetrazine-3,6-dicarboxylate functionalized with two dansyl chloride resulting in symmetrical arrangements as illustrated in scheme 10/Figure 10.
(Scheme 9) shows the PNA pentamer with five functionalized Reppe anhydrides (dienophile compound) (RE-PNA) after ligation with dimethyl-1,2,4,5-tetrazine-3,6-dicarboxylate (diene compound). The DARinv mechanism is described in detail[13] and the ligation procedure is documented in the footnote.5
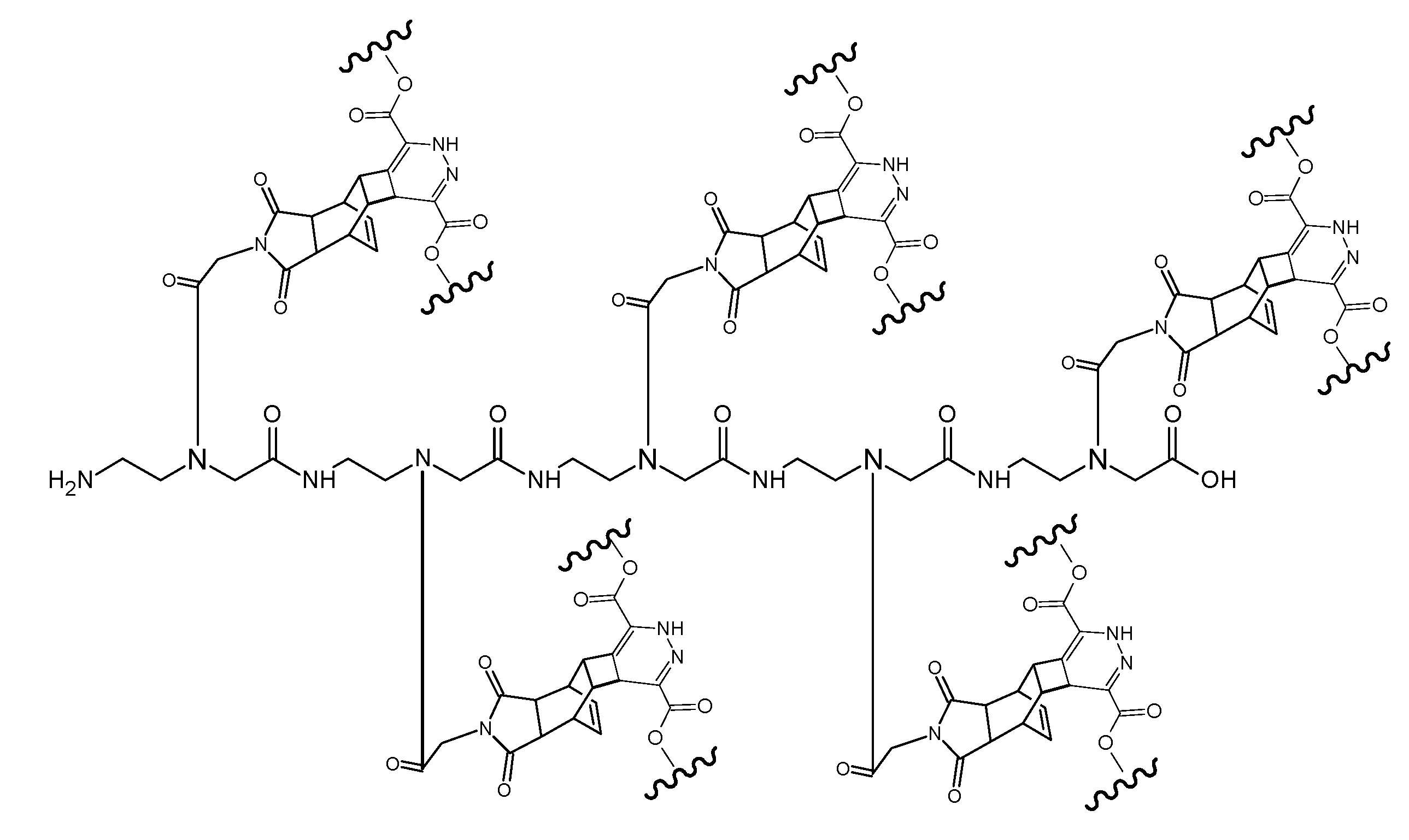
(Scheme 10) shows the ligation product of the pentamer (RE-PNA)5 molecule after the complete DARinv reaction with the diene compound dimethyl-1,2,4,5-tetrazine-3,6-dicarboxylate derivatized with the fluorescent marker dansyl chloride [5-(dimethylamino)naphthalene-1-sulfonyl chloride] connected by an ethylene diamine linker. The synthesis procedure is documented in the footnote.6
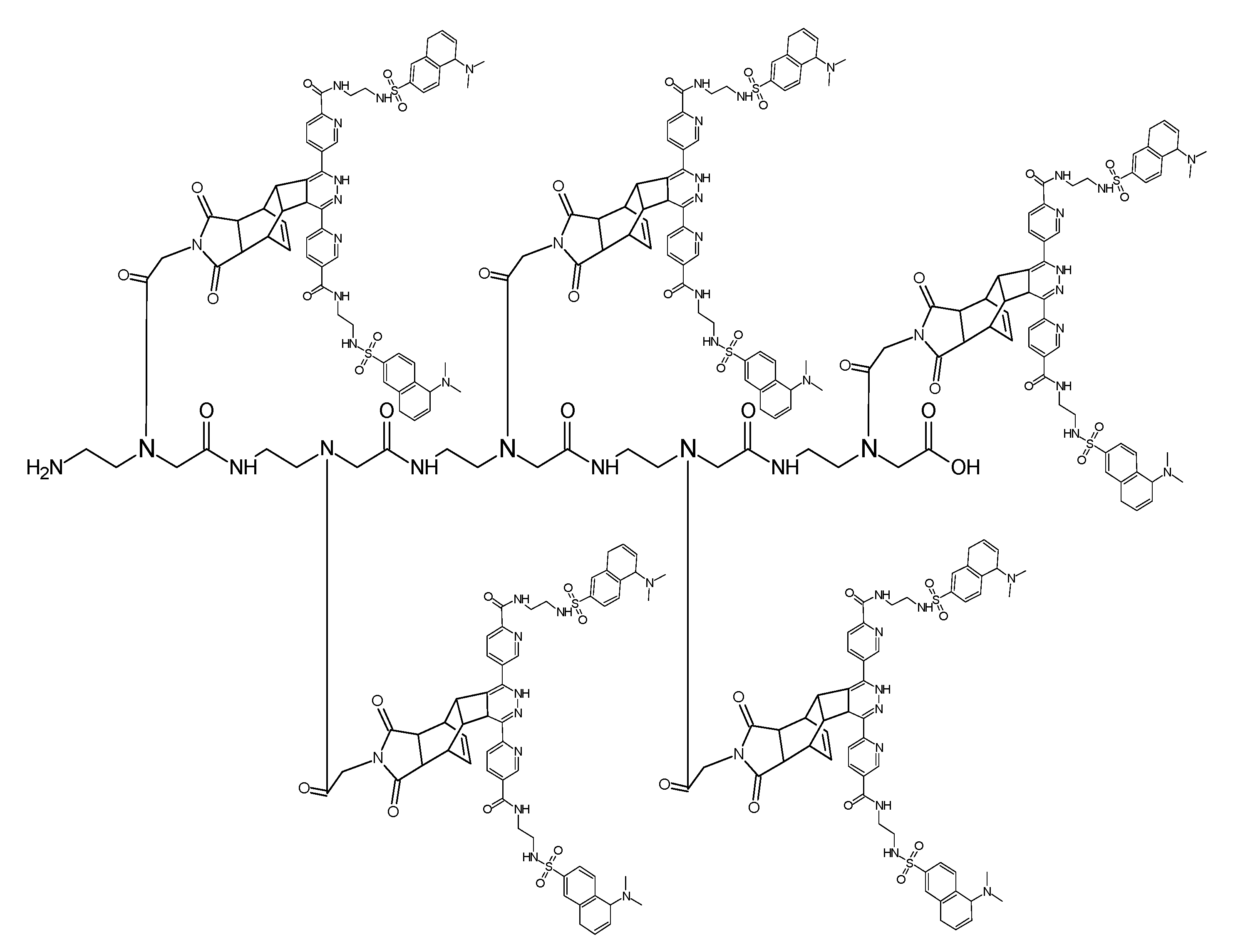
(Scheme 11) shows the ligation product of the PNA heptamer molecule generated after two steps: the first ligation step (A) with the Reppe molecule as dienophile and dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate as a diene, while the second ligation step (B) was the reaction of the pentenoic acid group with the tetrazine functionalized twofold with dansyl (symmetrical molecule). To avoid sterical interactions which can hamper the ligation processes all dienophile compounds were spatially separated with a PNA monomer functionalized with a cyclopentane group.
lists the synthesized components which were used, other than the norbonenyl functionalized PNA building block, for ligation reactions.
lists the compounds used as a carrier system for the DARinv ligation reaction in living cells.
Ligation reaction of a PNA heptamer functionalized with different reactive dienophiles
This ligation uses a PNA heptamer consisting of dienophiles with different reactivities like the Reppe anhydride and the pentenoic acid. In turn, these are separated by a cyclopentane building block avoiding possible steric interactions which could restrict the ligation efficiency. The ligation begins with chemical reaction of the Reppe anhydrides cyclobutene, the dienophile with the highest reactivity. After the reaction was complete, the second ligation reaction with the pentenoic acid was started. The sequenced ligation reactions A and B are described detailed in the footnote.7
Discussion
Here we demonstrate the synthesis of exemplary PNA building blocks suitable for a broad spectrum of ligation reactions as listed in table 1 for which the DARinv is predestined. For future applications the rapid and selective ligation must also bear dedicated requirements e.g. in living systems stable educts, intermediates, and products respectively. To meet these demands the PNA can be considered as a promising candidate: As demonstrated by its chemical structure, it resembles neither a peptide nor a nucleic acid, which results in a stability against enzymatic degradation by peptidases and nucleases. For this case, these properties justify the development of PNA based molecules with completely new functions appropriate for ligation reactions in the “Click chemistry”, instead of using conventional PNA harboring nucleobases. The multifaceted spectrum of applications of Click Chemistry is comprehensively detailed in the Volume 39, Issue 4 of the ChemSocRev in 2010.
Our new molecules could be functionalized, as diene-, or dienophile-compounds by coupling to the glycine's N-terminus which in turn is linked to an ethylendiamine N-monosubstituted Fmoc. In this manuscript we used the Tetracyclo-[5.4.21,7.O2,6.O8,11]3,5-dioxo-4-aza-9,12-tridecadiene, well known as “Reppe anhydride”, moderately derivatized for coupling at the PNA backbone, representing a suitable dienophile-compound candidate 11 (RE-PNA). Its chemical reactions, as well as the corresponding multi-faced range, are comprehensively documented.[45] We focused our studies on the RE-PNA pentamer. It is also important to note that the use of the Fmoc-protected PNA-monomer derivatives like RE-PNA avoids both, an unnecessary expansion of the molecule after ligation, and also associated undesired reactions and steric effects. Using the example of the RE-PNA monomer, the functionalized PNA monomers should be considered as candidates for powerful molecules which allow rapid and complete ligation reactions in aqueous solution, at room temperature and without catalytic support. Furthermore, using these functionalized PNA monomers like Fmoc-protected RE-PNA the PNA synthesis itself can be carried out by automated conventional solid phase peptide synthesis, which avoids unnecessary coupling steps and results in high yields and quality. Also in future pharmacological applications, as yet impracticable, this example could establish a platform for expanded use in the PNA, the DNA and the RNA world.
Conclusion
Here we like to emphasize the simple rationale of click chemistry for a use in medical applications. We describe the synthesis of complex functional molecules made by simple methods like the solid phase synthesis using the functionalized monomers.
The “Click chemistry” demands not expansive educts but proper products for the irreversible DARinvers ligation chemistry. This chemical reaction produces solely nitrogen as a by-product.
Efficient Click chemistry does not depend of stringent reaction conditions, takes place preferentially in aqueous solutions and is therefore useful for reaction in living cells.
With the functionalization of PNA building blocks and oligomer synthesis we can realize high local concentrations of diagnostic or/and therapeutic molecules at the desired target site.
Acknowledgements
This work was supported in part by grant from the Deutsche Krebshilfe Foundation (Project No. 106335).
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Miller SL. Peptide nucleic acids and prebiotic chemistry. Nat Struct Biol. 1997;4:167-9
2. Nielsen PE. Peptide nucleic acids and the origin of life. Chem Biodivers. 2007;4:1996-2002
3. Nielsen PE, Egholm M, Berg RH. et al. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991;254:1497-500
4. Huisgen R. Theory of 1,3-Dipolar Cycloadditions. In: (ed.) Padwa A. 1,3-Dipolar Cycloaddition Chemistry. New York: Wiley. 1984:1-176
5. Lin FL, Hoyt HM, van HH. et al. Mechanistic investigation of the staudinger ligation. J Am Chem Soc. 2005;127:2686-95
6. Seelig B, Jaschke A. Site-specific modification of enzymatically synthesized RNA: Transcription initiation and Diels-Alder reaction. Tetrahedron Letters. 1997;38:7729-32
7. Kolb HC, Finn MG, Sharpless KB. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew Chem Int Ed Engl. 2001;40:2004-21
8. Finn MG, Fokin VV. Click chemistry: function follows form. Chem Soc Rev. 2010;39:1231-2
9. Bachmann WE, Chemerda JM. The Diels Alder Reaction of 1-Vinyl-6-Methoxy-3,4-Dihydronaphthalene with Citraconic Anhydride. J Americ Chem Soc. 1948;70:1468-73
10. Alder K, Diels O. [Otto Diels and Kurt Alder; winners of the Nobel Prize in chemistry.]. Cienc Invest. 1951;7:143-4
11. Stocking EM, Williams RM. Chemistry and biology of biosynthetic Diels-Alder reactions. Angew Chem Int Ed Engl. 2003;42:3078-115
12. Blackman ML, Royzen M, Fox JM. Tetrazine ligation: fast bioconjugation based on inverse-electron-demand Diels-Alder reactivity. J Am Chem Soc. 2008;130:13518-9
13. Waldeck W, Wiessler M, Ehemann V. et al. TMZ-BioShuttle--a reformulated temozolomide. Int J Med Sci. 2008;5:273-84
14. Ibrahim-Ouali M. Diels-Alder route to steroids and associated structures. Steroids. 2009Feb;74(2):133-62
15. Carboni RA, Lindsey RV. Reactions of Tetrazines with Unsaturated Compounds - A New Synthesis of Pyridazines. J Americ Chem So. 1959;81:4342-6
16. Nielsen PE, Egholm M. An introduction to peptide nucleic acid. Curr Issues Mol Biol. 1999;1:89-104
17. Nielsen PE, Egholm M, Berg RH. et al. Peptide nucleic acids (PNAs): potential antisense and anti-gene agents. Anticancer Drug Des. 1993;8:53-63
18. Corey DR. Peptide nucleic acids: expanding the scope of nucleic acid recognition. Trends Biotechnol. 1997;15:224-9
19. Stein CA, Subasinghe C, Shinozuka K. et al. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 1988;16:3209-21
20. Johansson HE, Belsham GJ, Sproat BS. et al. Target-specific arrest of mRNA translation by antisense 2'-O-alkyloligoribonucleotides. Nucleic Acids Res. 1994;22:4591-8
21. Heidenreich O, Gryaznov S, Nerenberg M. RNase H-independent antisense activity of oligonucleotide N3 '-- > P5 ' phosphoramidates. Nucleic Acids Res. 1997;25:776-80
22. Egholm M, Nielsen PE, Buchardt O. et al. Recognition of Guanine and Adenine in DNA by Cytosine and Thymine Containing Peptide Nucleic-Acids (PNA)(1,2). J AMER CHEM SOC. 1992;114:9677-8
23. Summerton J, Weller D. Morpholino antisense oligomers: design, preparation, and properties. Antisense Nucleic Acid Drug Dev. 1997;7:187-95
24. Koshkin AA, Nielsen P, Meldgaard M. et al. LNA (locked nucleic acid): An RNA mimic forming exceedingly stable LNA: LNA duplexes. Journal of the American Chemical Society. 1998;120:13252-3
25. Nielsen PE. Peptide Nucleic-Acid (PNA) a Structural DNA Mimic. PROG BIOPHYS MOL BIOL. 1996;65:103
26. Demidov VV, Potaman VN, Frank Kamenetskii MD. et al. Stability of peptide nucleic acids in human serum and cellular extracts. Biochem Pharmacol. 1994;48:1310-3
27. Egholm M, Buchardt O, Nielsen PE. et al. Peptide Nucleic Acids (PNA). Oligonucleotide Analogues with an Achiral Peptide Backbone. J Am Chem Soc. 1992;114:1895-7
28. Braun K, von Brasch L, Pipkorn R. et al. BioShuttle-mediated plasmid transfer. Int J Med Sci. 2007;4:267-77
29. Nielsen PE. Applications of peptide nucleic acids. Curr Opin Biotechnol. 1999;10:71-5
30. Mah C, Byrne BJ, Flotte TR. Virus-based gene delivery systems. Clin Pharmacokinet. 2002;41:901-11
31. Mahato RI. Non-viral peptide-based approaches to gene delivery. J Drug Target. 1999;7:249-68
32. Filipovska A, Eccles MR, Smith RAJ. et al. Delivery of antisense peptide nucleic acids (PNAs) to the cytosol by disulphide conjugation to a lipophilic cation. Febs Letters. 2004;556:180-6
33. Gait MJ. Peptide-mediated cellular delivery of antisense oligonucleotides and their analogues. Cell Mol Life Sci. 2003;60:844-53
34. Wagner E. Effects of membrane-active agents in gene delivery. J Controlled Release. 1998;53:155-8
35. Ardhammar M, Norden B, Nielsen PE. et al. In vitro membrane penetration of modified peptide nucleic acid (PNA). J Biomol Struct Dyn. 1999;17:33-40
36. Koppelhus U, Nielsen PE. Cellular delivery of peptide nucleic acid (PNA). Adv Drug Deliv Rev. 2003;55:267-80
37. Braun K, Pipkorn R, Waldeck W. Development and Characterization of Drug Delivery systems for Targeting Mammalian Cells and Tissues: A Review. Curr Med Chem. 2005;12:1841-58
38. Thomson SA, Josey JA, Cadilla R. et al. Fmoc Mediated Synthesis of Peptide Nucleic-Acids. TETRAHEDRON. 1995;51:6179-94
39. Reppe W, Schlichting O, Klager K. et al. Cyclisierende Polymerisation von Acetylen I. Justus Liebigs Annalen der Chemie. 1948;560:1-92
40. Atherton E, Sheppard RC. In: Undenfriend S, Meienhofer J, editors. The Peptides. Orlando: Academic Press. 1987:1-38
41. Merriefield RB. Solid Phase Peptide Synthesis. I The Synthesis of a Tetrapeptide. J Americ Chem Soc. 1963;85:2149-54
42. Carpino LA, Han GY. The 9-Fluorenylmethoxycarbonyl Amino-Protecting Group. J ORG CHEM. 1972;37:3404-9
43. Braun K, Peschke P, Pipkorn R. et al. A biological transporter for the delivery of peptide nucleic acids (PNAs) to the nuclear compartment of living cells. J Mol Biol. 2002;318:237-43
44. Wiessler M, Waldeck W, Kliem C. et al. The Diels-Alder-reaction with inverse-electron-demand, a very efficient versatile click-reaction concept for proper ligation of variable molecular partners. Int J Med Sci. 2009;7:19-28
45. Pipkorn R, Waldeck W, Didinger B. et al. Inverse-electron-demand Diels-Alder reaction as a highly efficient chemoselective ligation procedure: Synthesis and function of a BioShuttle for temozolomide transport into prostate cancer cells. J Pept Sci. 2009;15:235-41
Footnotes
tert-butyl 3-[(2-aminoethyl)amino]glycine: Ethylenediamine (0.72 mol) 6 was pre-filled in a 5-fold molar excess in 40 ml chloroform and kept on ice. Then, with continuous stirring, a mixed solution of 20 ml chloroform and 0.144 mol chloride acetic acid tert-butyl ester 7 was added over a period of 90 minutes. The reaction mix was stirred over night at room temperature and then the product 8 was rinsed twice with water and desiccated. (The solvent was removed with a rotary evaporator.) Fmoc-C2-glycine-tert-butyl ester: The complete reaction product (0.1127 mol) tert-butyl 3-[(2-aminoethyl)amino]glycine 8 was consecutively used for chemical reaction with 0.1127 mol N,N-diisopropylethylamine in 500 ml dichloromethane. Then 0.1127 mol Fmoc-succinimide dissolved in 200ml dichloromethane were added dropwise over a period of 4 hours. After 1 hour a clouding of the reaction solution and separation of a substance could be observed. The reaction solution was stirred during the whole weekend and then, after rinsing fivefold with 200 ml 1 N HCl and once more with saturated solution of sodium chloride, the precipitate was desiccated. During the concentration of the solution a slow-going crystallization was observed. The crystalline product Fmoc protected-[(2-aminoethyl)glycine] tert-butyl ester 9 was washed manifold with ether and subsequently desiccated.
Coupling of the Fmoc-C2-glycine-tert-butyl ester with the Reppe anhydride: 2 mmol of the Fmoc-C2-glycine-tert-butyl ester 9 and 4 mmol N,N-diisopropylethylamine were pre-filled in 10ml dichloromethane and consecutively 5 ml dichloromethane was added by the dropping funnel stirring constantly over a period of 30 minutes. The yellow coloured product was concentrated by the rotary evaporator. The residue featuring a glass-like consistency was dissolved in dichloromethane and purified by silica gel column chromatography. Chloroform and ethanol were used for elution at a ratio of 95:5. Cleavage of the tert-butyl group: 2 mmol tert-butyl protected Fmoc-PNA building block 10 functionalized with 5 the glycine acetic chloride derivative of the Reppe anhydride was dissolved in 5 ml dichloromethane and 5 ml trifluoroacetic acid (TFA) and simultaneously 5 ml TFA dissolved in dichloromethane were added successively by a dropping funnel. The reaction batch was stirred continuously over night and the reaction's completeness was examined using thin-layer chromatography. The yellow coloured product was concentrated by rotary evaporator and covered with a layer of ether. The product {scheme 3: 11 [RE-PNA], scheme 6: 16} precipitates voluminously, depending on the quantity the precipitation process can take up to two days. In this case the precipitation should run at a temperature of 4°C.
Synthesis of the Fmoc-pentenoic acid-PNA. For synthesis of the pentenoic acid PNA (scheme 3), equimolar (2 mmol) Glycine and pentenoic acid were transformed. In the N-ethylisopropylamine (4 mmol) (Huening's base) / glycine mix the pentenoic acid chloride dissolved in dichloromethane was added in drops over night.
Solid phase synthesis of the “Reppe anhydride”-PNA pentamer. To perform the solid phase peptide synthesis (SPPS)[41] of PNA modules we employed the Fmoc-strategy[42] in a fully automated peptide synthesizer A433 (Perkin Elmer). The synthesis was carried out on a 0.05 mmol Tenta Gel R Ram (Rapp Polymere) 0.19 mmol/g of substitution. As coupling agent 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) was used. A typical synthetic cycle consisted of a single 30 minute coupling of 3 equivalents of monomer to the growing PNA chain, followed by capping of the unreacted free amines with acetic anhydride. The protected PNA resins were treated with 20% piperidine in dimethylformamide over 5 minutes and then washed thoroughly with dimethylformamide. Cleavage and deprotection of the resins were effected by treatment with 90% trifluoroacetic acid and 10% triethylsilane.
Ligation of (RE-PNA)5 with dimethyl-1,2,4,5-tetrazine-3,6-dicarboxylate. 1.723 mg (1 µmol) of the (RE-PNA)5 pentamer were pre-filled and reacted with 1.089 mg (5.5 µmol) dimethyl-1,2,4,5-tetrazine-3,6-dicarboxylate dissolved in 0.5 ml dichloromethane. The progress of the chemical reaction can be monitored by changes in colour. The end of the reaction is indicated by decolourization after a few minutes.
Ligation of the (RE-PNA)5 with dimethyl-1,2,4,5-tetrazine-3,6-dicarboxylate dansyl chloride connected via an ethylene diamine linker. 1.723 mg (1 µmol) of the PNA pentamer were pre-filled and reacted with 4.807 mg of the dimethyl-1,2,4,5-tetrazine-3,6-dicarboxylate derivatized with dansyl as fluorescence marker (5.5 µmol) dissolved in 0.5 ml DMSO. After a reaction time of 10 min a brightening of the magenta stained solution was observed. To complete the chemical reaction the suspension was allowed to stand over night.
A. 2,005 mg (1 µmol) of the PNA heptamer were pre-filled and reacted with 0.396 mg (1.0 µmol) of dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate dissolved in 0.5 ml dichloromethane under stirring. The reaction vessel was allowed to stand until decolorization was complete after circa 10 min. B. In a second step 0.874 mg of the tetrazine derivatized twofold with dansyl, was dissolved in a few drops of DMSO and then added to the reaction product of A. The reaction mixture was shaken and then allowed to settle over night.
Author contact
![]() Corresponding author: Dr. Klaus Braun, Im Neuenheimer Feld 280, German Cancer Research Center, Dep. Of Medical Physics in Radiology, D-69120 Heidelberg, Germany. Tel. No.: +49 6221 42 2495; Fax No.: +49 6221 42 3326; E-mail: k.braunde
Corresponding author: Dr. Klaus Braun, Im Neuenheimer Feld 280, German Cancer Research Center, Dep. Of Medical Physics in Radiology, D-69120 Heidelberg, Germany. Tel. No.: +49 6221 42 2495; Fax No.: +49 6221 42 3326; E-mail: k.braunde

 Global reach, higher impact
Global reach, higher impact