Impact Factor
ISSN: 1449-1907
Int J Med Sci 2009; 6(4):168-176. doi:10.7150/ijms.6.168 This issue Cite
Research Paper
Elevated Serum Levels of Arachidonoyl-lysophosphatidic Acid and Sphingosine 1-Phosphate in Systemic Sclerosis
1. Department of Health Chemistry, Institute of Health Bioscience, The University of Tokushima Graduate School, 1-78-1 Shomachi, Tokushima 770-8505, Japan;
2. Veterans Affairs Medical Center, Memphis, Tennessee, USA;
3. Division of Rheumatology, Department of Medicine, University of Tennessee Health Science Center, Memphis, Tennessee, USA;
4. Department of Physiology, University of Tennessee Health Science Center, 894 Union Ave., Memphis, Tennessee, USA
Received 2009-4-7; Accepted 2009-6-2; Published 2009-6-5
Abstract
Systemic sclerosis (SSc) is an often fatal disease characterized by autoimmunity and inflammation, leading to widespread vasculopathy and fibrosis. Lysophosphatidic acid (LPA), a bioactive phospholipid in serum, is generated from lysophospholipids secreted from activated platelets in part by the action of lysophospholipase D (lysoPLD). Sphingosine 1-phosphate (S1P), a member of the bioactive lysophospholipid family, is also released from activated platelets. Because activated platelets are a hallmark of SSc, we wanted to determine whether subjects with SSc have altered serum lysophospholipid levels or lysoPLD activity. Lysophospholipid levels were measured using mass spectrometric analysis. LysoPLD activity was determined by quantifying choline released from exogenous lysophosphatidylcholine (LPC). The major results were that serum levels of arachidonoyl (20:4)-LPA and S1P were significantly higher in SSc subjects versus controls. Furthermore, serum LPA:LPC ratios of two different polyunsaturated phospholipid molecular species, and also the ratio of all species combined, were significantly higher in SSc subjects versus controls. No significant differences were found between other lysophospholipid levels or lysoPLD activities. Elevated 20:4 LPA, S1P levels and polyunsaturated LPA:LPC ratios may be markers for and/or play a significant role in the etiology of SSc and may be future pharmacological targets for SSc treatment.
Keywords: scleroderma, lysophospholipids, LPA, S1P, LPC, lysophospholipase D, fibrosis
Introduction
Systemic sclerosis (SSc) is an often fatal disease characterized by autoimmunity and inflammation, leading to widespread vasculopathy and fibrosis of multiple organs (1). There are two distinct subsets of the disease, limited and diffuse, which are based on the extent of skin involvement. These subsets may have different clinical and laboratory findings (2). However, one of the hallmarks of the vasculopathy associated with both subsets of SSc is platelet activation, which has been implicated as a key mediator of the fibrosis that underlies SSc (3).
Phospholipid growth factors (PLGFs) are a family of lipids with growth factor-like properties. Lysophosphatidic acid (LPA) is a member of the PLGF family. LPA targets cells through at least six well-characterized extracellular receptors and one nuclear receptor (the PPARγ receptor) (4, 5). LPA is found at physiologically and pathophysiologically significant concentrations in plasma and serum, respectively. LPA is typically produced by the action of plasma lysophospholipase D (lysoPLD), also known as autotaxin, on lysophosphatidylcholine (LPC) (6). In serum, activated platelets are one of the primary sources of LPA, which is produced via the action of lysoPLD on LPC and other lysophospholipids (7). LPA has been found to stimulate cell division and migration and to inhibit apoptosis (8). We have previously determined that LPA can stimulate lung fibroblast to myofibroblast differentiation (9). A similar differentiation step is critical to the pathogenesis of SSc, in that myofibroblasts are one of the primary sources of SSc fibrotic tissue (10, 11). Rho-associated kinases (ROCK), which are activated by LPA, have recently been found to stimulate myofibroblast differentiation from cultured human skin SSc fibroblasts. In addition, ROCK stimulated the production of extracellular matrix by these cells (12).
Sphingosine 1-phosphate (S1P), another major member of the PLGF family, acts as an agonist to membrane receptors that have close evolutionary links to several of the LPA receptors. S1P has been shown to have significant effects on lymphocytes. Both B- and T-cells require S1P activity to allow their movement out of lymphoid organs, and thymocytes require S1P receptor activation for release from the thymus (13). Although there is currently no known association between S1P and SSc, SSc has an autoimmune component with reported lymphocyte involvement that suggests there may be a role for S1P in the development and/or progression of SSc.
In view of the high level of platelet activation seen in SSc (14), along with the increased myofibroblast activity and impaired myofibroblast apoptosis, we hypothesized that elevated PLGFs play a role in the pathogenesis of SSc, and specifically that LPA would be elevated in SSc subjects relative to healthy controls. Therefore, we designed this study to determine whether differences exist in serum LPA concentrations and in lysoPLD activities of SSc and control subjects. We chose to look at a range of different LPA species because of the likelihood that we would find differences in either a particular species or class of LPA (15). In addition to LPA measurement, we also measured and compared S1P concentrations in the blood of SSc versus control subjects because of the likely autoimmune pathogenesis of SSc and the link between S1P and immune cell function. Because of significant differences that are present between limited and diffuse SSc, we also examined the differences in lipid products and lysoPLD activity between subjects with these two subgroups of the disease. Finally, we measured in SSc and control subjects the concentrations of additional bioactive lysophospholipids, including lysophosphatidylethanolamine (LPE), lysophosphatidylinositol (LPI) and lysophosphatidylserine (LPS), which are precursors of LPA.
Subjects and Methods
Ethics Statement. Written consent for participation in the study was obtained from all participants in accordance with the Helsinki II declaration, and the protocol was approved by the UTHSC Institutional Review Board.
Subjects. SSc subjects with a history of limited or diffuse SSc were recruited from the Rheumatology Clinic of the University of Tennessee Health Science Center (UTHSC). All SSc subjects met the 1980 ACR classification criteria for clinical diagnosis of limited or diffuse SSc (16). Healthy controls were recruited from UTHSC staff. Those with a history of an organ or stem cell transplant, those who had used prednisone, cyclophosphamide, D-penicillamine, cyclosporine A, methotrexate, azathioprine, or other immune modulator therapies within one month of study start, and those who were less than 18 years old were excluded from the study.
Preparation of serum samples. After an overnight fast, blood was collected from 10 patients with SSc (7 with diffuse disease and 3 with limited disease) and from 13 healthy controls. Serum was drawn off after the collected blood had been left 1 h at room temperature and then spun at 2500 rpm for 15 min. Samples were stored at -80 oC until overnight shipping (on dry ice) to Japan for biochemical analysis. Analyses were performed in a blinded fashion. The laboratory performing the analyses was provided the sample key only after the analyses were complete and reported.
Lipid and lysoPLD measurements. Phospholipids. 1-heptadecanoyl (17:0)-LPC and C17-S1P were purchased from Avanti Polar Lipids (Alabaster, AL, USA). 17:0-LPA was prepared from 17:0-LPC by the action of phospholipase D from Streptomyces chromofuscus, as described previously (17). 17:0-LPE was prepared from 17:0-LPC and ethanolamine hydrochloride by a transphosphatidylation reaction with phospholipase D from Actinomadura sp. (Seikagaku Kogyo; Tokyo, Japan) according to the method for preparation of phosphatidylglycerol from phosphatidylcholine (18). In brief, a mixture of 0.1 ml of 5 M ethanolamine hydrochloride/0.2 M acetate buffer (pH 5.5) containing 0.1 mmol 17:0-LPC, 0.05 ml of 0.22 M CaCl2/0.02 M NaCl, and 0.1 ml of phospholipase D solution (8 mg/ml) in 0.2 M acetate buffer was incubated while being stirred for 2 h at 30oC. After the reaction was stopped by addition of 2 ml chloroform/methanol mixture (1:1, v/v), the mixture was centrifuged at 1300 x g for 10 min. Lipids recovered into the organic layer were separated by thin-layer chromatography on a silica gel plate (Silica gel G60; Merck; Darmstadt, Germany) developed with chloroform/methanol/20% ammonia (60:35:8, v/v). Lipid bands were detected by spraying of primuline under UV light. 17:0-LPE was extracted from silica in the corresponding lipid band by the method of Bligh and Dyer (19). Purities of 17:0-LPA and 17:0-LPE recovered from the silica were checked by liquid chromatography-tandem mass spectrometry (LC-MS-MS) as described below.
Lipid extraction. Lipids were extracted from human serum samples by the modified method of Bligh and Dyer (19) after adjusting the aqueous phase pH to 9-9.5 with 20% ammonium hydroxide. To the lipid extract were added 5 nmol 17:0-LPC, 0.05 nmol 17:0-LPE, 0.1 nmol 17:0-LPA, and 0.2 nmol C17 S1P. Most of the lipids, including LPC and LPE, were extracted into the organic layer. The lipid extract was dried under a stream of nitrogen gas, reconstituted with 0.5 ml of methanol/water mixture (1:1, v/v) containing 5 mM ammonium formate, and termed the neutral lipid fraction. The remaining aqueous layer was acidified to pH 2-2.5 with 1 N hydrochloric acid, and acidic lipids such as LPA, LPI and LPS and S1P were extracted into the organic layer by the method of Bligh and Dyer (19). The second lipid extract (acidic lysophospholipid fraction) was dried down under a stream of nitrogen gas and dissolved in 0.1 ml of acetonitrile/isopropanol/methanol/water (1:1:1:1, v/v) mixture containing 0.2% formic acid for LC-MS-MS.
LC-MS-MS of lysophospholipids. LC-MS-MS was performed on a quadrupole-linear iontrap hybrid MS, 4000 QTRAPTM (Applied Biosystems/MDS Sciex; Concord, ON, Canada), with an Agilent 1100 LC system combined with an autosampler (Agilent Technologies; Wilmington, DE, USA). Separation of neutral lipid fractions by LC was achieved using an Agilent ZORBAX Eclipse XDB-C18 column (50 mm x 1 mm; 3.5-μm particle size silica). The composition of the mobile phase was methanol/water (4:1, v/v) containing 5 mM ammonium formate, which was pumped at a flow rate of 0.1 ml/min for an isocratic elution. Separation of acidic lysophospholipids by LC was performed with a Tosoh TSK-ODS-100Z column (150 mm x 2 mm; silica with 5-μm particle size) developed with methanol/water (19:1, v/v) containing 5 mM ammonium formate at a flow rate of 0.22 ml/min in an isocratic elution mode. Routinely, 5 μl aliquots of test solutions were applied to the mass spectrometer for analysis. Lysophospholipids were analyzed by multiple reaction monitoring (MRM) in a positive ion mode for LPC and LPE or in a negative ion mode for LPA, LPI, LPS, and S1P. In the positive ion MRM, Q1 and Q3 were set for the protonated molecular ion and [phosphorylcholine]+ at m/z 184 for LPC or [M - 141]+ for LPE. In the negative ion MRM, Q3 was set to [cyclic glycerol phosphate]- at m/z 153 for LPA and LPS, [phosphorylinositol - H2O]- at m/z 241 for LPI or [PO3]- at m/z 79 for S1P, in combination with the deprotonated molecular ion as Q1 for all lysophospholipids tested. Amounts of the different molecular species of LPC and LPE were calculated from the ratios of their areas of positive ions to those of 17:0-LPC or LPE internal standards. Similarly, the amounts of molecular species of LPA were calculated from the ratios of their peak areas of negative ions to that of 17:0-LPA, an internal standard. The amounts of molecular species of LPS and LPI were calculated from both their peak areas of negative ions to that of 17:0-LPA and the correction factors for LPI and LPS. These correction factors were determined to be 10.5 and 6.4, respectively, based on both the extraction efficiencies of 16:0- and 18:1-LPS and bovine liver LPI, and the relative ion efficiency for LPI and LPS against 17:0-LPA by MRM under our conditions.
Measurement of lysoPLD activity. LysoPLD activity in serum was measured by the enzyme-linked fluorometric method for determination of choline produced together with LPA from exogenously added 0.15 mM 18:2-LPC. In our assay, performed on a 96-well microplate, 0.05 ml of 3.3-fold diluted serum was mixed with 0.025 ml of saline and 0.025 ml of LPC solution at 0.6 mM in saline containing 0.25% bovine serum albumin. The mixtures in the wells were incubated at 37 oC for 9 or 24 h, and were then mixed with 0.2 ml of assay buffer. The assay buffer was composed of 0.1 ml of Tris-HCl buffer (pH 8.5), 0.04 ml of 7.5 mM 3-(4-hydroxyphenyl)propionic acid, 0.02 ml of 25 U/ml choline oxidase and 0.04 ml of 2 U/ml horseradish peroxidase. With the aid of a standard line obtained with 0, 0.1, 0.3, 1, 3, 10, and 30 nmol/ml of water solution of choline chloride, the choline concentration was determined from the intensity of fluorescence measured at 320 nm (excitation) and 404 nm (emission), and lysoPLD activity was calculated as nmol choline/ml of serum. Alternatively, serum lysoPLD activity of subjects with diffuse SSc and healthy controls was measured by quantifying LPA production using LC-MS-MS as described above.
Statistical analysis. Results are expressed as means ± S.E. Analyses were performed using the Kruskal-Wallis test using Excel Statcel software. All analyses were prespecified. P < 0.05 was considered statistically significant.
Results
Ten female SSc subjects and 14 female age- and race-matched healthy controls were included in this study. Among the SSc subjects, seven had diffuse disease and three had limited disease. The mean duration of disease in the SSc subjects was 10.3 years, with a range of 3-28 years. The mean age of the SSc subjects was 47 years. The mean age of the controls was 46 years. Table 1 shows the characteristics of the study subjects.
Characteristics of individual study subjects
| Gender | Race | Age at Collection | Group | Disease Type | Disease Duration (yrs) |
|---|---|---|---|---|---|
| F | B | 57 | CON | ||
| F | W | 53 | CON | ||
| F | W | 55 | CON | ||
| F | B | 58 | CON | ||
| F | W | 23 | CON | ||
| F | W | 30 | CON | ||
| F | W | 57 | CON | ||
| F | W | 52 | CON | ||
| F | W | 56 | CON | ||
| F | W | 55 | CON | ||
| F | W | 49 | CON | ||
| F | B | 38 | CON | ||
| F | As | 27 | CON | ||
| F | W | 50 | SSC | Diffuse | 3 |
| F | W | 50 | SSC | Diffuse | 4 |
| F | B | 64 | SSC | Diffuse | 9 |
| F | W | 52 | SSC | Diffuse | 7 |
| F | W | 49 | SSC | Diffuse | 3 |
| F | B | 36 | SSC | Diffuse | 28 |
| F | W | 21 | SSC | Diffuse | 11 |
| F | W | 57 | SSC | Limited | 19 |
| F | W | 48 | SSC | Limited | 10 |
| F | W | 44 | SSC | Limited | 9 |
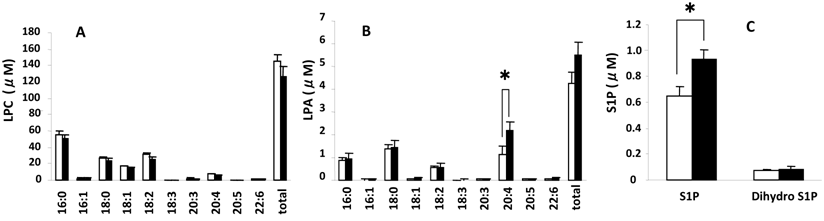
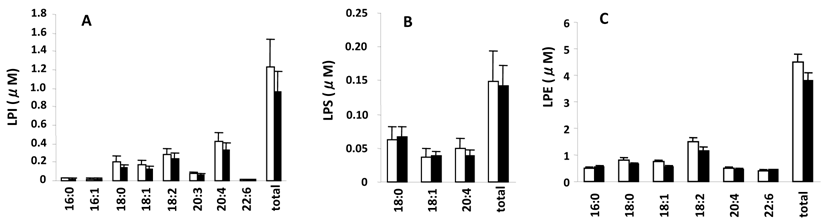
Measured serum concentrations of LPC, LPA, S1P, and dihydrosphingosine 1-phosphate (dihydro S1P), which is identical to S1P except that it lacks one double bond and acts as an agonist for all S1P receptors but with a 20-fold lower affinity for S1P2 (20, 21), from the control and SSc groups are summarized in Fig. 1. LPC (Fig. 1A), a precursor of LPA, had the highest concentrations (16:0>18:2>18:0>18:1 and 5 minor species) followed by LPA (Fig. 1 B) (18:2>20:4>16:0=18:1 and 5 minor species) and then S1P (Fig. 1C) (S1P>dihydro S1P). Figure 2 shows serum concentrations of LPE, LPI, and LPS from control and SSc subjects. Of these lysophospholipids, LPE had the highest concentrations (18:2>18:1>16:0=20:4 and 3 minor species), which were less than the concentrations of LPA, followed by LPI (20:4>18:2>18:0>18:1 and 5 minor species) and then LPS (18:0>18:1>20:4). Thus, the most predominant precursor for LPA produced by serum lysoPLD would be LPC. It is noteworthy that the percentages of saturated molecular species of LPC were higher than those of LPA (Fig. 1A and B).
The serum level of 20:4-LPA was significantly higher in the SSc group (Fig. 1B) versus the control group as well as in the limited SSc group versus control (2.54 ± 0.15 nmol/ml vs. 1.15 ± 0.37 nmol/ml for control, P= 0.026), with no differences measured between controls and the diffuse SSc group (1.99 ± 0.49 nmol/ml, P = 0.088). In addition, the S1P levels were significantly higher in SSc vs. control groups (Fig. 1C), and also in the diffuse SSc group versus controls (data not shown). Essentially no differences were observed for serum levels of LPC, LPE, LPS, and LPI, as shown in Figs. 1A and 2A, B, and C, respectively.
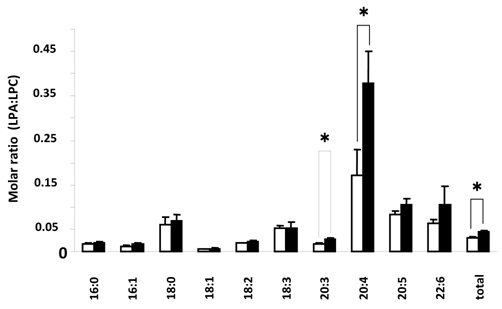
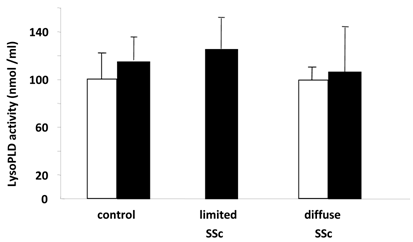
Figure 3 shows LPA:LPC ratios for the different species of these lysophospholipids. These ratios in part can show the conversion status of LPC to LPA for the individual species and for the combined total of species, with values closer to 1 indicating more conversion of LPC to LPA. Here we found a significant difference in the total LPC/total LPA ratio between control and SSc (P < 0.01) groups. There was also a significant difference between control and diffuse SSc groups (P < 0.02, data not shown). In addition, the values for 20:4 ratios were significantly different between control and SSc groups (P < 0.01), control and limited SSc (P < 0.02, data not shown) groups, and control and diffuse SSc (P < 0.05, data not shown) groups. In addition, there was a significant difference in the 20:3 ratio between control and SSc groups (P < 0.05) and control and limited SSc groups (P < 0.05, data not shown). No significant differences were found between groups in the lysoPLD activity toward 150 μM 18:2-LPC (Fig. 4). There were also no significant differences between total and individual levels of LPA produced during 12 h incubation of sera from SSc patients and control subjects (Fig. 4).
Serum levels of different molecular species of LPC, LPA, S1P and dihydro S1P. Open and closed bars represent the mean values +/- SE for 13 control and 10 SSc subjects, respectively. * P<0.05
Serum levels of different molecular species of LPI, LPS, and LPE. Open and closed bars represent the mean values +/- SE for 13 control and 10 SSc subjects, respectively.
Comparison of serum LPA:LPC ratio in control versus SSc subjects. Open and closed bars represent mean values +/- SE for 13 control and 10 SSc subjects, respectively. * P<0.05
Serum lysoPLD activity in human control and SSc subjects. LysoPLD activity in serum from subjects with limited SSc (n=3), diffuse SSc (n=7) and healthy controls (n=11) was measured by quantifying choline release (closed bars). Alternatively, LysoPLD activity in serum of subjects with diffuse SSc (n=4) and healthy controls (n=4) was measured by quantifying LPA production (open bars). No significant differences were found between any groups.
Discussion
For this study, we chose to measure not only total LPA levels in SSc versus control subjects, but also to measure several different specific saturated and unsaturated LPA species. It is now known that the different species of LPA have variable specificities to LPA receptors as well as differing potencies to cause cell specific cell responses (22-25). In addition, LPA agonists and antagonists are being modeled after different LPA species (26-28).
The major findings of this study were significantly elevated concentrations of 20:4 LPA and S1P in the serum of SSc subjects versus controls. The total serum LPA:LPC ratio, and within this, the subgroups of 20:3 and 20:4 LPA:LPC ratios, were also elevated in the SSc subjects versus controls. No significant differences in other lysophospholipid mediators, including LPE, LPI, and LPS, were found between control and SSc subjects. In addition, there were no differences observed in lysoPLD enzymatic activity between the SSc subjects and controls.
The serum level of LPA appears to be controlled by a balance between LPA-producing and LPA-degrading enzymatic activities. We postulate that the elevated LPA and LPA:LPC ratios found in SSc subjects may be linked to reduced activity of a postulated LPA-degrading lysophospholipase in the serum of SSc subjects that would preferentially hydrolyze highly polyunsaturated LPAs, such as the 20:4 species, over other LPA species. Increased LPA synthesis is likely not responsible for these elevated values because we found no increase in lysoPLD activity in any of the SSc groups. Our findings of significantly higher LPA:LPC ratios in the polyunsaturated species over saturated and monounsaturated species in serum samples from SSc patients and control subjects is consistent with a previous finding that sn-1-lyso-LPC, formed via hydrolysis of phosphatidylcholine by phospholipase A1, is a superior substrate for lysoPLD in human serum and plasma as compared to sn-2-lyso-LPC, which is generated by hydrolysis of phosphatidylcholine by phospholipase A2 (29). It will be interesting to measure PLA1 vs. PLA2 activity in control and diffuse SSc subjects once the molecular identity of PLA1 is determined.
Among the many different LPA subtypes measured in this study, 20:4 LPA was consistently elevated in the SSc subjects (both limited and diffuse) versus control subjects. 20:4 LPA has been used in a number of studies as an example of an sn-1 unsaturated LPA. Overall, 20:4 has been shown to have broad specificity for the different LPA receptors (23). In regard to SSc, 20:4 LPA has been found to have a high platelet-activating potency (24, 25). While activated platelets are a common feature of SSc, and activated platelets are known to release LPA, the presence of elevated 20:4 LPA could act in a positive feedback manner to perpetuate platelet activation. From an immune standpoint, unsaturated LPAs, with 20:4 LPA being one of the species tested, were found to be chemotactic to immature (but not mature) dendritic cells. This response was mediated at least in part through the LPA3 receptor (30). This is potentially important, given recent evidence for altered antigen processing in SSc (31).
When stratifying by SSc disease types, the total serum LPA:LPC ratio and serum 20:4 LPA:LPC ratio were significantly elevated in the diffuse SSc subjects versus controls. Although we had a small sample size of subjects with limited SSc, we found that serum 20:4 LPA and the 20:3 and 20:4 LPA:LPC ratios were significantly higher in the limited SSc group versus control subjects. In addition, elevated serum S1P concentrations approached significance in diffuse SSc and limited SSc subjects versus controls (P = 0.05 and 0.09, respectively). These LPA:LPC ratio and S1P patterns all follow the general pattern of these parameters being higher in SSc subjects versus controls.
Platelets are known to play a pathological role as a main source of circulating S1P by accumulating and releasing S1P after local blood coagulation, whereas erythrocytes are considered to be the main source of plasma S1P (32). It is quite likely that the elevated level of S1P in sera from SSc patients is due to the activation of platelets during blood coagulation in vitro. The biological/clinical significance of our findings relates to both the immune etiology and fibrotic disposition of SSc. In regard to the immune system, the elevated S1P concentrations found in SSc subjects may relate to the lymphocyte involvement in the autoimmune genesis of SSc. S1P is required for lymphocyte egress from lymphoid organs; in addition, thymocytes require S1P receptor activation to be released from the thymus (13). Therefore, elevated S1P may act to increase the pool of immune cells available for autoimmune responses. Interestingly, an experimental S1P agonist, FTY720, is being tested as a possible pharmacological agent to treat immune rejection following transplants and autoimmune diseases (33). Although it acts as an S1P receptor agonist, FTY720 actually leads to S1P receptor internalization, leaving the receptors unavailable for binding with S1P and thus antagonizing the effects of S1P. The result is the trapping of lymphocytes within lymph nodes and Peyer's patches and a marked reduction of lymphocytes in the circulation (34). Thus, it is theoretically possible that treatment with FTY720 or other agents directed at S1P might have effects in SSc.
Elevated LPA and LPA/LPC ratios may be involved in the fibrotic component of SSc. A recent study found that elevated plasma LPA levels and higher serum lysoPLD activity were associated with hepatitis C and its associated liver fibrosis (35). Serum LPA was not examined. The authors of this study postulate that elevated LPA contributed to the hepatic fibrosis through its proliferative and anti-apoptotic effect on hepatic stellate cells. In a second recent study linking LPA to a fibrotic pathology, LPA was found to be elevated in the bronchoalveolar lavage fluid of patients with idiopathic pulmonary fibrosis, and inhibition of the LPA1 receptor reduced the chemotactic activity of this fluid towards fibroblasts (36). The authors speculate that increased vascular leakage associated with LPA1 receptor activation may lead to increased fibrin deposition and a corresponding fibrotic cascade in injured airspaces. They also note that S1P reduces vascular permeability (37). It is possible that elevations in LPA and/or S1P concentrations may have altered vascular permeability in our SSc subjects, although this awaits further study.
Our previous work indicates that LPA-induced myofibroblast differentiation may be involved in SSc fibrotic activity and possibly pathological fibrosis in general. Myofibroblasts are cells with smooth muscle characteristics (they contain a smooth muscle actin and are contractile) and are typically derived from fibroblasts or epithelial cells. In SSc, they also appear to be derived from circulating blood-derived mononuclear cells (38). Myofibroblasts are widely accepted to be major contributors to tissue and organ fibrosis (39-41). Not only did we demonstrate that LPA could trigger fibroblast to myofibroblast differentiation (9), but we have also described an LPA-activated Cl- current (IClLPA) that is active in myofibroblasts but not myofibroblast precursors. We have observed IClLPA in myofibroblasts from the lung (9) and cornea (42, 43) and from the skin, heart, and liver (unpublished data). We have demonstrated that blocking IClLPA activity can prevent fibroblast to myofibroblast differentiation (9).
In conclusion, we measured 10 different LPC and LPA species (2 saturated and 8 unsaturated) along with S1P and lysoPLD activity in the serum of control subjects and subjects with confirmed SSc. We demonstrated significantly increased serum 20:4 LPA and S1P concentrations in SSc subjects versus controls, along with elevated LPA:LPC ratios of two different unsaturated (but not saturated) phospholipid species, as well as the ratio of all species combined (total). We also determined that there was no difference in the enzymatic activity of lysoPLD, which generates LPA from LPC, between control and SSc subjects. Our results are potentially of real clinical significance in a disease for which there remains no cure and no directed therapies. Although we can control some manifestations of the disease, no pharmacological therapies are currently available that alter the underlying systemic fibrosis that is the hallmark of SSc. Our study suggests that directed pharmacological inhibition of LPA and S1P receptors (novel LPA and S1P receptor agonists and antagonists are currently under development) (34, 44) may represent an innovative pathway for targeting the fibrosis and autoimmunity in SSc.
Limitations
The major limitation of this study is the relative rarity of the disease and subsequent difficulty in recruiting large numbers of SSc subjects, especially with both diffuse and limited disease, making it difficult to adequately power these comparisons. Despite this limitation, we were able to see significant results between the groups tested, indicating what is likely clinically significant differences in the levels of these bioactive lipids and the LPA:LPC ratios between control and SSc subjects.
Acknowledgements
The authors would like to thank Dr. Gabor Tigyi for his helpful suggestions. This work was funded by the Scleroderma Foundation (MW), and by a grant-in-aid from the Ministry of Education, Culture, Sports, Sciences and Technology of Japan (AT).
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. The Journal of Clinical Investigation. 2007;117:557-67
2. Wollheim F. Classification of systemic sclerosis. Visions and reality. Rheumatology (Oxford). 2005;44:1212-6
3. Kowal-Bielecka O, Kowal K, Lewszuk A, Bodzenta-Lukaszyk A, Walecki J, Sierakowski S. Beta thromboglobulin and platelet factor 4 in bronchoalveolar lavage fluid of patients with systemic sclerosis. Ann Rheum Dis. 2005;64:484-6
4. Meyer zu Heringdorf D, Jakobs KH. Lysophospholipid receptors: signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim Biophys Acta. 2007;1768:923-40
5. Tabata K, Baba K, Shiraishi A, Ito M, Fujita N. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochemical and Biophysical Research Communications. 2007;363:861-6
6. Tokumura A, Harada K, Fukuzawa K, Tsukatani H. Involvement of lysophospholipase D in the production of lysophosphatidic acid in rat plasma. Biochim Biophys Acta. 1986;875:31-8
7. Aoki J. Mechanisms of lysophosphatidic acid production. Semin Cell Dev Biol. 2004;15:477-89
8. Birgbauer E, Chun J. New developments in the biological functions of lysophospholipids. Cell Mol Life Sci. 2006;63:2695-701
9. Yin Z, Watsky MA. An LPA-activated Cl- current in lung myofibroblasts. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1110-6
10. Jelaska A, Arakawa M, Broketa G, Korn JH. Heterogeneity of collagen synthesis in normal and systemic sclerosis skin fibroblasts. Increased proportion of high collagen-producing cells in systemic sclerosis fibroblasts. Arthritis Rheum. 1996;39:1338-46
11. Rajkumar VS, Howell K, Csiszar K, Denton CP, Black CM, Abraham DJ. Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis. Arthritis Res Ther. 2005;7:R1113-23
12. Akhmetshina A, Dees C, Pileckyte M, Szucs G, Spriewald BM, Zwerina J. et al. Rho-associated kinases are crucial for myofibroblast differentiation and production of extracellular matrix in scleroderma fibroblasts. Arthritis Rheum. 2008;58:2553-64
13. Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V. et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355-60
14. Postlethwaite AE, Chiang TM. Platelet contributions to the pathogenesis of systemic sclerosis. Curr Opin Rheumatol. 2007;19:574-9
15. Baker DL, Desiderio DM, Miller DD, Tolley B, Tigyi GJ. Direct quantitative analysis of lysophosphatidic acid molecular species by stable isotope dilution electrospray ionization liquid chromatography-mass spectrometry. Analytical Biochemistry. 2001;292:287-95
16. Masi A, Subcommittee For Scleroderma Criteria of the American Rheumatism Association Diagnostic, Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum. 1980;23:581-90
17. Tokumura A, Iimori M, Nishioka Y, Kitahara M, Sakashita M, Tanaka S. Lysophosphatidic acids induce proliferation of cultured vascular smooth muscle cells from rat aorta. The American Journal of Physiology. 1994;267:C204-10
18. Sato R, Itabashi Y, Fujishima H, Okuyama H, Kuksis A. Simple synthesis of diastereomerically pure phosphatidylglycerols by phospholipase D-catalyzed transphosphatidylation. Lipids. 2004;39:1025-30
19. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology. 1959;37:911-7
20. Tamama K, Kon J, Sato K, Tomura H, Kuwabara A, Kimura T. et al. Extracellular mechanism through the Edg family of receptors might be responsible for sphingosine-1-phosphate-induced regulation of DNA synthesis and migration of rat aortic smooth-muscle cells. The Biochemical Journal. 2001;353:139-46
21. Watterson KR, Berg KM, Kapitonov D, Payne SG, Miner AS, Bittman R. et al. Sphingosine-1-phosphate and the immunosuppressant, FTY720-phosphate, regulate detrusor muscle tone. FASEB J. 2007;21:2818-28
22. Baker DL DD, Miller DD, Tolley B, Tigyi GJ. Direct quantitative analysis of lysophosphatidic acid molecular species by stable isotope dilution electrospray ionization liquid chromatography-mass spectrometry. Anal Biochem. 2001;292:287-95
23. Bandoh K, Aoki J, Taira A, Tsujimoto M, Arai H, Inoue K. Lysophosphatidic acid (LPA) receptors of the EDG family are differentially activated by LPA species. Structure-activity relationship of cloned LPA receptors. FEBS Letters. 2000;478:159-65
24. Rother E, Brandl R, Baker DL, Goyal P, Gebhard H, Tigyi G. et al. Subtype-selective antagonists of lysophosphatidic Acid receptors inhibit platelet activation triggered by the lipid core of atherosclerotic plaques. Circulation. 2003;108:741-7
25. Tokumura A, Sinomiya J, Kishimoto S, Tanaka T, Kogure K, Sugiura T. et al. Human platelets respond differentially to lysophosphatidic acids having a highly unsaturated fatty acyl group and alkyl ether-linked lysophosphatidic acids. The Biochemical Journal. 2002;365:617-28
26. Gajewiak J, Tsukahara R, Tsukahara T, Fujiwara Y, Yu S, Lu Y. et al. Alkoxymethylenephosphonate analogues of (Lyso) phosphatidic acid stimulate signaling networks coupled to the LPA2 receptor. ChemMedChem. 2007;2:1789-98
27. Jiang G, Xu Y, Fujiwara Y, Tsukahara T, Tsukahara R, Gajewiak J. et al. Alpha-substituted phosphonate analogues of lysophosphatidic acid (LPA) selectively inhibit production and action of LPA. ChemMedChem. 2007;2:679-90
28. Qian L, Xu Y, Simper T, Jiang G, Aoki J, Umezu-Goto M. et al. Phosphorothioate analogues of alkyl lysophosphatidic acid as LPA3 receptor-selective agonists. ChemMedChem. 2006;1:376-83
29. Sano T, Baker D, Virag T, Wada A, Yatomi Y, Kobayashi T. et al. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J Biol Chem. 2002;277:21197-206
30. Chan LC, Peters W, Xu Y, Chun J, Farese RVJr, Cases S. LPA3 receptor mediates chemotaxis of immature murine dendritic cells to unsaturated lysophosphatidic acid (LPA). Journal of Leukocyte Biology. 2007;82:1193-200
31. Chen M, Dittmann A, Kuhn A, Ruzicka T, von Mikecz A. Recruitment of topoisomerase I (Scl-70) to nucleoplasmic proteasomes in response to xenobiotics suggests a role for altered antigen processing in scleroderma. Arthritis Rheum. 2005;52:877-84
32. Kihara A, Igarashi Y. Production and release of sphingosine 1-phosphate and the phosphorylated form of the immunomodulator FTY720. Biochim Biophys Acta. 2008;1781:496-502
33. Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: Mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacology & Therapeutics. 2007;115:84-105
34. Brinkmann V, Lynch KR. FTY720: targeting G-protein-coupled receptors for sphingosine 1-phosphate in transplantation and autoimmunity. Curr Opin Immunol. 2002;14:569-75
35. Watanabe N, Ikeda H, Nakamura K, Ohkawa R, Kume Y, Aoki J. et al. Both plasma lysophosphatidic acid and serum autotaxin levels are increased in chronic hepatitis C. J Clin Gastroenterol. 2007;41:616-23
36. Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z. et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nature Medicine. 2008;14:45-54
37. Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H. et al. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. American Journal of Respiratory and Critical Care Medicine. 2004;169:1245-51
38. Postlethwaite AE, Shigemitsu H, Kanangat S. Cellular origins of fibroblasts: possible implications for organ fibrosis in systemic sclerosis. Curr Opin Rheumatol. 2004;16:733-8
39. Abraham DJ, Eckes B, Rajkumar V, Krieg T. New developments in fibroblast and myofibroblast biology: implications for fibrosis and scleroderma. Curr Rheumatol Rep. 2007;9:136-43
40. Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol. 2007;257:143-79
41. Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807-16
42. Wang J, Carbone LD, Watsky MA. Receptor-mediated activation of a depolarizing Cl- current by lysophosphatidic acid and sphingosine-1-phosphate in cultured corneal keratocytes. Invest Ophthalmol Vis Sci. 2002;43:3202-8
43. Watsky M. Lysophosphatidic acid, serum and hyposmolarity activate Cl- currents in corneal keratocytes. Am J Physiol (Cell Physiology). 1995;38:C1385-C93
44. Yamamoto T, Fujita K, Asari S, Chiba A, Kataba Y, Ohsumi K. et al. Synthesis and evaluation of isoxazole derivatives as lysophosphatidic acid (LPA) antagonists. Bioorganic & Medicinal Chemistry Letters. 2007;17:3736-40
Author biography
Dr Akira Tokumura heads the department of Pharmaceutical Health Chemistry (University of Tokushima, Japan). Since the end of 1970's, Dr. Tokumura has published many pioneering studies describing the physiological activities of lysophosphatidic acid, the central member of lysophospholipid mediator family, and its production by autotaxin/lysophospholipase D. During the FASEB Summer Conference on Lysophospholipids in 2003, Dr. Tokumura was recognized for his outstanding contributions to the field of lysophospholipid chemistry and biology.
Dr. Mitchell Watsky is a professor in the Department of Physiology at the University of Tennessee Health Science Center. Since the early 1980's, Dr. Watsky has published numerous studies examining the influence of LPA and S1P on ion channels during wound healing. This basic research, initially focused on corneal keratocytes and myofibroblasts, branched out to examine fibroblasts located in numerous organ systems, and led to the current translational study examining lysophospholipids in systemic sclerosis. Dr. Watsky's other research interests include cell signaling, bone markers and osteoporosis, and development of an artificial cornea. Dr. Watsky was awarded the University of Tennessee Science Alliance Faculty Award in 2001, and the Marta Marx Eradication of Scleroderma Award by the National Scleroderma Foundation in 2005.
![]() Correspondence to: Mitchell Watsky, Department of Physiology, University of Tennessee Health Science Center, 894 Union Ave., Memphis, Tennessee, 38002 USA. Phone: 1-901-448-8206; Fax: 1-901-448-7126; E-mail: mwatskyutmem.edu
Correspondence to: Mitchell Watsky, Department of Physiology, University of Tennessee Health Science Center, 894 Union Ave., Memphis, Tennessee, 38002 USA. Phone: 1-901-448-8206; Fax: 1-901-448-7126; E-mail: mwatskyutmem.edu

 Global reach, higher impact
Global reach, higher impact