3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2009; 6(6):305-311. doi:10.7150/ijms.6.305 This issue Cite
Research Paper
Replacement of cisplatin with nedaplatin in a definitive 5-fluorouracil/cisplatin-based chemoradiotherapy in Japanese patients with esophageal squamous cell carcinoma
1. School of Pharmacy and Pharmaceutical Sciences, Mukogawa Women's University, Nishinomiya, Japan;
2. Graduate School of Pharmaceutical Sciences, Kyoto University, Kyoto, Japan;
3. Kobe University Graduate School of Medicine, Kobe, Japan;
4. Faculty of Pharmaceutical Sciences, Kyoto Pharmaceutical University, Kyoto, Japan
Received 2009-6-23; Accepted 2009-9-25; Published 2009-9-28
Abstract
Objective: The effects of replacing cisplatin (CDDP) with cis-diammineglycolatoplatinum (nedaplatin, NDP), a second-generation platinum complex, on the pharmacokinetics of 5-fluorouracil (5-FU) were investigated in Japanese patients with esophageal squamous cell carcinoma, who were treated with a definitive 5-FU/CDDP-based chemoradiotherapy.
Methods: Fifty-six patients were enrolled, 49 treated with CDDP and 7 treated with NDP. A course consisted of continuous infusion of 5-FU at 400 mg/m2/day for days 1-5 and 8-12, infusion of CDDP or NDP at 40 mg/m2/day on days 1 and 8, and radiation at 2 Gy/day on days 1 to 5, 8 to 12, and 15 to 19, with a second course repeated after a 2-week interval. Plasma concentrations of 5-FU were determined by high performance liquid chromatography at 5 PM on days 3, 10, 38 and45, and at 5 AM on days 4, 11, 39 and 46.
Results and conclusions: The circadian rhythm in plasma concentrations of 5-FU observed in the case of CDDP was altered when NDP was used instead. The clinical response can be predicted by monitoring plasma concentrations of 5-FU in the CDDP group, but not in the NDP group.
Keywords: nedaplatin, chemoradiotherapy, esophageal squamous cell carcinoma, 5-fluorouracil, plasma concentration
Introduction
A clinical report published in 1999, the RTOG (Radiation Therapy Oncology Group) 85-01 trial involving 134 patients with T1-3, N0-1 and M0 esophageal cancer, is of great interest in terms of clinical outcome because it demonstrated a 5-year survival rate of 26 % [1-4]. This treatment consists of infusion of 5-fluorouracil (5-FU) and cisplatin (CDDP), and concurrent radiation, without pre- or post-surgical resection. Simultaneously in Japan, a modified version was proposed by Ohtsu and his co-workers for advanced metastatic esophageal cancer [5,6]. Two independent clinical investigations have shown curative potential using this regimen for unresectable esophageal squamous cell carcinoma (ESCC) with T4 or M1a [5,6]. A long-term evaluation of efficacy and toxicity with 139 patients resulted in a complete response (CR) rate of 56%, along with a 5-year survival rate of 29% [7-9]. Currently, a definitive 5-FU/CDDP-based chemoradiotherapy (CRT) is recognized as one of the most promising treatments for esophageal cancer [10].
A series of studies has been performed to find a marker predictive of clinical outcome after treatment with a definitive 5-FU/CDDP-based CRT [11-13]. A total of 8 measurements of the plasma concentration of 5-FU were made per patient, and it was concluded that the average value was predictive of clinical response, but not of severe acute leucopenia, stomatitis and cheilitis. Additionally, it has been suggested that clinical response and severe acute toxicities may be predicted on the basis of genetic polymorphisms.
CDDP is one of the antitumor agents most widely used against several types of solid tumors. However, its clinical use is limited by its potent nephrotoxicity, which can lead to acute renal failure. Nedaplatin (NDP), cis-diammineglycolatoplatinum, is a second-generation platinum complex that is approximately 10 times as soluble in water as CDDP [14-16]. As such, NDP is considered to have more pronounced activity against solid tumors, but less nephrotoxicity and gastrointestinal toxicity than CDDP [14]. In phase II clinical studies, NDP was found to be highly effective against solid tumors, including non-small cell lung cancer, small cell lung cancer, head and neck cancer and esophageal cancer [15]. The replacement of CDDP with NDP might be of value for a certain subpopulation of patients. Although little information is yet available, it was recently reported that NDP was comparable to CDDP with regards to clinical response and survival, and also to acute and late toxicity in the treatment of ESCC [16]. In this study, the effects of replacing CDDP with NDP on the pharmacokinetics of 5-FU were investigated in ESCC patients treated with a definitive 5-FU/CDDP-based CRT.
Patients and Methods
Patients
Fifty-six ESCC patients were enrolled in this study based on the following criteria: 1) ESCC treated at Kobe University Hospital from August 2002 to June 2006; 2) clinical stage T1 to T4, N0 or N1, and M0 or M1a according to the International Union Against Cancer tumor node metastasis (TNM) classification; 3) age less than 85 years; 4) an Eastern Cooperative Oncology Group performance status of 0 to 2; 5) adequate bone marrow, marrow, renal, and hepatic function; 6) no prior chemotherapy; 7) no severe medical complications; and 8) no other active malignancies (except early cancer). The tumors were histologically confirmed to be primary, and no patients with recurrence were included in this study.
Protocol
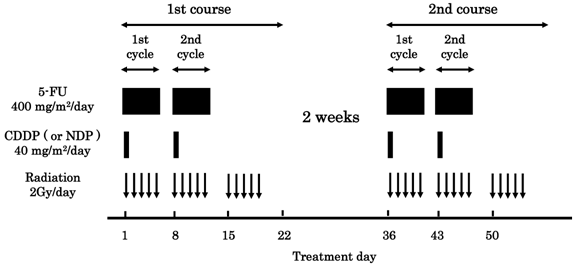
The protocol is presented in Figure 1. A course consisted of continuous infusion of 5-FU at 400 mg/m2/day for days 1-5 and 8-12, infusion of CDDP or NDP at 40 mg/m2/day on days 1 and 8, and radiation at 2 Gy/day on days 1 to 5, 8 to 12, and 15 to 19, with a second course repeated after a 2-week interval [5,6]. If disease progression/recurrence was observed, either salvage surgery, endoscopic treatment, or another regimen of chemotherapy was scheduled. Forty-nine of 56 patients were treated with CDDP (the CDDP group), and the remaining 7 patients were treated with NDP (the NDP group). This study was conducted with the authorization of the institutional review board and followed the medical research council guidelines of Kobe University.
Protocol of a definitive 5-fluorouracil (5-FU)/ cisplatin (CDDP) or nedaplatin (NDP)-based chemoradiotherapy. One course of treatment consisted of protracted venous infusions of 5-FU (400 mg/m2/day for days 1-5 and 8-12) and CDDP (or NDP) (40 mg/m2/day on days 1 and 8), and radiation (2 Gy/day on days 1-5, 8-12, and 15-19), with a second course (days 36-56) was repeated after a 2-week interval.
Pharmacokinetics of 5-FU
Aliquots (5 mL) of blood were collected into etylenediaminetetraacetic acid-treated tubes at 5 PM on days 3, 10, 38 and 45, and at 5 AM on days 4, 11, 39 and 46 [11-13]. The plasma concentration of 5-FU was determined by high-performance liquid chromatography as described previously [11-13]. The apparent elimination half-life of 5-FU is approximately 10 minutes [17], and the plasma concentration will reach a steady-state within a few hours of starting continuous infusion. The systemic exposure to 5-FU during each of 4 cycles was assessed as the area under the concentration time curve for 120 hours (AUC120h), calculated as 120 hours x the average of 2 measurements within a cycle.
Clinical Response
A CR was defined as the complete disappearance of all measurable and assessable disease at the first evaluation, which was performed 1 month after the completion of CRT to determine whether the disease had progressed. The clinical response was evaluated by endoscopy and chest and abdominal computed tomography (CT) scans in each course. A CR at the primary site was evaluated by endoscopic examination when all of the following criteria were satisfied on observation of the entire esophagus: 1) disappearance of the tumor lesion; 2) disappearance of ulceration (slough); and 3) absence of cancer cells in biopsy specimens. If small nodes of 1 cm or less were detected on CT scans, the recovery was defined as an “uncertain CR” after confirmation of no progression for at least 3 months. An “uncertain CR” was included as a CR when calculating the CR rate. When these criteria were not satisfied, a non-CR was assigned. The existence of erosion, a granular protruded lesion, an ulcer scar, and 1.2 w/v% iodine/glycerin-voiding lesions did not prevent an evaluation of CR. The evaluations were performed every month for the first 3 months, and when the criteria for CR were not satisfied at 3 months, the result was changed to non-CR. Follow-up evaluations were performed thereafter every 3 months for 3 years by endoscopy and CT scan. After 3 years, patients were seen every 6 months. During the follow-up period, a routine course of physical examinations and clinical laboratory tests was performed to check the patient's health.
Severe Acute Toxicities
A definitive 5-FU/CDDP-based CRT is associated with acute toxicities, predominantly leucopenia, stomatitis, and cheilitis [5-9,18]. Toxicity was evaluated using criteria defined by the Japan Clinical Oncology Group [19]. These criteria were based on the National Cancer Institute Common Toxicity Criteria. Toxicity was assessed on a 2 to 3 day basis during the CRT and subsequent hospitalization period and on every visit after the completion of CRT. Episodes of leucopenia, stomatitis, and cheilitis during the first 2 courses and subsequent 2 weeks (until day 70) were recorded as acute toxicities and those of grade 3 or more as severe acute toxicities.
Data Analysis and Statistics
All values reported are the mean±standard deviation (SD). The association of disease stage with the rates of CR and severe acute toxicities were analyzed with Fisher's exact test. Circadian variations of plasma concentrations of 5-FU were analyzed with the Wilcoxon signed-rank test. The unpaired Student's t-test/Welch's test or Mann-Whitney's U test was used for two-group comparisons of the plasma concentrations or AUC120h values of 5-FU. P values of less than 0.05 (two tailed) were considered to be significant.
Results
Demographic and clinicopathologic characteristics of 56 ESCC patients are summarized in Table 1. The ratio of T1/T2/T3/T4 was 17/6/21/12, that of N0/N1 was 23/33, and that of M0/M1a was 45/11, resulting in a stage I/II/III/IVa ratio of 13/10/22/11. There was no significant difference between the 2 groups; the CDDP group (N=49) and the NDP group (N=7).
The results of clinical outcome are summarized in Table 2. The overall CR rate was 44.6%, and depended on disease stage; 84.6%, 70.0%, 27.3% and 9.1% for stage I, II, III and IVa, respectively (P<0.05). NDP was comparable to CDDP with respect to clinical response, but the treatment with NDP achieved a CR at stage IVa (data not shown). Episodes of severe acute leucopenia, stomatitis and cheilitis occurred in 42.9%, 12.5% and 14.3% of cases, respectively, and each rate was independent of disease stage (data not shown). Replacement of CDDP with NDP had no effect on the rates of these severe acute toxicities (data not shown).
Demographic and Clinicopathologic Characteristics of 56 Japanese Patients with Esophageal Squamous Cell Carcinoma
| Characteristics | Values |
|---|---|
| Age, yr | 64.3±7.5 (48 -78) |
| Height, cm | 163.1±6.7 (150-180) |
| Weight, kg | 55.9±9.4 (33-79) |
| Sex | Male/Female = 51/5 |
| Race | Japanese |
| Performance status | 0/1/2/unknown = 28/22/4/2 |
| Histological type | squamous cell carcinoma |
| Differentiation | well/moderate/poor/unknown = 8/31/9/8 |
| TNM score | T1/T2/T3/T4 = 17/6/21/12 |
| N0/N1 = 23/33 | |
| M0/M1a = 45/11 | |
| Stage | I/II/III/IVa = 13/10/22/11 |
The values are the mean±SD, with the range in parentheses. TNM score: tumor, node, metastasis. Patients with noncervical primary tumors with positive supraclavicular lymph nodes were defined as M1a.
Clinical Outcome in 56 Japanese Patients with Esophageal Squamous Cell Carcinoma
| N | % | |
|---|---|---|
| Clinical Response | ||
| Complete response (CR) rate | 25 | 44.6 |
| Partial response (PR) rate | 24 | 42.9 |
| Severe Acute Toxicities | ||
| Leucopenia | 24 | 42.9 |
| Stomatitis | 7 | 12.5 |
| Cheilitis | 8 | 14.3 |
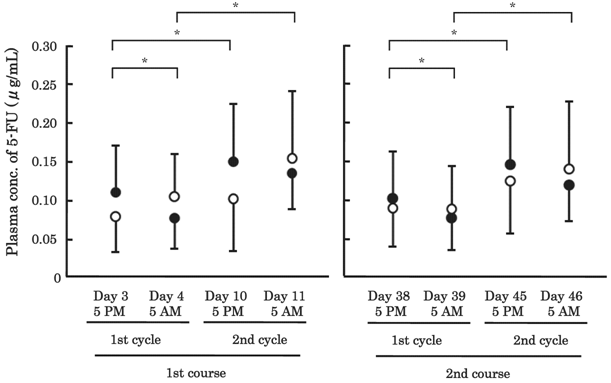
The plasma concentrations of 5-FU are shown in Figure 2. The values of AUC120h are summarized in Table 3. In the 1st cycle/1st course, plasma concentrations of 5-FU were significantly lower at 5 AM (0.076±0.040 μg/mL) than at 5 PM (0.109±0.060 μg/mL) in the CDDP group (P<0.05, β=0.907). A similar tendency was observed in the 2nd cycle/1st course (P=0.134, β=0.390). In the NDP group, however, concentrations tended to be higher at 5 AM than at 5 PM in both the 1st and 2nd cycle/1st course (P=0.249, β=0.106, P=0.463, β=0.138, respectively), whereas the AUC120h value of 5-FU in the CDDP group was almost the same as that in the NDP group in the 1st as well as 2nd cycle/1st course (Table 3). In the 1st course, the plasma concentrations of 5-FU at both 5 PM and 5 AM were significantly higher in the 2nd cycle than the 1st cycle in the CDDP group (P<0.05, β=0.951, P<0.05, β=0.999, respectively). Similarly in the NDP group, the concentration of 5-FU tented to increase in the 2nd cycle, but not significantly (P=0.116, β=0.205, P=0.173, β=0.211, respectively). These phenomena found in the 1st course were also found in the 2nd course, for both groups.
The correlation between the CR rate and the plasma concentration of 5-FU was evaluated, and the results obtained with the average value of 8 measurements are summarized in Table 4. In the CDDP group, the plasma concentrations of 5-FU were significantly higher in the patients with CR than those with non-CR (P<0.05), but the inclusion of 7 patients treated with NDP resulted in no statistically significant difference (P=0.090). The association with severe acute toxicities was also evaluated, and the results on leucopenia are summarized in Table 5. There was no difference in the plasma concentrations of 5-FU between the patients with and without severe acute leucopenia, in either groups. Similarly, the plasma concentrations of 5-FU in the patients with severe acute stomatitis or cheilitis were comparable to those in the patients without (data not shown).
Area Under the Concentration-Time Curve Values (AUC120h, mg*h/L) of 5-Fluorouracil (5-FU) in 56 Japanese Patients with Esophageal Squamous Cell Carcinoma
| CDDP | NDP | |
|---|---|---|
| N=49 | N=7 | |
| 1st cycle / 1st course | 11.1±4.8 | 11.0±4.6 |
| 2nd cycle / 1st course | 16.8±6.4 | 15.3±7.3 |
| 1st cycle / 2nd course | 10.7±5.2 | 10.6±4.4 |
| 2nd cycle / 2nd course | 16.0±5.4 | 15.9±6.8 |
CDDP: cisplatin, NDP: nedaplatin. Systemic exposure to 5-FU was assessed as the AUC120h, calculated as 120 hours x the average of 2 measurements. There was no significant difference between the 2 groups at each of the 4 cycles.
Plasma Concentrations of 5-Fluorouracil (5-FU) in the Patients with and without a Complete Response (CR).
| CR | non-CR | ||||
|---|---|---|---|---|---|
| N | 5-FU, μg/mL | N | 5-FU, μg/mL | P | |
| CDDP | 23 | 0.124±0.035 | 26 | 0.105±0.044 | 0.045 |
| NDP | 2 | 0.078 , 0.117 | 5 | 0.116±0.038 | - |
| total | 25 | 0.122±0.035 | 31 | 0.107±0.043 | 0.090 |
CDDP: cisplatin, NDP: nedaplatin. The average of 8 measurements made per patient is listed as the data. In the CDDP group, plasma concentrations of 5-FU were significantly higher in the patients with CR than those without (non-CR), but the inclusion of 7 patients treated with NDP resulted in no significant differences.
Plasma Concentrations of 5-Fluorouracil (5-FU) in the Patients with and without Severe Acute Leucopenia.
| Severe Acute Leucopenia | No Severe Acute Leucopenia | ||||
|---|---|---|---|---|---|
| N | 5-FU, μg/mL | N | 5-FU, μg/mL | P | |
| CDDP | 21 | 0.116±0.036 | 28 | 0.113±0.033 | 0.785 |
| NDP | 3 | 0.114±0.053 | 4 | 0.109±0.021 | 0.869 |
| total | 24 | 0.115±0.037 | 32 | 0.112±0.031 | 0.746 |
CDDP: cisplatin, NDP: nedaplatin. The average of 8 measurements made per patient is listed as the data. There was no difference between the patients with and without severe acute leucopenia, in either group.
Plasma concentrations of 5-fluorouracil (5-FU) in 56 patients with esophageal cancer. A total of 8 measurements were made per patient: 5 PM on days 3, 10, 38 and45, and 5 AM on days 4, 11, 39 and 46. Closed circle: the cisplatin (CDDP) group (N=49), open circle: the nedaplatin (NDP) group (N=7). The bars represent the SD. * P<0.05; significant differences were observed in the CDDP group, but not in the NDP group.
Discussion
Esophageal cancer is the 8th most common cancer in the world and one of the most lethal [10]. Symptoms include dysphagia, odynophagia, and progressive weight loss. The two predominant histological subtypes are adenocarcinoma and squamous cell carcinoma, and treatment depends on the location of the primary tumor, the disease stage, patient characteristics and co-morbidities, and occasionally, histological subtype. There is no consensus on an optimal treatment strategy for esophageal cancer, and treatments include surgical procedures, radiation, chemotherapy, and combinations thereof [10]. In patients with localized squamous cell carcinoma, a definitive 5-FU/CDDP-based CRT is one of the most promising ways to achieve a complete pathologic response. The treatment might be improved further through modification of the treatment schedule, dose escalation and the replacement of 5-FU and CDDP. Capecitabine or tegafur/uracil might provide better results than 5-FU, and oxaliplatin and NDP are potential substitutes for CDDP.
In this study, we investigated the effects of replacing CDDP with NDP in 56 ESCC patients treated with a definitive 5-FU/CDDP-based CRT, and found no significant differences in clinical outcome, i.e., the CR rate and the severe acute toxicities, in the NDP group, when compared with the CDDP group. Although multi-center, cross-over style clinical investigations should be conducted on the replacement, NDP may be beneficial to ESCC patients, especially those with renal disease. Yamashita et al. [16] also reported that NDP did not differ from CDDP with regards to overall survival, progression-free survival and severe acute leucopenia in the treatment of locally advanced and metastatic esophageal cancer.
Herein, it was clarified that NDP has substantial effects on the pharmacokinetics of 5-FU. It is well-known that there is a circadian rhythm in drug metabolism, cellular proliferation and physiological function, and the suprachiasmatic nuclei, a hypothalamic pacemaker clock, is important for the rhythm [20-22]. As a result, both the toxicity and efficacy of over 30 anticancer agents vary as a function of dosing time [20-22]. More than 80 % of the administered 5-FU is eliminated by the rate-limiting enzyme, dihydropyrimidine dehydrogenase (DPD). The DPD activity is found in most tissues, but is highest in the liver. The activity of DPD of diurnally active cancer patients varies significantly during a 24-hour time period, and is greatest from midnight to early morning [21-24], being consistent with the findings of this study. However, in the NDP group, the pattern of circadian rhythm in 5-FU pharmacokinetics was certainly different from that in the CDDP group, although the AUC120h values were not altered (Table 3). The interaction of DPD with CDDP might be different from that of NDP, but there is no rational explanation for these phenomena. Further clinical and non-clinical investigations should be conducted.
The plasma concentrations of 5-FU were predictive of clinical response, but not of severe acute toxicities, in the CDDP group (Tables 4, 5), however the inclusion of 7 patients treated with NDP affected predictions, presumably because clinical response cannot be predicted on the basis of plasma concentrations of 5-FU in the NDP group. A number of clinical investigations on colorectal cancer and head and neck cancer have revealed that the plasma concentrations of 5-FU were associated with treatment efficacy and toxicity, and the target level of 5-FU concentrations to ensure a certain efficacy was presented [25]. The target level might be proposed also for ESCC, but when using NDP instead of CDDP, it is necessary to look for some marker capable of indicating clinical response.
In conclusion, only a small number of patients were enrolled in this study, especially in the NDP group, and we had no conclusions on the replacement of CDDP with NDP in terms of clinical outcome after the definitive 5-FU/CDDP-based CRT. The circadian rhythm in plasma concentrations of 5-FU observed with CDDP was altered when NDP was used instead, and clinical response can be predicted on the basis of the plasma concentrations of 5-FU in the CDDP group, but not in the NDP group.
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research and Service Innovation Program from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Competing Interest
The authors declare that no conflict of interest exists.
References
1. Cooper JS, Guo MD, Herskovic A. et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623-7
2. Herskovic A, Martz K, Al-Sarraf M. et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Eng J Med. 1992;326:1593-8
3. Begg C, Cho M, Eastwood S. et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276:637-9
4. Al-Sarraf M, Martz K, Herskovic A. et al. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an intergroup study. J Clin Oncol. 1997;15:277-84
5. Ohtsu A, Boku N, Muro K. et al. Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous cell carcinoma of the esophagus. J Clin Oncol. 1999;17:2915-21
6. Kaneko K, Ito H, Konishi K. et al. Definitive chemoradiotherapy for patients with malignant stricture due to T3 or T4 squamous cell carcinoma of the oesophagus. Br J Cancer. 2003;88:18-24
7. Tahara M, Ohtsu A, Hironaka S. et al. Clinical impact of criteria for complete response (CR) of primary site to treatment of esophageal cancer. Jpn J Clin Oncol. 2005;35:316-23
8. Ishikura S, Nihei K, Ohtsu A. et al. Long-term toxicity after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 2003;21:2697-702
9. Kumekawa Y, Kaneko K, Ito H. et al. Late toxicity in complete response cases after definitive chemoradiotherapy for esophageal squamous cell carcinoma. J Gastroenterol. 2006;41:425-32
10. Sakaeda T, Yamamori M, Kuwahara A. et al. Pharmacokinetics and pharmacogenomics in esophageal cancer chemoradiotherapy. Adv Drug Deliv Rev. 2009;61:388-01
11. Miki I, Tamura T, Nakamura T. et al. Circadian variability of pharmacokinetics of 5-fluorouracil and CLOCK T3111C genetic polymorphism in patients with esophageal carcinoma. Ther Drug Monit. 2005;27:369-74
12. Okuno T, Tamura T, Yamamori M. et al. Favorable genetic polymorphisms predictive of clinical outcome of chemoradiotherapy for stage II/III esophageal squamous cell carcinoma in Japanese. Am J Clin Oncol. 2007;30:252-7
13. Sakaeda T, Yamamori M, Kuwahara A. et al. VEGF G-1154A is predictive of severe acute toxicities during chemoradiotherapy for esophageal squamous cell carcinoma in Japanese patients. Ther Drug Monit. 2008;30:497-503
14. Kato H, Fukuchi M, Manda R. et al. Efficacy and toxicity of nedaplatin and 5-FU with radiation treatment for advanced esophageal carcinomas. Anticancer Res. 2003;23:3493-8
15. Yamada H, Maki H, Takeda Y. et al. Evaluation of combined nedaplatin and docetaxel therapy for human head and neck cancer in vivo. Anticancer Res. 2006;26:989-94
16. Yamashita H, Nakagawa K, Tago M. et al. Radiation therapy combined with cis-diammine-glycolatoplatinum (nedaplatin) and 5-fluorouracil for Japanese stage II-IV esophageal cancer compared with cisplatin plus 5-fluorouracil regimen: a retrospective study. Dis Esophagus. 2006;19:15-9
17. Heggie GD, Sommadossi JP, Cross DS. et al. Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile. Cancer Res. 1987;47:2203-6
18. Hironaka S, Ohtsu A, Boku N. et al. Nonrandomized comparison between definitive chemoradiotherapy and radical surgery in patients with T(2-3) N(any) M(0) squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys. 2003;57:425-33
19. Tobinai K, Kohno A, Shimada Y. et al. Toxicity grading criteria of the Japan Clinical Oncology Group (The Clinical Trial Review Committee of the Japan Clinical Oncology Group). Jpn J Clin Oncol. 1993;23:250-7
20. Milano G, Chamorey AL. Clinical pharmacokinetics of 5-fluorouracil with consideration of chronopharmacokinetics. Chronobiol Int. 2002;19:177-89
21. Lévi F, Focan C, Karaboué A. et al. Implications of circadian clocks for the rhythmic delivery of cancer therapeutics. Adv Drug Deliv Rev. 2007;59:1015-35
22. Altinok A, Lévi F, Goldbeter A. Identifying mechanisms of chronotolerance and chronoefficacy for the anticancer drugs 5-fluorouracil and oxaliplatin by computational modeling. Eur J Pharm Sci. 2009;36:20-38
23. Harris BE, Song R, Soong SJ. et al. Relationship between dihydropyrimidine dehydrogenase activity and plasma 5-fluorouracil levels with evidence for circadian variation of enzyme activity and plasma drug levels in cancer patients receiving 5-fluorouracil by protracted continuous infusion. Cancer Res. 1990;50:197-201
24. Zeng ZL, Sun J, Guo L. et al. Circadian rhythm in dihydropyrimidine dehydrogenase activity and reduced glutathione content in peripheral blood of nasopharyngeal carcinoma patients. Chronobiol Int. 2005;22:741-54
25. Highlights from; 5-fluorouracil drug management pharmacokinetics and pharmacogenomics workshop; Orlando, Florida; January 2007. Clin Colorectal Cancer. 2007;6:407-22
Author contact
![]() Correspondence to: Toshiyuki Sakaeda, Ph.D., Center for Integrative Education of Pharmacy Frontier (Frontier Education Center), Graduate School of Pharmaceutical Sciences, Kyoto University 46-29 Yoshidashimoadachi-cho, Sakyo-ku, Kyoto 606-8501, Japan. Tel: +81-75-753-9560, Fax: +81-75-753-4502, E-Mail: sakaedatkyoto-u.ac.jp
Correspondence to: Toshiyuki Sakaeda, Ph.D., Center for Integrative Education of Pharmacy Frontier (Frontier Education Center), Graduate School of Pharmaceutical Sciences, Kyoto University 46-29 Yoshidashimoadachi-cho, Sakyo-ku, Kyoto 606-8501, Japan. Tel: +81-75-753-9560, Fax: +81-75-753-4502, E-Mail: sakaedatkyoto-u.ac.jp

 Global reach, higher impact
Global reach, higher impact