Impact Factor
ISSN: 1449-1907
Int J Med Sci 2010; 7(4):197-208. doi:10.7150/ijms.7.197 This issue Cite
Research Paper
Preparation of RGD-modified Long Circulating Liposome Loading Matrine, and its in vitro Anti-cancer Effects
1. The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang 310003, China
2. Department of Chemical and Biological Engineering, Zhejiang University, Hangzhou, Zhejiang 310027, China
Received 2010-5-13; Accepted 2010-6-9; Published 2010-6-14
Abstract
Aim: To prepare RGD-modified long circulating liposome (LCL) loading matrine (RGD-M-LCL) to improve the tumor-targeting and efficacy of matrine. Methods: LCL which was prepared with HSPC, cholesterol, DSPE-PEG2000 and DSPE-PEG-MAL was modified with an RGD motif confirmed by high performance liquid chromatography (HPLC). The encapsulation efficiency of RGD-M-LCL was also detected by HPLC. MTT assay was used to examine the effects of RGD-M-LCL on the proliferation of Bcap-37, HT-29 and A375 cells. The percentage of apoptotic cells and morphological changes in Bcap-37 cells treated with RGD-M-LCL were detected by Annexin-V-FITC/PI affinity assay and observed under light microscope, respectively. Results: Spherical or oval single-chamber particles of uniform sizes with little agglutination or adhesion were observed under transmission electronic microscope. The RGD motif was successfully coupled to the DSPE-PEG-MAL on liposomes, as confirmed by HPLC. An encapsulation efficiency of 83.13% was obtained when the drug-lipid molar ratio was 0.1, and the encapsulation efficiency was negatively related to the drug-lipid ratio in the range of 0.1~0.4, and to the duration of storage. We found that, compared with free matrine, RGD-M-LCL had much stronger in vitro activity, leading to anti-proliferative and pro-apoptotic effects against cancer cells (P<0.01). Conclusion: RGD-M-LCL, a novel delivery system for anti-cancer drugs, was successfully prepared, and we demonstrated that the use of this material could augment the effects of matrine on cancer cells in vitro.
Keywords: Matrine, Liposomes, Cyclic arginine- glycine-aspartic acid, Drug delivery systems.
Introduction
Matrine, the major active component of the traditional Chinese medicine Sophora flavescens, has been used to treat jaundice, reduce liver enzyme activity, and prevent hepatic fibrosis (1,2). In recent years, studies have indicated that matrine also inhibits tumor cell proliferation (3), and induces cellular differentiation (4) and apoptosis (5). Our previous study showed that matrine could inhibit proliferation and induce apoptosis in human malignant melanoma A375 cells in a dose-dependent manner. Furthermore, it effectively suppressed their adhesion and invasiveness in vitro (6). However, like most low molecular weight drugs, matrine is able to freely traverse in and out of blood vessels to distribute to non-tumor tissues, resulting in accumulation in the liver, spleen or kidneys, diminishing its effects on cancer cells. In addition, consistent with the results of other research groups, our previous study also showed that matrine had fewer side-effects and broader indications compared with conventional anti-cancer drugs. However, its anti-tumor activity was moderate, and its half maximal inhibitory concentration (IC50) was about 2.01mM (6).
In order to improve the tumor-specificity and efficacy of anti-tumor drugs, liposomes have been used extensively as delivery systems in both pre-clinical and clinical applications. Liposomes allow for enhanced drug delivery into tumors, and improve the accumulation of drugs in tumors via the enhanced permeability and retention (EPR) mechanism (7). However, the use of conventional vesicles cannot fully overcome their binding with serum components and uptake by mononuclear phagocyte system (MPS). To overcome such problems, long circulating liposomes, modified with a hydrophilic or a glycolipid such as poly (ethylene glycol) (PEG) or monosialoganglioside (GM1), have been developed in the past several years. The presence of PEG on the surface of the liposomal carrier has been shown to extend blood-circulation time while reducing MPS uptake (8). Furthermore, several novel types of liposomes, such as ligand- or antibody-mediated liposomes (9,10), have been developed to further enhance their tumor-specificity and therapeutic efficacy. The use of these specific “vector” molecules showing affinity toward certain receptors or antigens highly expressed on the surface of the target tumor cells enhances their uptake and activity. Among the most commonly targeted are the integrin receptors, including α5ß1, αγß3 or α4ß1, which are highly expressed on breast, colon and rectal cancer, and melanoma cells (11-13). Therefore, the arginine-glycine-aspartic acid (RGD) peptide, which is a ligand of several integrin receptors, has been used to modify oncolytic viral vectors, delivery vehicles, and the resulting molecules have been used as probes and radiotracers for tumor imaging (14-18). However, linear RGD-peptides are susceptible to chemical degradation, so the peptides were cyclized to confer rigidity and increase stability (19).
In this study, we prepared liposomes loaded with matrine using hydrogenated soybean phosphatidylcholine (HSPC), cholesterol, 1,2- distearoyl-sn-glycero-3-phosphoethanolamine -N-[PEG(2000)] (DSPE-PEG2000) and maleimide-[poly (ethylene glycol)]-1,2-dioleoyl-sn -glycero-3-phosphoethanolamine (DSPE-PEG-MAL), and modified them with RGD. The novel therapeutic approach was then evaluated by in vitro testing in human cancer cell lines. We observed that RGD-M-LCL had increased anti-proliferative activity and increased the induction of apoptosis in cancer cells compared to free matrine, supporting the further development of this novel delivery system for cancer therapy.
Materials and Methods
Preparation of RGD-modified long circulating liposome (LCL) loading matrine (RGD-M-LCL)
Lipids composed of HSPC (Lipoid, Germany), cholesterol (Lipoid, Germany), DSPE-mPEG2000 (Avanti, USA) and DSPE-PEG-MAL (Avanti, USA) in a molar ratio of 2:1:0.1:0.01, were dissolved in chloroform:methanol (9:1 vol/ vol) in a round-bottom flask. The solvent was evaporated to form a lipid film under reduced pressure and constant rotation (Rotovapor R-200, Buchi, Switzerland) at 40°C. The lipid film was hydrated with 300mmol/L citric acid (pH 4.0) at 62°C for 1 h, and in a magnetic stirrer for another 1 h, followed by ultrasonication in an ice bath for 15 min. Then the sample of LCL was delivered to the Zhejiang California International NanoSystems Institute, and was processed by a microfluidizer (Microfluidics, USA). After microfluidizing, the LCL suspension was stored at 4°C until use.
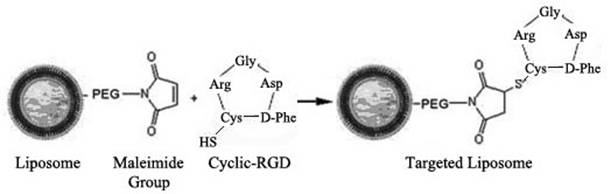
The Cyclic-RGD peptide (Arg-Gly-Asp-D-Phe-Cys, purity assayed by HPLC to be >98%) was synthesized by the Chinese Peptide Company (Hangzhou, China) and dissolved in 50 mM HEPES buffer (pH 6.5) at 1mg/mL, then the peptide was reacted with the LCL suspension with maleimide functional groups in a molar ratio of 1:10 (RGD:maleimide) at room temperature overnight to prepare RGD-LCL. In this step, the sulfhydryl (-SH) group of cystine in the Cyclic-RGD couples to the maleimide groups at the distal end of DSPE-PEG-MAL on the liposomes (Figure 1) (20). Then, referring to the pH-gradient method (21), matrine (purity determined to be >99% by HPLC, Nanjing Tcm Institute Of Chinese Materia Medica, China) was added to the RGD-LCL in a drug-lipid ratio of 0.1, 0.2 or 0.4, respectively, and mixed with NaH2PO4 (pH 7.0) to obtain a desired pH gradient (inside pH 4.0, outside pH7.0). Subsequently, the mixture was incubated under argon at 60°C for 30 min, and then the generation of RGD-modified long circulating liposome loading matrine (RGD-M-LCL) was complete. Some RGD-M-LCL in a drug-lipid ratio of 0.1 was stored at 4°C to determine its stability, particle size and the efficiency of drug-loading.
Schematic representation of the coupling reaction between the maleimide group on the distal end of the PEG chain on the LCL and the -SH group in the cyclic RGD peptide (19).
HPLC analysis of the coupling of RGD to LCL
Free Cyclo-RGD (250μg/mL or 15μg/mL) and conjunctive RGD-LCL equivalent to 15μg/mL RGD were analyzed by HPLC to ascertain the status of RGD. A Hypersil-BDS-C18-column (4.0 × 250 mm, Thermo, USA) was used with a mobile phase consisting of 0.05% trifluoroacetic acid in water (eluant A) and 0.05% trifluoroacetic acid in acetonitrile (eluant B). The eluant gradient was set from 10% to 60% B in 50 min, and subsequently back to 10% B over 5 min (19). The detection wavelength was 214nm, the flow rate was 1mL/min, and the injection volume was 20μL.
Lyophilization of RGD-M-LCL and measurement of its particle size
RGD-M-LCL was freeze-dried by a cryoprotectant of sugar in a sugar-lipid quality ratio of 2.0 (22), then was redissolved with DMEM. The size of the liposomes was measured before and after freeze-drying by a Zetasizer Nano (Malvern, United Kingdom). In addition, the samples were delivered to the Electronic Microscope Centre of Huajiachi Campus, Zhejiang University. Subsequently, liposomes were dyed with 3% phosphotungstic acid for negative staining, and then deposited to a copper screen for observation under transmission electronic microscope (Tecnai-co, Philip, the Netherlands) to evaluate their shape.
Encapsulation efficiency and loading-drug stability of the RGD-M-LCL
Matrine solutions at different concentrations (1.95 μg/mL, 3.91 μg/mL, 7.81 μg/mL, 15.63 μg/mL, 31.25 μg/mL, 62.5 μg/mL, 125 μg/mL and 250 μg/mL) were prepared. A Hypersil-BDS-C18-column was used with a mobile phase consisting of acetonitrile-alcohol-0.2% triethylamine (8:2:90, pH adjusted to 3.0 with phosphoric acid), the detection wavelength was 210nm, the flow rate was 1mL/min and the injection volume was 20μL. The unloaded matrine from different RGD-M-LCL samples obtained immediately after encapsulation, one or two weeks after encapsulation, or following re-dissolution after freeze-drying, was separated by mini-gel columns of Sephadex-G50 prepared in advance. In brief, incubation mixtures of 200μL containing unloaded matrine and RGD-M-LCL were added onto mini-gel columns. Then particles of RGD-M-LCL were collected by centrifugation at 2000rpm/min for 3min, while the free matrine was still isolated in the columns because of their differences in the sizes of the molecules. The amount of matrine collected from the RGD-M-LCL samples was determined by HPLC assay under the same conditions. At the same time, total matrine present in each group before separation was also measured by HPLC. The encapsulation efficiency was calculated as Efficiency = Matrine separated from liposomes (encapsulated)/matrine in unseparated liposomes (total) ×100%.
Cell culture
The A375 melanoma cell line was purchased from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). The breast cancer Bcap-37 and colon cancer HT-29 cell lines were maintained in our lab. A375 cells and Bcap-37 cells were grown in RPMI1640 medium (GIBCO, USA) containing 10% heat-inactivated fetal bovine serum (Nuoding, China), and the HT-29 cells were grown in DMEM medium (GIBCO, USA) also containing 10% heat-inactivated fetal bovine serum. All of the cells were incubated at 37°C in a humidified atmosphere with 50 mL/L CO2. RGD-M-LCL and RGD-LCL were re-dissolved with DMEM or RPMI1640 after freeze-drying.
Effects of RGD-M-LCL on cell viability using MTT) assay
Briefly, cells (5×103 per well) were seeded in 96-well plates (Corning, USA). After subculturing them for 24 h, the cells were treated with matrine of different concentrations (0.0625 mg/mL, 0.125 mg/mL, 0.25 mg/mL, 0.5 mg/mL) or RGD-M-LCL with matrine of equivalent concentrations. The control group consisted of cells in culture medium only. Experiments were carried out in triplicate. In order to observe the toxicity of RGD-LCL without matrine on cells, Bcap-37 cells were also treated with RGD-LCL of different concentrations (0.3125 mg/mL, 0.625 mg/mL, 1.25 mg/mL, and 2.5 mg/mL), and RGD-M-LCL of different concentrations equivalent to these RGD-LCL concentrations. After exposure for 48h, 100 μL of MTT (1 mg/mL) (Sigma-Aldrich, USA) was added to each well and the plates were incubated for an additional 4 h at 37°C. The MTT solution was removed by aspiration, and 150 μL of dimethylsulfoxide (DMSO) (Sigma-Aldrich) was added to each well. Finally, the absorbance of each well was measured at 570 nm. All MTT assays were repeated two times. The relative growth rate was calculated as A570 (test)/A570 (control). We assumed that the average A570 values of the control group were equal to 1, and then generated a histogram of cellular viability according to the relative growth rate following the different treatments.
Morphological observation
Bcap-37 cells were seeded in 96-well plates for 24 h before treatments as follows: matrine at 0.03215 mg/mL or 0.0625 mg/mL, RGD-LCL at 0.625 mg/mL or 1.25 mg/mL, or RGD-M-LCL equivalent to the same concentrations of matrine and RGD-LCL. After being exposed to the different treatments for 48h, the Bcap-37 cells were observed under inverted light microscope (Olympus, Tokyo, Japan).
Effect of RGD-M-LCL on cellular apoptosis
The Annexin-V-fluorescein isothiocyanate/propidium iodide (Annexin-V-FITC/PI) double staining assay was used to detect cellular apoptosis. Bcap-37 cells were equally distributed into culture flasks, and treated with either culture medium only, matrine at 0.03215 mg/mL, RGD-LCL at 0.625 mg/mL, or RGD-M-LCL equivalent to these concentrations of matrine and RGD-LCL. After 24 h of treatment, the cells were collected, washed with cold phosphate-buffered saline (PBS), and resuspended at 2 × 106 cells /mL in Annexin-V binding buffer. The supernatant (100 μL/tube) was incubated with 5 μL of Annexin-V-FITC (Invitrogen, USA) and 5 μL of PI (Invitrogen, USA) for 15 min at room temperature in dark. Binding buffer (400 μL) was then added to each tube, followed by cytometric analysis (Coulter-XL, USA) within 1 h of staining. All experiments were repeated three times.
Statistical analysis
The SAS statistical software was used for statistical analyses. The results are expressed as the means ± standard deviations, and samples were subjected to multiple analysis of variance. The Dunnett-t test was performed to compare the mean between each test group and control group, and the SNK-q test was performed to compare the means between either two test groups. P < 0.05 was considered to be statistically significant.
Results
The RGD motif was successfully coupled to DSPE-PEG-MAL on liposomes
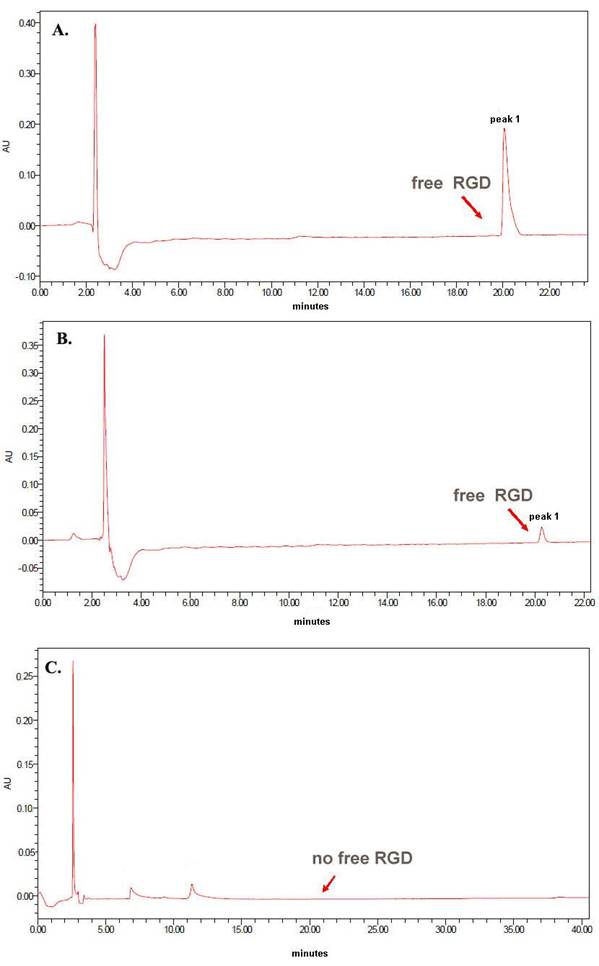
HPLC analysis showed that free RGD at concentrations of 250 μg/mL or 15 μg/mL eluted with a retention time of ~20 minutes, and the peak area was dose-dependent. However, when RGD was conjugated to liposomes at the same concentration (15 μg/mL) following the coupling step, there was no significant peak for the free RGD around 20 minutes (Figure 2), indicating successful coupling of the RGD to the surface of the liposomes.
Assay of particle sizes and morphological observation of RGD-M-LCL
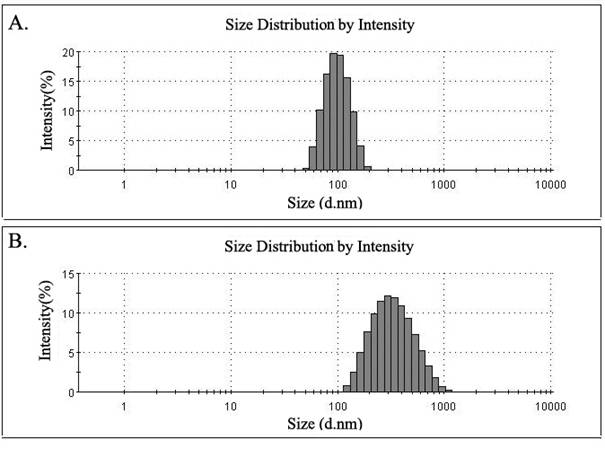
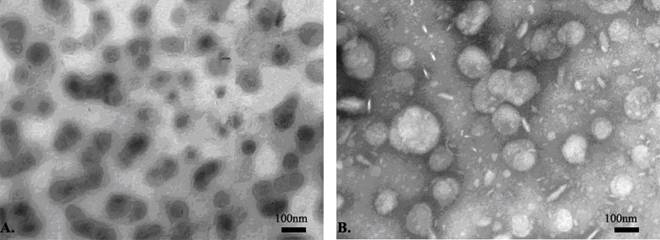
The average size of liposomes processed by the microfluidizer was 97.59±1.93nm, with the liposomes appearing as a light milky-white and translucent suspension. Liposomes were found to be spherical or oval single-chamber particles of uniform sizes with little agglutination or adhesion under transmission electronic microscope. They showed little change in appearance or size when stored for one week, two weeks or four weeks at 4°C. However, the average size of liposomes re-dissolved after freeze-drying increased to 295.77±5.52nm, and presented as larger particles with some agglutination under transmission electronic microscope (Figure 3-4, Table 1).
HPLC confirmation of RGD coupling to the liposomes. (A) Free RGD at 250 μg/mL eluted with a retention time of ~20 minutes; (B) Free RGD at 15 μg/mL also eluted with a retention time of ~20 minutes, and the peak areas of the peaks in A and B demonstrate dose-dependence; (C) The liposome sample modified with the same concentration of 15 μg/mL RGD following the coupling step showed no significant peak for the free RGD around 20 minutes.
Particle size of liposomes. The size of the liposomes was measured with a Zetasizer Nano using a dynamic light scattering technique. (A) The average size of liposomes immediately after encapsulation was 95.37nm, and the distribution width was 27.24nm; (B) The average size of liposomes following re-dissolution after freeze-drying was 301.9nm, and the distribution width was 165.8nm.
The ultrastructural morphology and size of liposomes observed under electron microscope (×3700). (A) After microfluidizing, the liposomes presented as spherical or oval single-chamber particles of uniform sizes with little agglutination or adhesion. (B) After the liposomes were re-dissolved following freeze-drying, they presented with increased size and some agglutination.
Encapsulation efficiency and loading-drug stability of RGD-M-LCL
The peak areas for matrine in the concentration range from 1.95-250 μg/mL was linear in the HPLC assay, and the linear regression equation was Y=28.715X+33.322, with a correlation coefficient of 0.9997. As shown in Table 1, the encapsulation efficiency decreased with the increase in drug-lipid ratio, indicating that there was a significant effect of the drug-lipid ratio on the encapsulation efficiency. During the process of preservation of liposomes, the encapsulation efficiency also decreased due to the release of water-soluble drugs. Although the size of liposomes after freeze-drying increased, their encapsulation efficiency was still maintained at a high level.
Influence of the drug-lipid ratio and storage on the encapsulation efficiency and size of liposomes.
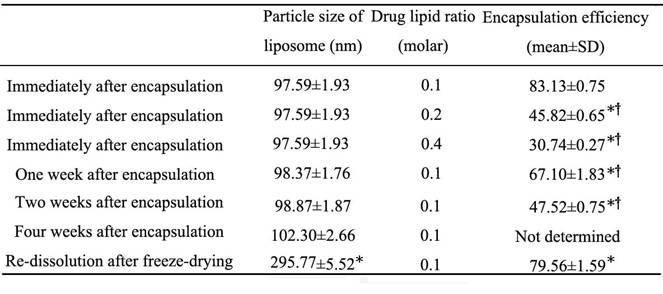
* Compared with the group analyzed immediately after encapsulation, P<0.01; †compared with the group re-dissolved after freeze-drying, P<0.01.
RGD-M-LCL augments the anti-proliferative and pro-apoptotic effects of matrine in cancer cells
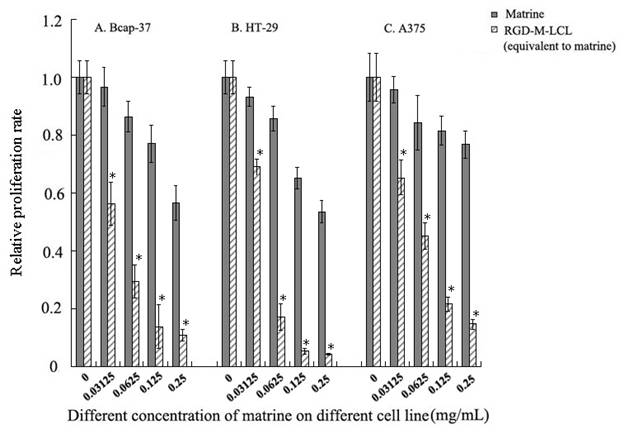
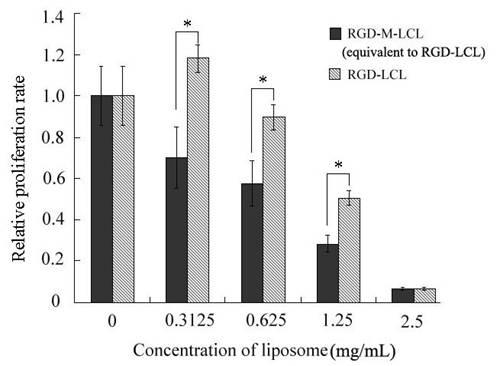
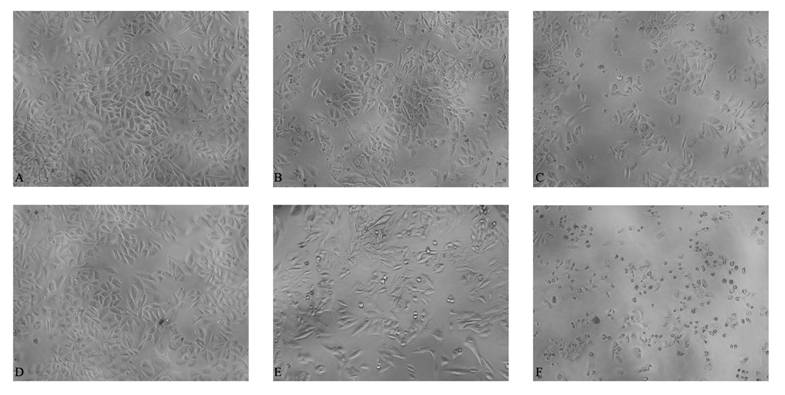
RGD-M-LCL significantly inhibited the growth of Bcap-37, HT-29 and A375 cells, compared with cells treated with the free matrine (P<0.01). Furthermore, the effects of the RGDF-M-LCL were dose-dependent (Figure 5). When Bcap-37 cells were used to assess the toxicity of RGD-LCL, we observed cytotoxicity of liposomes beginning at the 1.25 mg/mL or 2.5 mg/mL concentrations. Indicating that the liposomes themselves have some cytotoxic activity, we did not observe any significant differences between RGD-LCL and the equivalent RGD-M-LCL group at 2.5 mg/mL. However, in the 0.3125 mg/mL or 0.625 mg/mL groups that had low concentrations of RGD-LCL, there were no significant inhibitory effects induced by the liposomes on Bcap-37 cells. In contrast, the RGD-M-LCL equivalent to 0.3125 mg/mL or 0.625mg/mL RGD-LCL significantly inhibited the growth of the cancer cells (P<0.01, Figure 6). We observed under an inverted light microscope that, compared with free matrine and RGD-LCL, there was more pyknosis and a greater cell-free zone in cells treated with RGD-M-LCL of equivalent concentrations (Figure 7).
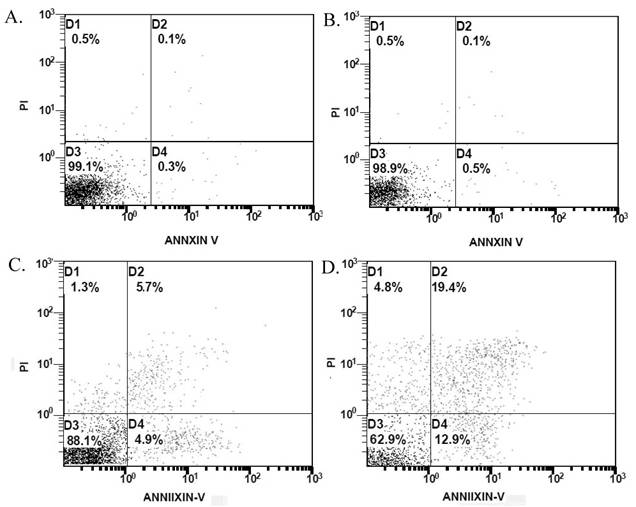
The flow cytometric results demonstrated that, compared with the control group, treatment with matrine alone at 0.03125 mg/mL had no effect on apoptosis in the cultured cancer cells (P>0.05) (Figure 8). However, the percentage of apoptotic cells in cultures treated with 0.625mg/mL RGD-LCL was increased in comparison with the control group (12.25±2.33% vs 0.90±0.51%, P<0.05). Furthermore, the percentage of apoptotic cells in the RGD-M-LCL group was 35.40±2.68%, which was significantly higher than that in the control, 0.03125 mg/mL matrine and 0.625 mg/mL RGD-LCL groups (P<0.05), indicating that the combination of RGD-LCL and matrine leads to enhanced cytotoxic effects.
RGD-M-LCL led to a significant increase in the inhibition of cancer cell growth in a dose-dependent manner. Three cell lines, Bcap-37, HT-29 and A375, were treated with various concentrations of matrine and RGD-M-LCL equivalent to the same concentrations of matrine for 48h, and the A570 values following incubation with MTT were measured by an enzyme-labeling analyzer. Data were compared to the control group (assumed to be 1.0), and the relative viability of each experimental group was plotted. *P < 0.01 when compared with the equivalent matrine group.
Comparison of the growth inhibitory effects of RGD-M-LCL and RGD-LCL on Bcap-37 cells. Bcap-37 cells were treated with various concentrations of RGD-LCL and RGD-M-LCL (equivalent to the same concentration of RGD-LCL) for 48h, and were again plotted as the ratio to the control group. In the cells treated with 0.3125 mg/mL or 0.625 mg/mL of RGD-LCL, the inhibitory effect of liposomes on Bcap-37 cells was minimal, while the RGD-M-LCL equivalent to the same dose of RGD-LCL strongly inhibited the growth of the cancer cells. However, in the cells treated with 1.25 mg/mL or 2.5 mg/mL of RGD-LCL, RGD-LCL itself was moderately cytotoxic to the Bcap-37 cells. *P < 0.01 when compared to the equivalent RGD-LCL group.
Morphological observation of Bcap-37 cells in different treatment groups. Bcap-37 cells were treated with different concentrations of free matrine, RGD-LCL or RGD-M-LCL for 48 h and observed with an inverted light microscope at low power (10 × 10). Compared with free matrine or RGD-LCL groups, there was more pyknosis and a larger cell-free zone in the cells treated with the equivalent RGD-M-LCL. (A) matrine (0.03125 mg/mL), (B) RGD-LCL (0.625 mg/mL), (C) RGD-M-LCL (equivalent to matrine of 0.03125 mg/mL and RGD-LCL of 0.625 mg/mL), (D) matrine (0.0625 mg/mL), (E) RGD-LCL (1.25 mg/mL), and (F) RGD-M-LCL (equivalent to matrine of 0.0625 mg/mL and RGD-LCL of 1.25 mg/mL).
Representative results of apoptosis in Bcap-37 cells in different treatment groups. Bcap-37 cells were treated with different treatment of culture medium, free matrine, RGD-LCL or RGD-M-LCL for 24 h, then were stained with Annexin-V-FITC and PI, and assayed by flow cytometry. Compared with the control group, matrine at 0.03125 mg/mL had no effect on the apoptosis of cancer cells. However, RGD-LCL at 0.625 mg/mL could induce apoptosis in the Bcap-37 cells. Furthermore, treatment with RGD-M-LCL led to apoptosis in 37.1% of the cells, which was, significantly higher than the extent of apoptosis seen in the control, matrine and RGD-LCL groups. (A) control group, (B) matrine at 0.03125 mg/mL, (C) RGD-LCL at 0.625 mg/mL, (D) RGD-M-LCL equivalent to these concentration of matrine and RGD-LCL.
Discussion
Liposomes are well-recognized drug delivery vehicles that utilize the fusion of their phospholipid bilayer membrane to the cellular plasma membrane to enhance the delivery and subsequent therapeutic activity of anticancer drugs. However, conventional liposomes are rapidly cleared by the reticular endothelial system in vivo. Furthermore, liposomes interact with plasma proteins, resulting in poor specific targeting and a short circulation time. An efficient drug delivery system is needed that can 1) protect drugs from clearance and degradation to keep sufficient circulation-action time in vivo, 2) suppress nonspecific uptake by normal or non-target tissues, 3) mediate accumulation of drugs within the target tissues and cells (23).
To achieve these three goals, we first prepared LCL modified with PEG to lengthen their circulation time in vivo and improve their passive targeting. Derivatives of PEG provide a hydrophilic surface for the liposomes and reduce their interactions with plasma proteins. Furthermore, this addition increased the molecules' steric hindrance, shielding them from being recognized by the reticular endothelial system (24). Compared with normal tissues, tumor tissues have characteristics such as an enlarged interspace between vascular endothelial cells (about 400~800nm), increased vascular permeability, and reduced lymphatic return, which make liposomes of 200~400nm accumulate within tumor tissues through the EPR effect (25). This accumulation keeps a higher concentration of the drug in the tumor than in other tissues, and increases its duration of action. Second, we modified the surface of the liposomes with the RGD motif to further improve their active tumor targeting through the endocytotic mechanism mediated by specific ligands.
In previous studies, PS Reddy et al (14) inserted an RGD motif into the knob domain of an adenovirus so that a re-target vector used integrin as a cellular receptor to dramatically increase the level of transduction of tumor cells. Similarly, Schmieders et al (26) constructed nanoparticles loaded with siRNA and polyethyleneimine (PEI) that were PEGylated with an RGD peptide ligand as a means to target the tumor neovasculature expressing integrins, and used them to deliver siRNA against vascular endothelial growth factor receptor-2 (VEGF-R2). Besides rapid growth, tumor tissues also undergo extensive neovascularization, and depend on the development of new blood vessels to supply nutrients and growth factors. Integrins are also overexpressed in proliferating endothelial cells, while they are not expressed on quiescent endothelial cells in normal tissues or blood vessels (27), providing a further target for the molecules. Based on these studies, we supposed that a drug delivery system modified with RGD could not only target tumor cells, but could also target the tumor neovasculature through the specific ligand-receptor binding, to achieve improved effects.
In the present study, we prepared LCL with HSPC, cholesterol, DSPE-PEG2000 and DSPE-PEG-MAL, and modified them with an RGD motif. The prepared liposomes were processed by a microfluidizer and stored at 4°C. The liposomes were stable for at least one month at this temperature, with minimal changes in size, although the particle size increased when the liposomes were reconstitutes in solvent.
Because matrine is a strongly hydrophilic and weakly alkaline drug, the encapsulation efficiency of liposomes for loading matrine is very low when prepared by routine methods, such as a thin film dispersal method or a reverse evaporation method. To achieve better encapsulation, a pH-gradient method based on the Henderson-Hasselbalch theory can be used. After a desired pH gradient is obtained by adjusting the pH values between the liposomes and external medium, the weakly acidic or alkaline drugs can cross the phospholipid membrane in molecular form following the transmembrane pH gradient, to become encapsulated within liposomes in ion form (28). We used this method to encapsulate matrine into liposomes, and detected their encapsulation efficiency. Our results indicated that the encapsulation efficiency was negatively related to the drug-lipid ratio in the range of 0.1~0.4. An encapsulation efficiency of 83.13% was obtained when the drug-lipid ratio was 0.1. However, the encapsulation efficiency decreased with the duration of storage due to the release of water-soluble drugs. Lyophilization of the liposomes was thus used to improve their storage stability. Although the size of the liposomes after freeze-drying increased from 100nm to 300nm, their encapsulation efficiency was still maintained at a high level. Our study suggested that for liposomes of water-soluble drugs, the pH-gradient mediated active loading method, and lyophilization of liposomes, might be optimal methods for preparation and preservative, respectively.
In our preliminary validation of the anti-tumor effects of RGD-M-LCL in vitro, we observed that the molecule strongly inhibited the growth of Bcap-37, HT-29 and A375 cells, three cell lines representing different tissues expressing integrin receptors. This activity was significantly stronger than that observed when cells were treated with free matrine. In order to demonstrate that the activity of the molecule was due to the complexed matrine, rather than the RGD-LCL, we treated Bcap-37 cells with RGD-LCL of different concentrations and RGD-M-LCL equivalent to RGD-LCL. We observed that high concentrations of RGD-LCL alone induced cytotoxicity, but that the complex alone had minimal toxicity at lower concentrations, indicating that most of the activity is attributable to the complexed matrine. More importantly, RGD-M-LCL was much more cytotoxic than matrine alone. We presumed that this was because RGD-M-LCL could accumulate more matrine within the target cells, leading to increased anti-cancer activity. In addition, RGD-M-LCL also induced more apoptosis than the equivalent dose of free matrine or RGD-LCL, although the RGD-LCL did also induce apoptosis to a lesser extent. We speculated that this was because the RGD conjugated to the surface of liposomes, just like the exogenous RGD peptide, not only had the desired homing effect to target tumor cells and deliver anti-tumor drugs, but also had direct effects on cell viability and apoptosis (29,30). This will need to be confirmed in further studies, and the mechanism underlying this effect is unclear.which needed further confirmation.
In conclusion, we have successfully prepared RGD-modified long circulating liposome loaded with matrine (RGD-M-LCL), and confirmed that RGD-M-LCL had much stronger anti-cancer effects than free matrine or RGD-LCL alone. This study provides an effective model drug for subsequent research on the pharmacokinetics, in vivo efficacy and targeting mechanism of the RGD-LCL drug-loaded system.
Acknowledgements
This project was supported by the Science and Technology Program of Traditional Chinese Medicine of Zhejiang Province (2007YA015). We greatly appreciate Mr. Qianglin Duan from Tongji Hospital of Tongji University for critical reading of the manuscript.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Sun YN, Xu B, Huang ZQ. et al. Effect of matrine on levels of TGF-β_1, TNF-α, HA and PcIII in sera of chronic hepatitis B patients. Chin J Modern Med. 2004;14:41-5
2. Wu CX, Chen YH, Zheng ZX. Observation on efficacy of matrine with dansheng on liver fibrosis. J Pract Med. 2006;22:1567-8
3. Ma L, Wen S, Zhan Y. et al. Anticancer effects of the Chinese medicine matrine on murine hepatocellular carcinoma cells. Planta Med. 2008;74:245-51
4. Zhang LP, Jiang JK, Tam JW. et al. Effects of matrine on proliferation and differentiation in K-562 cells. Leuk Res. 2001;25:793-800
5. Jiang H, Hou C, Zhang S, et l. Matrine upregulates the cell cycle protein E2F-1 and triggers apoptosis via the mitochondrial pathway in K562 cells. Eur J Pharmacol. 2007;559:98-108
6. Liu XY, Fang H, Yang ZG. et al. Matrine inhibits invasiveness and metastasis of human malignant melanoma cell line A375 in vitro. Int J Dermatol. 2008;47:448-56
7. Gabizon AA, Shmeeda H, Zalipsky S. Pros and cons of the liposome platform in cancer drug targeting. J Liposome Res. 2006;16:175-83
8. Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomed. 2006;1:297-315
9. Mai J, Song S, Rui M. et al. A synthetic peptide mediated active targeting of cisplatin liposomes to Tie2 expressing cells. J Control Release. 2009;139:174-81
10. EIBayoumi TA, Torchilin VP. Tumor-targeted nanomedicines: enhanced antitumor efficacy in vivo of doxorubicin-loaded, long-circulating liposomes modified with cancer-specific monoclonal antibody. Clin Cancer Res. 2009;15:1973-80
11. Lin HY, Lansing L, Merillon JM. et al. Integrin alphavbeta3 contains a receptor site for resveratrol. FASEB J. 2006;20:1742-4
12. Ellis LM. A targeted approach for antiangiogenic therapy of metastatic human colon cancer. Am Surg. 2003;69:3-10
13. Nakamura T, Sato K, Hamada H. Effective gene transfer to human melanomas via integrin-targeted adenoviral vectors. Hum Gene Ther. 2002;13:613-26
14. Reddy PS, Ganesh S, Yu DC. Enhanced gene transfer and oncolysis of head and neck cancer and melanoma cells by fiber chimeric oncolytic adenoviruses. Clin Cancer Res. 2006;12:2869-78
15. Wang Y, Wang X, Zhang Y. et al. RGD-modified polymeric micelles as potential carriers for targeted delivery to integrin-overexpressing tumor vasculature and tumor cells. J Drug Target. 2009;17:459-67
16. Xiong XB, Huang Y, Lu WL. et al. Enhanced intracellular delivery and improved antitumor efficacy of doxorubicin by sterically stabilized liposomes modified with a synthetic RGD mimetic. J Control Release. 2005;107:262-75
17. Huo T, Du X, Zhang S. et al. Gd-EDDA/HYNIC-RGD as an MR molecular probe imaging integrin alphanubeta3 receptor-expressed tumor-MR molecular imaging of angiogenesis. Eur J Radiol. 2010;73:420-7
18. Liu S. Radiolabeled multimeric cyclic RGD peptides as integrin alphavbeta3 targeted radiotracers for tumor imaging. Mol Pharm. 2006;3:472-87
19. Bogdanowich-Knipp SJ, Chakrabarti S, Williams TD. et al. Solution stability of linear vs. cyclic RGD peptides. J Pept Res. 1999;53:530-41
20. Nallamothu R, Wood GC, Pattillo CB. et al. A tumor vasculature targeted liposome delivery system for combretastatin A4: design, characterization, and in vitro evaluation. AAPS Pharm Sci Tech. 2006;7:E32
21. Qiu L, Jing N, Jin Y. Preparation and in vitro evaluation of liposomal chloroquine diphosphate loaded by a transmembrane pH-gradient method. Int J Pharm. 2008;361:56-63
22. Maitani Y, Aso Y, Yamada A. et al. Effect of sugars on storage stability of lyophilized liposome/DNA complexes with high transfection efficiency. Int J Pharm. 2008;356:69-75
23. Yagi N, Manabe I, Tottori T. et al. A nanoparticle system specifically designed to deliver short interfering RNA inhibits tumor growth in vivo. Cancer Res. 2009;69:6531-8
24. Lee CM, Choi Y, Huh EJ. et al. Polyethylene glycol (PEG) modified 99mTc-HMPAO-liposome for improving blood circulation and biodistribution: the effect of the extent of PEGylation. Cancer Biother Radiopharm. 2005;20:620-8
25. Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur J Pharm Biopharm. 2009;71:409-19
26. Schiffelers RM, Ansari A, Xu J. et al. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 2004;32:e149
27. Rhim JH, Tosato G. Targeting the tumor vasculature to improve the efficacy of oncolytic virus therapy. J Natl Cancer Inst. 2007;99:1739-41
28. Haran G, Cohen R, Bar LK. et al. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim Biophy Acta. 1993;1151:201-15
29. Dijkgraaf I, Kruijtzer JA, Frielink C. et al. Synthesis and biological evaluation of potent alphavbeta3-integrin receptor antagonists. Nucl Med Biol. 2006;33:953-61
30. Okrój M, Dobrzańska-Paprocka Z, Rolka K. et al. In vitro and in vivo analyses of the biological activity of RGD peptides towards Ab Bomirski melanoma. Cell Mol Biol Lett. 2003;8:873-84
Author contact
![]() Corresponding author: Li-ming Ruan, The First Affiliated Hospital, College of Medicine, Zhejiang University, #79 Qingchun Road, Hangzhou, Zhejiang 310003, China. Tel: +8613957121201; Email: doc1998net. Mei-hua Sui, Department of Chemical and Biological Engineering, Zhejiang University, Hangzhou, Zhejiang 310027, China. Tel: +8615888847026; email: suimedu.cn
Corresponding author: Li-ming Ruan, The First Affiliated Hospital, College of Medicine, Zhejiang University, #79 Qingchun Road, Hangzhou, Zhejiang 310003, China. Tel: +8613957121201; Email: doc1998net. Mei-hua Sui, Department of Chemical and Biological Engineering, Zhejiang University, Hangzhou, Zhejiang 310027, China. Tel: +8615888847026; email: suimedu.cn

 Global reach, higher impact
Global reach, higher impact