3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2008; 5(5):248-262. doi:10.7150/ijms.5.248 This issue Cite
Review
The usefulness of circulating adipokine levels for the assessment of obesity-related health problems
Department of Public Health, Faculty of Medicine, University of Toyama, 2630 Sugitani, Toyama 930-0194, Japan.
Received 2008-7-6; Accepted 2008-8-27; Published 2008-8-29
Abstract
Because the prevalence of obesity has increased dramatically in recent years, one of the key targets of public health is obesity and its associated pathological conditions. Obesity occurs as a result of white adipose tissue enlargement, caused by adipocyte hyperplasia and/or hypertrophy. Recently, endocrine aspects of adipose tissue have become an active research area and these adipose tissue-derived factors are referred to as adipokines. These adipokines interact with a range of processes in many different organ systems and influence a various systemic phenomena. Therefore, dysregulated production of adipokines has been found to participate in the development of metabolic and vascular diseases related to obesity. The obese state is also known to be associated with increased local and systemic inflammation. Adipokines influence not only systemic insulin resistance and have pathophysiological roles in the metabolic syndrome and cardiovascular disease, but also contribute toward an increase in local and systemic inflammation. Thus, circulating levels of adipokines can be used as high-throughput biomarkers to assess the obesity-related health problems, including low grade inflammation. This review focuses on the usefulness of measuring circulating adipokine levels for the assessment of obesity-related health problems.
Keywords: Adipokine, biomarker, insulin resistance, metabolic syndrome, obesity.
1. Introduction
The prevalence of obesity has increased dramatically as a result of our modern lifestyle and is one of the most important targets of public health programs [1]. Accumulating evidence derived from both clinical and experimental studies highlight the association of obesity with a number of chronic diseases such as type II diabetes mellitus (T2DM), atherosclerosis and cardiovascular disease (CVD). T2DM is a problem not only in developed countries but is also becoming an urgent problem in developing countries owing to the worldwide increase in obesity [2]. Therefore, there is considerable effort to understand the underlying biology of these disease states and to identify the contributing risk factors.
The clustering of CVD risk factors, most notably the simultaneous presence of obesity, T2DM, dyslipidemia, and hypertension was recognized as an important pathophysiological state [3-5]. The coexistence of these diseases has been termed the metabolic syndrome (MS). Insulin resistance (IR) is well known to be a key feature of MS, and is strongly associated with excess adiposity, especially in the intra-abdominal region. Individuals with MS are at increased risk for the development of CVD and other diseases related to plaque formation in artery walls, resulting in stroke and peripheral vascular disease. Because the prevalence of these diseases is increasing, high throughput assessment of disease states accompanied with obesity or MS are important issues from the public health point of view.
Excess white adipose tissue (WAT) is linked to obesity-related health problems. It is also recognized that obesity is accompanied by chronic, low-level inflammation of WAT [6, 7]. Inflammation has been considered to be associated with the development of IR and MS [8]. Recently, WAT has been recognized as an important endocrine organ that secretes a wide variety of biologically active adipokines [9-11]. Since some of these adipokines greatly influence insulin sensitivity, glucose metabolism, inflammation and atherosclerosis, they may provide a molecular link between increased adiposity and the development of T2DM, MS and CVD. The signals from WAT are thought to directly connect with IR and inflammation. It is expected, therefore, that circulating levels of adipokines may be useful as biomarkers to evaluate the risk of other disease states associated with obesity.
This review describes the usefulness and clinical significance of circulating adipokine levels. First, I focused on three representative adipokines associated with IR, namely adiponectin, retinol binding protein 4 (RBP4) and resistin. Next, I discuss the inflammation-related markers such as tumor necrosis factor (TNF) α, interleukin (IL)-6 and C-reactive protein (CRP). Because leptin has not been recognized directly to be related with IR and inflammation, description of this adipokine was excluded. Finally, I have summarized the significance of other molecules, followed by a brief discussion for future research.
2. Adipose tissue as a secretory organ
In 1993, it was discovered that TNFα expression was up-regulated in WAT of obese mice [12]. The role of WAT as a hormone-producing organ became well recognized in 1994 with the discovery of leptin as an adipocyte-secreted protein [13]. Systemic analysis of the active genes in WAT, by constructing a 3'-directed complementary DNA library, revealed a high frequency of genes encoding secretory proteins. Of the gene group classified by function, approximately 20–30% of all genes in WAT encode secretory proteins [14].
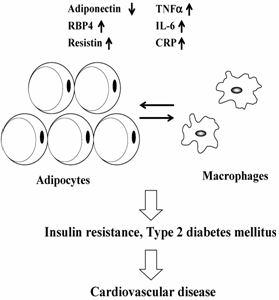
In adults, most organ systems have reached their final size and are programmed to be maintained at steady state. However, WAT is unique because of its almost unlimited expansion potential. Thus, WAT can become one of the largest organs in the body, and the total amount of an adipokine secreted from WAT may affect whole-body homeostasis. WAT contains various types of cells that include preadipocytes, adipocytes and stromal vascular cells. Moreover, bone marrow-derived macrophages home to WAT in obesity [6, 7]. The massive increase in fat mass leads to a dysregulation of circulating adipokine levels that may have pathogenic effects associated with obesity. Thus, dysregulated secretion of adipokines, not only from adipocytes but also from macrophages in WAT, will contribute to the pathogenesis of obesity by triggering IR and systemic inflammation (Fig. 1). It is expected, therefore, that circulating levels of adipokines can be used as a high-throughput biomarker to assess obesity-related health problems.
Schematic representation of mechanisms linking adipokine dysreguation and cardiovascular disease in obese state. See text for abbreviations.
3. Adiponectin
Adiponectin is the most abundantly expressed adipokine in WAT [14]. The average levels of adiponectin in human plasma are 5–10 μg/ml [15]. Adiponectin is a multifunctional protein that exerts pleiotropic insulin-sensitizing effects. It lowers hepatic glucose production [16] and increases glucose uptake and fatty acid oxidation in skeletal muscle [17]. Moreover, adiponectin may possess anti-atherogenic properties by inhibiting the expression of adhesion molecules and smooth muscle cell proliferation, as well as suppressing the conversion of macrophages to foam cells [18, 19]. An anti-inflammatory role of adiponectin has also been reported [20].
A number of studies reported the significance of circulating levels of adiponectin (Table 1). Unlike most adipokines, adiponectin mRNA in WAT and serum levels are decreased in obesity [21]. Adiponectin is the only adipokine that is known to be down-regulated in obesity. Plasma concentrations are negatively correlated with body mass index (BMI) [15]. A longitudinal study in primates suggests that adiponectin decreases with weight gain as animals become obese [22]. In contrast, weight loss results in significant increases in circulating adiponectin levels [23, 24]. In addition to the association with whole-body fat mass, adiponectin levels differ with the distribution of body fat. Plasma levels of adiponectin exhibit strong negative correlations with intra-abdominal fat mass [25]. Visceral, but not subcutaneous abdominal fat, was reported to be inversely associated with plasma adiponectin levels in healthy women [26]. A low waist to hip ratio has been reported to be associated with high levels of plasma adiponectin independent of the body fat percentage [27].
Clinical studies of circulating adiponectin levels
| Subjects | Major findings | References |
|---|---|---|
| Obese subjects | Decreased in obese subjects | Hu et al., (1996) [21] |
| Arita et al., (1999) [15] | ||
| Patients with CVD | Decreased in patients with CVD | Ouchi et al., (1999) [37] |
| Nondiabetic and T2DM subjects | Decreased in T2DM patients | Hotta et al., (2000) [28] |
| Obese subjects | Increased after weight loss | Yang et al., (2001) [23] |
| Caucasians and Pima Indians | Associated with IR | Weyer et al., (2001) [153] |
| Pima Indian | Low plasma concentration precedes a decrease in insulin sensitivity | Stefan et al., (2002) [29] |
| Pima Indian | Decreased in T2DM patients | Lindsay et al., (2002) [30] |
| Pima Indian children | An inverse relationship to adiposity | Stefan et al., (2002) [154] |
| Nondiabetic Japanese women | Negative correlation with serum triglyceride | Matsubara et al., (2002) [155] |
| Obese subjects | Increased after weight loss | Bruun et al., (2003) [24] |
| Middle-aged population | Associated with intra-abdominal fat | Cnop et al., (2003) [25] |
| Nondiabetic white volunteers | Positive correlation with HDL-cholesterol | Tschritter et al., (2003) [31] |
| Hypertensive patients | Correlation with vasodilator response | Ouchi et al., (2003) [34] |
| Japanese men | Decreased in patients with CVD | Kumada et al., (2003) [38] |
| Japanese subjects | Connected with endothelial dysfunction | Shimabukuro et al., (2003) [156] |
| Japanese subjects | Decreased in patients with T2DM | Daimon et al., (2003) [157] |
| Apparently healthy individuals | Associated with the risk of T2DM | Spranger et al., (2003) [158] |
| Asian Indians with IGT | Low adiponectin was a strong predictor of T2DM | Snehalatha et al., (2003) [159] |
| Nonobese and obese subjects | Correlation with advantageous lipid profile | Baratta et al., (2004) [32] |
| Japanese men | Decreased in hypertensive men | Iwashima et al., (2004) [33] |
| Male participants | High adiponectin was associated with lower risk of myocardial infarction | Pischon et al., (2004) [39] |
| Whites and African Americans | Higher adiponectin was associated with a lower incidence of T2DM | Duncan et al., (2004) [160] |
| Patients with CVD | Decreased in patients with CVD | Nakamura et al., (2004) [161] |
| Pregnant women | Decreased in patients with gestational DM | Ranheim et al., (2004) [162] |
| Nondiabetic subjects | Obesity-independent association of IR with adiponectin levels | Abbasi et al., (2004) [163] |
| Obese individuals | Decreased in subjects with MS | Xydakis et al., (2004) [164] |
| Healthy premenopausal women | Associated with visceral fat mass | Kwon et al., (2005) [26] |
| Obese juveniles | An inverse relation with the intima media thickness of common carotid arteries | Pilz et al., (2005) [40] |
| Patients with chronic heart failure | High adiponectin was a predictor of mortality | Kistorp et al., (2005) [42] |
| British women | No association with CVD risk | Lawlor et al., (2005) [43] |
| American Indian | No association with later development of CVD | Lindsay et al., (2005) [44] |
| Hispanic children | Inversely associated with IR | Butte et al., (2005) [52] |
| Patients with CVD | Decreased in patients with CVD | Rothenbacher et al., (2005) [165] |
| Middle-aged men | Positive association with lower fat mass | Buemann et al., (2005) [166] |
| Obese children | Low adiponectin was associated with components of MS | Winer et al., (2006) [36] |
| Older Black Americans | High adiponectin was associated with higher risk of CVD | Kanaya et al., (2006) [45] |
| Patients with CVD | High adiponectin was a predictor of mortality | Cavusoglu et al., (2006) [46] |
| Patients with CVD | High adiponectin was a predictor of mortality | Pilz et al., (2006) [50] |
| Pregnant women | Elevated with preeclampsia | Haugen et al., (2006) [59] |
| Patients with congestive heart failure | Positive correlation with disease severity | George et al., (2006) [167] |
| Caucasian | High adiponectin increased the risk of death from all causes | Laughlin et al., (2007) [48] |
| Aged men | High adiponectin increased the risk of death from all causes | Wannamethee et al., (2007) [49] |
| Patients with incident CVD | No association with the prognostic outcome | von Eynatten et al., (2008) [41] |
| General Dutch population | High levels of adiponectin predict mortality | Dekker et al., (2008) [51] |
Plasma adiponectin concentrations are lower in people with T2DM than in BMI-matched controls [28]. The plasma concentrations have been shown to correlate strongly with insulin sensitivity, which suggests that low plasma concentrations are associated with IR [29]. In a study of Pima Indians, a population that has one of the highest prevalence of obesity, IR and T2DM, individuals with high adiponectin levels were less likely to develop T2DM than those with low concentrations [30]. The high adiponectin concentration was, therefore, a predictive marker for the development of T2DM. Plasma concentrations of adiponectin are also reported to be associated with components of MS. High plasma concentrations of adiponectin were found to be related to an advantageous blood lipid profile [31, 32]. Plasma adiponectin levels are decreased in hypertensive humans, irrespective of the presence of IR [33]. Endothelium-dependent vasoreactivity is impaired in people with hypoadiponectinemia [34], which might be one of the mechanisms involved in hypertension in visceral obesity. A reciprocal association between CRP and adiponectin mRNA levels was reported in human WAT, suggesting that hypoadiponectinemia appears to contribute to low-grade systemic chronic inflammation [35]. All these mechanisms may underlie the protective effects against the progression of atherosclerosis of adiponectin. A recent study revealed that adiponectin may function as a biomarker for MS, even in childhood obesity [36]. Collectively, adiponectin has been recognized as a key molecule in MS and has the potential to become a clinically relevant parameter to be measured routinely at general medical check ups.
Plasma concentrations of adiponectin are also known to be lower in people with CVD than in controls, even after matching for BMI and age [37]. A case-control study performed in Japan revealed that the people with hypoadiponectinemia with the plasma levels less than 4 μg/ml had increased risk of CVD and multiple metabolic risk factors, indicating that hypoadiponectinemia is a key factor in MS [38]. Retrospective case-control studies have demonstrated that patients with the highest levels of adiponectin have a dramatically reduced 6-year risk of myocardial infarction compared with case controls with the lowest adiponectin levels, and this relationship persists even after controlling for family history, BMI, alcohol, history of diabetes and hypertension, hemoglobin A1c, CRP, and lipoprotein levels [39]. An inverse relationship between serum adiponectin levels and the intima media thickness of common carotid arteries was also reported [40]. These clinical studies clearly indicate that hypoadiponectinemia is a strong risk factor for CVD.
Although the above studies support the notion that adiponectin would protect against vascular diseases, recent epidemiological studies have failed to support this notion [41-51]. A recent prospective study reported adiponectin levels were not significantly associated with future secondary CVD events [41]. Thus, measurement of adiponectin may add no significant value to risk stratifications in patients with incident CVD, and effects of adiponectin may be more of importance in the early phases of atherosclerosis. Kistorp et al. reported that adiponectin was positively related to increased mortality in patients with chronic heart failure [42]. These authors suspect that the high adiponectin concentrations may reflect a wasting process in subjects with increased risk of death. Pilz et al. reported that high adiponectin levels predict all-cause, cardiovascular and noncardiovascular mortality [50]. A recent study also reported that a high adiponectin level was a significant predictor of all-cause and CVD mortality [51]. These authors hypothesized that a counter-regulatory increase in adiponectin occurs, which represents a defense mechanism of the body against cardiovascular alterations and a pro-inflammatory state associated with CVD. Thus, yet-unknown mechanisms may underlie the association between adiponectin and the risk of death, the prognostic value of adiponectin remains unresolved. Further prospective studies will be required to provide conclusive results about the association of adiponectin and mortality. It is also necessary to understand the underlying molecular mechanisms of elevated adiponectin concentrations in these disease states.
It must be highlighted that several physiological factors affect the circulating levels of adiponectin. First, aging, gender and puberty have effects on circulating adiponectin levels [52, 53]. An age-associated elevation of plasma adiponectin levels has been reported [51, 54]. Plasma adiponectin levels were significantly higher in female subjects, indicative of a sex hormone affect on circulating adiponectin levels [51, 55]. Adiponectin levels tend to decrease throughout puberty, which parallels the development of IR [36, 56]. Second, the glomerular filtration rate has been recognized as a strong inverse predictor of adiponectin. The clearance of adiponectin by the kidney may have a strong influence on its concentration [57]. Hence, high adiponectin levels may reflect impaired renal function. Last but not least, an increased adiponectin level has been suggested to act as a compensatory mechanism to dampen inflammation. Indeed, elevated plasma adiponectin concentrations are observed in several diseases associated with inflammation: arthritis [58], preeclampsia [59], and end-stage renal disease [60]. All of these factors must be considered when evaluating the clinical significance of circulating adiponectin levels in MS or vascular diseases related to obesity.
Circulating adiponectin forms several different complexes in the adipocyte before being secreted into the blood [61]. Commercial assays measure the total plasma concentration of adiponectin. Thus, the vast majority of clinical studies published to date have evaluated correlations between total adiponectin levels and various markers of MS. The most basic form of adiponectin secreted is the trimer. Adiponectin forms two higher-ordered structures through the noncovalent binding of two trimers (hexamers) and six trimers (18mers). The native protein circulates in serum as low molecular weight (LMW) hexamers and as larger multimeric structures of high molecular weight (HMW). Of these higher-ordered structures, the 18mer (HMW) form is assumed to act beneficial against IR; the function of the hexamer (LMW) form is suggested to play a pro-inflammatory role [55, 62]. Thus, the HMW form is more strongly associated with insulin sensitivity than is total adiponectin [63-65]. Overall, these results suggest that the assessment of total adiponectin may be insufficient and that the analysis of the levels of the multimeric forms should be favorable to assess the significance of adiponectin.
4. Retinol binding protein 4 (RBP4)
RBP4 is a protein that is the specific carrier for retinol in the blood. It is one of a large number of proteins that solubilize and stabilize the hydrophobic and labile metabolites of retinoids in aqueous spaces in both extra- and intracellular spaces. Its physiological function appears to be to bind retinol and prevent its loss through the kidneys. RBP4, although largely produced in liver, is also made by adipocytes, with increased levels in obesity contributing to impaired insulin action [66]. Studies in transgenic rodent models showed overexpression of human RBP4 or injection of recombinant RBP4 induced IR in mice, whereas RBP4 knockout mice showed enhanced insulin sensitivity [66]. The same authors reported that high plasma RBP4 levels are associated with IR states in humans and suggested that RBP4 is an adipokine responsible for obesity-induced IR and, thus, a potential therapeutic target in T2DM [66, 67]. Since then, a number of clinical studies have been conducted to assess the significance of circulating levels of RBP4 (Table 2).
Clinical studies of circulating RBP4 levels
| Subjects | Major findings | References |
|---|---|---|
| Obese and T2DM subjects | Elevated in subjects with T2DM | Yang et al., (2005) [66] |
| IGT and T2DM subjects | Correlation with the magnitude of IR | Graham et al., (2006) [67] |
| IGT and T2DM subjects | Elevated in subjects with IGT or T2DM than normal glucose tolerance | Cho et al., (2006) [68] |
| Caucasian menopausal women | No correlation with adiposity | Janke et al., (2006) [72] |
| Japanese subjects | No correlation with BMI | Takashima et al., (2006) [168] |
| IGT and T2DM subjects | No correlation with IR | Erikstrup et al., (2006) [169] |
| Chinese subjects | Correlation with the components of MS | Qi et al., (2007) [69] |
| Healthy women | Associated with visceral fat | Lee et al., (2007) [70] |
| Chinese subjects | Correlation with visceral adiposity | Jia et al., (2007) [71] |
| Non diabetic person | No correlation with IR | Yao-Borengasser et al., (2007) [73] |
| Subjects with BMI from 18 to 30 | Negative correlation with insulin sensitivity | Gavi et al., (2007) [74] |
| Caucasian without T2DM | Associated with liver fat | Stefan et al., (2007) [76] |
| Nondiabetic individuals | Reflected ectopic fat accumulation | Perseghin et al., (2007) [77] |
| Obese children | Associated positively with CRP | Balagopal et al., (2007) [170] |
| Subjects with morbid obesity | Reduction after weight loss | Haider et al., (2007) [171] |
| Obese women | Reduction after weight loss | Vitkova et al., (2007) [172] |
| Patients with T2DM | Associated with IR | Takebayashi et al., (2007) [173] |
| Women with polycystic ovary syndrome | Elevated than BMI-matched subjects | Tan et al., (2007) [174] |
| Nondiabetic men | Negatively associated with insulin secretion | Broch et al., (2007) [175] |
| Patients with chronic liver disease | Decreased compared with control subjects | Yagmur et al., (2007) [176] |
| Patients with T2DM or CVD | Associated with pro-atherogenic lipoprotein levels | von Eynatten et al., (2007) [177] |
Cho et al. reported that plasma concentrations of RBP4 were higher in people with impaired glucose tolerance (IGT) or T2DM than in people with normal glucose tolerance [68]. A recent cross-sectional study of 3289 middle-aged population showed that plasma RBP4 levels increased gradually with increasing numbers of MS components [69]. Similar to other adipokines, circulating levels of RBP4 is associated with body fat distribution rather than body weight per se. RBP4 was reported to be more highly correlated with waist-to-hip ratio or visceral fat areas than with BMI [67, 70, 71]. However, Janke et al. reported that, in human abdominal subcutaneous (sc) adipose tissue, RBP4 mRNA is down-regulated in obese women, whereas circulating RBP4 concentrations were similar in lean, overweight, and obese women [72]. Yao-Borengasser et al. also reported that neither sc adipose tissue RBP4 mRNA expression nor circulating RBP4 levels show any correlation with BMI [73]. It is not clear why such differences are present among similarly conducted human studies. These inconsistencies most likely result from differences in age, ethnicity, sample size, and assay methods used. For example, sex and age were found to be independent determinants of plasma RBP4 concentrations [68, 74]. A recent study suggested that the sandwich ELISA kit commercially available for the assessment of RBP4 may overestimate the circulating levels [75]. Those authors also claimed that competitive EIAs may underestimate serum RBP4 levels in the setting of IR owing to assay saturation. Thus, it is probable that the reported RBP4 associations would become clearer if more reliable assays were employed.
Two recent studies have indicated that high circulating RBP4 is associated with elevated liver fat and, presumably, hepatic insulin resistance [76, 77]. In rodents, only 20% of systemic RBP4 is produced by adipocytes, and RBP4 gene expression in adipocytes was 20% compared with expression in the liver [78]. Thus, it is possible that the increase in systemic RBP4 concentrations is not explained by increased RBP4 production in WAT. RBP4 is a transporter for retinol, which serves as a precursor for the synthesis of ligands for nuclear hormone receptors such as retinoid X receptor and retinoic acid receptor. Thus, circulating RBP4 can modulate metabolic pathways via these nuclear hormone receptors. Certainly, future prospective studies are needed to clarify whether a high RBP4 level plays a causal role in the development of MS, T2DM, and eventually for the development of CVD.
5. Resistin
After the identification of resistin as an adipokine in 2001 [79], several studies have been conducted to investigate the role and significance of this molecule. Resistin was discovered as a result of a hypothesis that WAT secretes a hormone that mediates IR and that insulin sensitizing drug thiazolidinediones act by suppressing the production of this hormone. Resistin is secreted by mature adipocytes in proportion to the level of obesity and acts on insulin-sensitive cells to antagonize insulin-mediated glucose uptake and utilization in mice. Treatment of wild-type mice with recombinant resistin resulted in IR, whereas administration of an anti-resistin antibody increased insulin sensitivity in obese and insulin-resistant animals [79]. However, human resistin is 59% homologous at the amino acid level to the mouse molecule, a relatively low degree of sequence conservation. Moreover, in contrast to mice, human resistin is expressed at lower levels in adipocytes but at higher levels in circulating blood monocytes [80]. As a result, there is still uncertainty about possible relationships between serum concentrations of resistin and markers of IR (Table 3).
Clinical studies of circulating resistin levels
| Subjects | Major findings | References |
|---|---|---|
| Healthy Greek students | Correlation with body fat mass | Yannakoulia et al., (2003) [81] |
| Non-diabetic subjects | Correlation with IR | Silha et al., (2003) [82] |
| Patients with essential hypertension | Elevated in T2DM patients | Zhang et al., (2003) [83] |
| Patients with inflammatory diseases | Correlation with inflammatory markers | Stejskal et al., (2003) [91] |
| Obese subjects | Correlation with BMI | Azuma et al., (2003) [178] |
| Lean and obese subjects | Increase in obese subjects | Degawa-Yamauchi et al., (2003) [179] |
| Women | No relation with fat mass or IR | Lee et al., (2003) [180] |
| Patients with T2DM | No correlation with IR | Pfutzner et al., (2003) [181] |
| Obese subjects | Not changed after weight loss | Monzillo et al., (2003) [182] |
| Diabetic subjects | Correlation with CRP | Shetty et al., (2004) [87] |
| Obese Caucasian subjects | Correlation with HOMA-R | Silha et al., (2004) [183] |
| Non obese subjects | Correlation with insulin sensitivity | Heilbronn et al., (2004) [184] |
| Pima Indians | Correlation with fat mass but not IR | Vozarova de Courten et al., (2004) [185] |
| Diabetic subjects | Elevated in T2DM patients | Youn et al., (2004) [186] |
| Japanese subjects | Elevated in T2DM patients | Fujinami et al., (2004) [187] |
| Patients with T2DM | Correlation with hepatic fat content | Bajaj et al., (2004) [188] |
| Women | Associated with the presence of CVD | Pischon et al., (2005) [88] |
| Subjects who had a family history of premature coronary artery disease | Correlation with the levels of inflammatory markers | Reilly et al., (2005) [90] |
| Japanese subjects | Associated with the presence and severity of CVD | Ohmori et al., (2005) [189] |
| Men | Correlation with CRP | Bo et al., (2005) [190] |
| Patients with rheumatoid arthritis | Elevated than the patients with osteoarthritis | Senolt et al., (2007) [89] |
The role of resistin in the pathophysiology of obesity and IR in humans is controversial. Several studies have shown positive correlations of circulating resitin levels with body fat mass [80, 81] or IR [82, 83]. However, the other studies found no relationship between resistin gene expression and body weight or insulin sensitivity [84-86]. These conflicting data may reflect variations in the study design and the lack of adjustment for potential confounding factors. It also seems possible that resistin is a marker for, or contributes to, IR in a specific population. The predominantly paracrine role of resistin might explain the weakness of the correlations between circulating resistin levels and some of the metabolic variables.
Two studies have shown that among the blood markers, the most significant association of the circulating resistin level was with plasma CRP [87, 88]. Thus, higher resistin levels may be a marker of systemic inflammation. Indeed, the circulating level of resistin is up-regulated in patients with rheumatoid arthritis [89]. The circulating resistin level is also reported to be an inflammatory marker of atherosclerosis [90]. Considering that the resistin concentration is elevated in the patients with severe inflammatory disease [91], hyperresistinemia may be a biomarker and/or a mediator of inflammatory states in humans. Overall, the resistin levels in humans are thought to correlate more closely with inflammation than with IR.
6. Inflammation-related molecules
Obesity is associated with a state of chronic, low-grade inflammation characterized by abnormal cytokine production and the activation of inflammatory signaling pathways in WAT [92]. Obese hypertrophic adipocytes and stromal cells within WAT directly augment systemic inflammation. Although WAT is usually populated with 5-10% macrophages, diet-induced weight gain causes a significant macrophage infiltration, with macrophages comprising up to 60% of all cells found in WAT in a rodent model [6]. Thus, several adipokines implicated in inflammation are cytokines which are produced by macrophages. The accumulation of WAT resident macrophages and elaboration of inflammatory cytokines have been implicated in the development of obesity-related IR. Indeed, increases in inflammatory cytokine expression by WAT are associated with a parallel increase in WAT macrophage content [6, 7, 93]. Thus, obesity leads to increased production of several inflammatory cytokines, which play a critical role in obesity-related inflammation and metabolic pathologies.
A number of studies have reported that several humoral markers of inflammation are elevated in people with obesity and T2DM [94, 95] (Table 4). Pfeiffer et al. showed that men with T2DM had higher TNFα concentrations compared with nondiabetic subjects [96]. However, several studies reported no association between circulating levels of TNFα and insulin sensitivity [97, 98]. Since there was no arteriovenous difference with TNFα [99], TNFα is considered to work mainly in an autocrine or paracrine manner, where the local concentrations would be more likely to exert its metabolic effects [99, 100]. Moreover, circulating TNFα has been reported to be associated with a soluble receptor that inhibits its biological activity [101], suggesting that the action of TNFα is primarily a local one. Therefore, it seems unlikely that the circulating levels of TNFα would be a good biomarker to reflect the IR state of the whole body.
Clinical studies of circulating inflammatory markers
| Subjects | Major findings | References |
|---|---|---|
| TNFα | ||
| Nondiabetic offsprings of T2DM patients | Not major contributing factor for obesity induced IR | Kellerer et al., (1996) [97] |
| Adult males | Elevated in patients with T2DM | Pfeiffer et al., (1997) [96] |
| Obese patients with T2DM | Correlation with the visceral fat area | Katsuki et al., (1998) [191] |
| T2DM subjects | Elevated in T2DM as compared to control | Winkler et al., (1998) [192] |
| Aged men | Correlation with BMI | Nilsson et al., (1998) [193] |
| Canadian population | Positive correlation with IR | Zinman et al., (1999) [194] |
| Obese subjects | Elevated in obese subjects than in controls | Corica et al., (1999) [195] |
| Normotensive obese patients | Elevated in patients with android obesity than gynoid obesity | Winkler et al., (1999) [196] |
| Obese subjects | No relationship with BMI | Kern et al., (2001) [98] |
| Premenopausal obese women | Reduced after weight loss | Ziccardi et al., (2002) [197] |
| Nondiabetic obese women | Associated with fat amount | Maachi et al., (2004) [198] |
| Premenopausal obese women | Reduced after weight loss | Marfella et al., (2004) [199] |
| IL-6 | ||
| White nondiabetic subjects | Correlation with BMI | Yudkin et al., (1999) [100] |
| Healthy middle-aged women | Associated with BMI | Hak et al., (1999) [200] |
| Obese nondiabetic women | Reduced after weight loss | Bastard et al., (2000) [103] |
| Obese subjects | Correlation with obesity and IR | Kern et al., (2001) [98] |
| Pima Indians | Correlation with IR | Vozarova et al., (2001) [102] |
| Premenopausal obese women | Reduced after weight loss | Ziccardi et al., (2002) [197] |
| Premenopausal obese women | Reduced after weight loss | Esposito et al., (2003) [104] |
| Obese patients | Reduced after weight loss | Kopp et al., (2003) [105] |
| Obese subjects | Reduced after weight loss | Monzillo et al., (2003) [182] |
| Premenopausal obese women | Reduced after weight loss | Giugliano et al., (2004) [106] |
| Nondiabetic offspring of patients with T2DM | Not associated with the components of MS | Salmenniemi et al., (2004) [110] |
| Premenopausal obese women | Reduced after weight loss | Marfella et al., (2004) [199] |
| Japanese men | Not associated with the components of MS | Matsushita et al., (2006) [111] |
| T2DM subjects | Associated with IR | Natali et al., (2006) [201] |
| Adolescents | Positive correlation with BMI | Herder et al., (2007) [135] |
| CRP | ||
| White nondiabetic subjects | Positive correlation with BMI | Yudkin et al., (1999) [100] |
| Healthy middle-aged women | Associated with BMI | Hak et al., (1999) [200] |
| Young adults | Elevated in obese person | Visser et al., (1999) [202] |
| Adult men | Correlation with body fat mass | Lemieux et al., (2001) [115] |
| Obese women | Reduced after weight loss | Heilbronn et al., (2001) [117] |
| Middle-aged men | Predictor of T2DM development | Freeman et al., (2002) [116] |
| Obese postmenopausal women | Reduced after weight loss | Tchernof et al., (2002) [118] |
| Premenopausal obese women | Reduced after weight loss | Ziccardi et al., (2002) [197] |
| Healthy obese women | Correlation with IR independent of obesity | McLaughlin et al., (2002) [203] |
| Premenopausal obese women | Reduced after weight loss | Esposito et al., (2003) [104] |
| Healthy American women | Prognostic marker to the MS | Ridker et al., (2003) [114] |
| Premenopausal obese women | Obesity is the major determinant of elevated CRP levels | Escobar-Morreale et al., (2003) [204] |
| Premenopausal obese women | Reduced after weight loss | Marfella et al., (2004) [199] |
| Obese subjects | Correlation with serum TNFα levels | Shadid et al., (2006) [86] |
| T2DM subjects | Associated with IR | Natali et al., (2006) [201] |
| Overweight women | Reduced after weight loss | Moran et al., (2007) [205] |
A considerable proportion of circulating IL-6 is derived from WAT, and WAT is estimated to produce about 25% of the systemic IL-6 in vivo [99]. Fasting plasma IL-6 concentrations were negatively correlated with the rate of insulin-stimulated glucose disposal in Pima Indians [102]. Bastard et al. reported that the IL-6 values were more strongly correlated with obesity and IR parameters than TNFα, and a very low-calorie diet induced significant decreases in circulating IL-6 levels in obese women [103]. Other studies have also showed that weight loss results in decreased circulating levels of IL-6 [104-106]. Although several reports have indicated that IL-6 plays a role in the development of IR [95, 107], some investigators have insisted that IL-6 prevents IR [108, 109]. Some of these discrepancies may be explained by the widely different characteristics of the study populations regarding age, sex, glucose tolerance status, and degree of obesity. Overall, the association of IL-6 and IR seems complex and IL-6 alone might not be an appropriate marker of IR or MS [110, 111].
IL-6 derived from visceral adipose tissue draining directly into the portal system and causes the obesity-associated rise of liver CRP production [112]. Although CRP was traditionally thought to be produced exclusively by the liver in response to inflammatory cytokines, emerging data indicate that CRP can also be produced by nonhepatic tissues. Adipocytes isolated from human WAT produced CRP in response to inflammatory cytokines [113]. Adiponectin has been suggested to play a role in modulating CRP levels. In fact, adiponectin knockout mice showed higher CRP mRNA levels in WAT compared with the wild-type mice [35]. Therefore, hypoadiponectinemia also appears to be responsible for a low-grade systemic chronic inflammatory state, which is closely related to high CRP levels.
Several studies have shown that CRP is more strongly associated with IR than either TNFα or IL-6 [110, 111, 114]. CRP has been reported to be associated with body fat and other inflammatory markers [86, 115]. Abundant evidence has accumulated to show that CRP is associated with MS and predicts T2DM and CVD events independently of traditional risk factors [114, 116]. Thus, elevated CRP levels in obesity, and the decreases associated with weight loss indicate a link between CRP and obesity-associated risks for CVD [104, 117, 118].
7. Chemokines: monocyte chemoattractant protein-1 and IL-8
Monocyte chemoattractant protein-1 (MCP-1) is a chemokine, which plays a pivotal role in the recruitment of monocytes and T lymphocytes to the sites of inflammation. MCP-1 is expressed in adipocytes and considered to be an adipokine [119, 120]. MCP-1 mediates the infiltration of macrophages into WAT in obesity and may play an important role in establishing and maintaining a proinflammatory state that predisposes to the development of IR and MS [121]. Macrophage infiltration into WAT is increased by the secretion of MCP-1, which is expressed by adipocytes, as well as by macrophages and other cell types, especially in obese, insulin-resistant subjects [122]. A number of studies have reported significantly higher circulating MCP-1 levels in obese [122, 123] or T2DM patients [124, 125]. Conversely, obese patients who lost weight showed decreased levels of MCP-1 [122, 126]. However, a recent study indicated that there was no difference in circulating MCP-1 levels between nonobese and obese subjects, when either abdominal venous or arterialized blood was analyzed [127]. Previous studies showed that plasma MCP-1 levels were influenced by numerous factors, including aging [128], hypertension [129], hypercholesterolemia [130], vascular disease [131], and renal failure [132]. Moreover, MCP-1 is also produced by other cell types, such as vascular smooth muscle cells, endothelial cells, fibroblasts, mesangial cells, and lymphocytes. Thus, undetectable conditions might have influenced the circulating MCP-1 levels, and it seems improbable that the circulating levels of MCP-1 merely reflect obesity-related disease states.
IL-8 is responsible for the recruitment of neutrophils and T lymphocytes into the subendothelial space and considered to be an atherogenic factor that leads to intimal thickening. IL-8 is produced and secreted by human adipocytes [133]. Plasma IL-8 levels are increased in obese subjects, linking obesity with increased cardiovascular risk [134]. The circulating IL-8 level is associated with obesity-related parameters such as BMI, waist circumference and CRP [123]. However, Herder et al. reported that, among the seven immunological mediators (IL-6, IL-18, TNFα, IL-8, MCP-1, IP-10, and adiponectin) expressed and secreted by WAT, high BMI was significantly associated with elevated circulating levels of IL-6, IL-18, and IP-10 as well as lower levels of adiponectin [135]. Thus, the clinical relevance of circulating levels of MCP-1 and IL-8 to predict obesity-related disease conditions is still unresolved.
8. Other molecules
Plasminogen activator inhibitor-1 (PAI-1) is an important endogenous inhibitor of tissue plasminogen activator and is a main determinant of fibrinolytic activity. PAI-1 contributes to the pathogenesis of atherothrombosis and CVD. Experimental data indicate that WAT has a capacity to produce PAI-1 [136]. Much of the elevation of circulating levels of PAI-1 in obesity is attributable to upregulated production from WAT [136-138]. The increased plasma PAI-1 levels in obesity and positive correlations with visceral fat depots are reported in several studies [139-142]. Conversely, weight loss is associated with reduced PAI-1 activity in obese subjects [143]. Hyperinsulinemia caused by IR may increase both adipocyte and hepatic synthesis of PAI, which could play a role in the development of the vascular complications [144, 145].
Obesity is associated with expansion of the capillary bed in regional fat depots. Adipocytes or other cell types present in WAT secrete angiogenic factors such as vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF), which act in an autocrine or paracrine manner within WAT but may have endocrine effects throughout the body. Serum VEGF levels were found to positively correlate with BMI [146, 147]. HGF has also been reported to be elevated in obese subjects [148] and elevated serum HGF in obese subjects is reduced with weight loss [149]. These angiogenic factors may be involved in the development of obesity-related metabolic disorders such as inflammation and CVD.
Cathepsin S was recently identified as a novel adipokine [150]. Cathepsin S is a cysteine protease that has the ability to degrade many extracellular elements and is involved in the pathogenesis of atherosclerosis [151]. Cathepsin S is secreted by adipocytes and its circulating levels are increased in obese subjects than in nonobese subjects [152]. Conversely, weight loss is associated with a decrease in circulating cathepsin S levels as well as WAT cathepsin S content [152]. Thus, cathepsin S could constitute a novel biomarker of adiposity that may be linked with enlarged WAT and may also play a role in vascular pathogenesis in obesity.
9. Conclusions
Obesity is recognized as a worldwide public health problem that contributes to a wide range of disease conditions. The development of a method for convenient prediction of obesity-related health problems represents a major challenge for public policy makers facing the epidemic of obesity. WAT is an endocrine organ that communicates with other tissues via secretion of adipokines. Adipokines, which integrate metabolic and inflammatory signals are attractive candidates for predicting the risk of CVD. With obesity, the production of most adipokines is enhanced, except for the anti-inflammatory and insulin-sensitizing effector, adiponectin. Enlarged adipocytes and macrophages embedded within WAT produce more RBP4, resistin and proinflammatory cytokines, such as TNFα and IL-6. Markers of inflammation including CRP have been proposed for use in clinical practice to aid in the identification of asymptomatic patients at high risk for CVD. Thus, measurement of adiponectin and inflammatory markers could be used to assess the risk of developing CVD.
It is important to note, however, that only a limited number of adipokines are released into the bloodstream at levels that are detectable with current assays, resulting in increased circulating levels in the obese state. Some adipokines acting in a paracrine or autocrine manner may play an important role; thus, circulating levels of the adipokines may represent only spillover from WAT and may not be associated with the disease condition. Moreover, except for adiponectin, many of the adipokines are not expressed exclusively in WAT. Thus, there remains uncertainty as to the most appropriate and optimal marker for use in clinical practice. Since various WAT in different regions may have unique characteristics related to differential expression of adipokines, different types of fat distribution may offer the explanations for the discrepancies observed between different studies. Further epidemiological studies with solid clinical end points are needed to determine which combination of adipokines can be a reliable risk marker for CVD and may provide an improved method for identifying persons at risk for future cardiovascular events. Elucidation of the significance of circulating adipokines may provide a therapeutic target for adipokine-based pharmacological and/or interventional therapies in obesity and related complications.
Abbreviations
BMI: body mass index; CRP: C-reactive protein; CVD: cardiovascular disease; IL: interleukin; IR: insulin resistance; MCP-1: monocyte chemoattractant protein-1; MS: metabolic syndrome; RBP4: retinol binding protein 4; T2DM: type 2 diabetes mellitus; TNF: tumor necrosis factor; WAT: white adipose tissue.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Mello MM, Studdert DM, Brennan TA. Obesity- the new frontier of public health law. N Engl J Med. 2006;354:2601-10
2. Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world- a growing challenge. N Engl J Med. 2007;356:213-5
3. Wingard DL, Barrett-Connor E, Criqui MH, Suarez L. Clustering of heart disease risk factors in diabetic compared to nondiabetic adults. Am J Epidemiol. 1983;117:19-26
4. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595-607
5. Ferrannini E, Haffner SM, Mitchell BD, Stern MP. Hyperinsulinemia: the key feature of a cardiovascular and metabolic syndrome. Diabetologia. 1991;34:416-22
6. Weisberg SP, McCann D, Desai M. et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796-808
7. Xu H, Barnes GT, Yang Q. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821-30
8. Garg R, Tripathy D, Dandona P. Insulin resistance as a proinflammatory state: mechanisms, mediators, and therapeutic interventions. Curr Drug Targets. 2003;4:487-92
9. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548-56
10. Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939-49
11. Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev. 2006;27:762-78
12. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87-91
13. Zhang Y, Proenca R, Maffei M. et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425-32
14. Maeda K, Okubo K, Shimomura I. et al. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun. 1996;221:286-9
15. Arita Y, Kihara S, Ouchi N. et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophy Res Commun. 1999;257:79-83
16. Berg AH, Combs TP, Du X. et al. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947-53
17. Yamauchi T, Kamon J, Minokoshi Y. et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288-95
18. Okamoto Y, Kihara S, Ouchi N. et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767-70
19. Shimada K, Miyazaki T, Daida H. Adiponectin and atherosclerotic disease. Clin Chim Acta. 2004;344:1-12
20. Szmitko PE, Teoh H, Stewart DJ, Verma S. Adiponectin and cardiovascular disease. Am J Physiol Heart Circ Physiol. 2007;292:H1655-63
21. Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697-703
22. Hotta K, Funahashi T, Bodkin NL. et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50:1126-33
23. Yang WS, Lee WJ, Funahashi T. et al. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001;86:3815-9
24. Bruun JM, Lihn AS, Verdich C. et al. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocrinol Metab. 2003;285:E527-33
25. Cnop M, Havel PJ, Utzschneider KM. et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459-69
26. Kwon K, Jung SH, Choi C, Park SH. Reciprocal association between visceral obesity and adiponectin: in healthy premenopausal women. Int J Cardiol. 2005;101:385-90
27. Staiger H, Tschritter O, Machann J. et al. Relationship of serum adiponectin and leptin concentrations with body fat distribution in humans. Obes Res. 2003;11:368-72
28. Hotta K, Funahashi T, Arita Y. et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595-9
29. Stefan N, Vozarova B, Funahashi T. et al. Plasma adiponectin concentration is associated with skeletal muscle insulin receptor tyrosine phosphorylation, and low plasma concentration precedes a decrease in whole-body insulin sensitivity in humans. Diabetes. 2002;51:1884-8
30. Lindsay RS, Funahashi T, Hanson RL. et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57-8
31. Tschritter O, Fritsche A, Thamer C. et al. Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes. 2003;52:239-43
32. Baratta R, Amato S, Degano C. et al. Adiponectin relationship with lipid metabolism is independent of body fat mass: evidence from both cross-sectional and intervention studies. J Clin Endocrinol Metab. 2004;89:2665-71
33. Iwashima Y, Katsuya T, Ishikawa K. et al. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004;43:1318-23
34. Ouchi N, Ohishi M, Kihara S. et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003;42:231-4
35. Ouchi N, Kihara S, Funahashi T. et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671-4
36. Winer JC, Zern TL, Taksali SE. et al. Adiponectin in childhood and adolescent obesity and its association with inflammatory markers and components of the metabolic syndrome. J Clin Endocrinol Metab. 2006;91:4415-23
37. Ouchi N, Kihara S, Arita Y. et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473-6
38. Kumada M, Kihara S, Sumitsuji S. et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85-9
39. Pischon T, Girman CJ, Hotamisligil GS. et al. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730-7
40. Pilz S, Horejsi R, Moller R. et al. Early atherosclerosis in obese juveniles is associated with low serum levels of adiponectin. J Clin Endocrinol Metab. 2005;90:4792-6
41. von Eynatten M, Hamann A, Twardella D. et al. Atherogenic dyslipidaemia but not total- and high-molecular weight adiponectin are associated with the prognostic outcome in patients with coronary heart disease. Eur Heart J. 2008;29:1307-15
42. Kistorp C, Faber J, Galatius S. et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756-62
43. Lawlor DA, Davey Smith G, Ebrahim S. et al. Plasma adiponectin levels are associated with insulin resistance, but do not predict future risk of coronary heart disease in women. J Clin Endocrinol Metab. 2005;90:5677-83
44. Lindsay RS, Resnick HE, Zhu J. et al. Adiponectin and coronary heart disease: the Strong Heart Study. Arterioscler Thromb Vasc Biol. 2005;25:e15-6
45. Kanaya AM, Wassel Fyr C, Vittinghoff E. et al. Serum adiponectin and coronary heart disease risk in older Black and White Americans. J Clin Endocrinol Metab. 2006;91:5044-50
46. Cavusoglu E, Ruwende C, Chopra V. et al. Adiponectin is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction in patients presenting with chest pain. Eur Heart J. 2006;27:2300-9
47. Pilz S, Maerz W, Weihrauch G. et al. Adiponectin serum concentrations in men with coronary artery disease: the LUdwigshafen RIsk and Cardiovascular Health (LURIC) study. Clin Chim Acta. 2006;364:251-5
48. Laughlin GA, Barrett-Connor E, May S, Langenberg C. Association of adiponectin with coronary heart disease and mortality: the Rancho Bernardo study. Am J Epidemiol. 2007;165:164-74
49. Wannamethee SG, Whincup PH, Lennon L, Sattar N. Circulating adiponectin levels and mortality in elderly men with and without cardiovascular disease and heart failure. Arch Intern Med. 2007;167:1510-7
50. Pilz S, Mangge H, Wellnitz B. et al. Adiponectin and mortality in patients undergoing coronary angiography. J Clin Endocrinol Metab. 2006;91:4277-86
51. Dekker JM, Funahashi T, Nijpels G. et al. Prognostic value of adiponectin for cardiovascular disease and mortality. J Clin Endocrinol Metab. 2008;93:1489-96
52. Butte NF, Comuzzie AG, Cai G. et al. Genetic and environmental factors influencing fasting serum adiponectin in Hispanic children. J Clin Endocrinol Metab. 2005;90:4170-6
53. Ong KK, Frystyk J, Flyvbjerg A. et al. Sex-discordant associations with adiponectin levels and lipid profiles in children. Diabetes. 2006;55:1337-41
54. Isobe T, Saitoh S, Takagi S. et al. Influence of gender, age and renal function on plasma adiponectin level: the Tanno and Sobetsu study. Eur J Endocrinol. 2005;153:91-8
55. Aso Y, Yamamoto R, Wakabayashi S. et al. Comparison of serum high-molecular weight (HMW) adiponectin with total adiponectin concentrations in type 2 diabetic patients with coronary artery disease using a novel enzyme-linked immunosorbent assay to detect HMW adiponectin. Diabetes. 2006;55:1954-60
56. Bottner A, Kratzsch J, Muller G. et al. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab. 2004;89:4053-61
57. Tentolouris N, Doulgerakis D, Moyssakis I. et al. Plasma adiponectin concentrations in patients with chronic renal failure: relationship with metabolic risk factors and ischemic heart disease. Horm Metab Res. 2004;36:721-7
58. Senolt L, Pavelka K, Housa D, Haluzik M. Increased adiponectin is negatively linked to the local inflammatory process in patients with rheumatoid arthritis. Cytokine. 2006;35:247-52
59. Haugen F, Ranheim T, Harsem NK. et al. Increased plasma levels of adipokines in preeclampsia: relationship to placenta and adipose tissue gene expression. Am J Physiol Endocrinol Metab. 2006;290:E326-33
60. Shoji T, Shinohara K, Hatsuda S. et al. Altered relationship between body fat and plasma adiponectin in end-stage renal disease. Metabolism. 2005;54:330-4
61. Pajvani UB, Du X, Combs TP. et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073-85
62. Mangge H, Almer G, Haj-Yahya S. et al. Nuchal thickness of subcutaneous adipose tissue is tightly associated with an increased LMW/total adiponectin ratio in obese juveniles. Atherosclerosis. 2008 [Epub ahead of print]
63. Fisher FF, Trujillo ME, Hanif W. et al. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia. 2005;48:1084-7
64. Hara K, Horikoshi M, Yamauchi T. et al. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006;29:1357-62
65. Liu Y, Retnakaran R, Hanley A. et al. Total and high molecular weight but not trimeric or hexameric forms of adiponectin correlate with markers of the metabolic syndrome and liver injury in Thai subjects. J Clin Endocrinol Metab. 2007;92:4313-8
66. Yang Q, Graham TE, Mody N. et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356-62
67. Graham TE, Yang Q, Bluher M. et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552-63
68. Cho YM, Youn BS, Lee H. et al. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care. 2006;29:2457-61
69. Qi Q, Yu Z, Ye X. et al. Elevated retinol-binding protein 4 levels are associated with metabolic syndrome in Chinese people. J Clin Endocrinol Metab. 2007;92:4827-34
70. Lee JW, Im JA, Lee HR. et al. Visceral adiposity is associated with serum retinol binding protein-4 levels in healthy women. Obesity. 2007;15:2225-32
71. Jia W, Wu H, Bao Y. et al. Association of serum retinol-binding protein 4 and visceral adiposity in Chinese subjects with and without type 2 diabetes. J Clin Endocrinol Metab. 2007;92:3224-9
72. Janke J, Engeli S, Boschmann M. et al. Retinol-binding protein 4 in human obesity. Diabetes. 2006;55:2805-10
73. Yao-Borengasser A, Varma V, Bodles AM. et al. Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab. 2007;92:2590-7
74. Gavi S, Stuart LM, Kelly P. et al. Retinol-binding protein 4 is associated with insulin resistance and body fat distribution in nonobese subjects without type 2 diabetes. J Clin Endocrinol Metab. 2007;92:1886-90
75. Graham TE, Wason CJ, Bluher M, Kahn BB. Shortcomings in methodology complicate measurements of serum retinol binding protein (RBP4) in insulin-resistant human subjects. Diabetologia. 2007;50:814-23
76. Stefan N, Hennige AM, Staiger H. et al. High circulating retinol-binding protein 4 is associated with elevated liver fat but not with total, subcutaneous, visceral, or intramyocellular fat in humans. Diabetes Care. 2007;30:1173-8
77. Perseghin G, Lattuada G, De Cobelli F. et al. Serum retinol-binding protein-4, leptin, and adiponectin concentrations are related to ectopic fat accumulation. J Clin Endocrinol Metab. 2007;92:4883-8
78. Tsutsumi C, Okuno M, Tannous L. et al. Retinoids and retinoid-binding protein expression in rat adipocytes. J Biol Chem. 1992;267:1805-10
79. Steppan CM, Bailey ST, Bhat S. et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307-12
80. Savage DB, Sewter CP, Klenk ES. et al. Resistin/Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes. 2001;50:2199-202
81. Yannakoulia M, Yiannakouris N, Bluher S. et al. Body fat mass and macronutrient intake in relation to circulating soluble leptin receptor, free leptin index, adiponectin, and resitin concentrations in healthy humans. J Clin Endocrinol Metab. 2003;88:1730-6
82. Silha JV, Krsek M, Skrha JV. et al. Plasma resistin, adiponectin and leptin levels in lean and obese subjects: correlations with insulin resistance. Eur J Endocrinol. 2003;149:331-5
83. Zhang JL, Qin YW, Zheng X. et al. Serum resistin level in essential hypertension patients with different glucose tolerance. Diabet Med. 2003;20:828-31
84. Janke J, Engeli S, Gorzelniak K. et al. Resistin gene expression in human adipocytes is not related to insulin resistance. Obes Res. 2002;10:1-5
85. Fehmann H, Heyn J. Plasma resistin levels in patients with type 1 and type 2 diabetes mellitus and in healthy controls. Horm Metab Res. 2002;34:671-3
86. Shadid S, Stehouwer CD, Jensen MD. Diet/Exercise versus pioglitazone: effects of insulin sensitization with decreasing or increasing fat mass on adipokines and inflammatory markers. J Clin Endocrinol Metab. 2006;91:3418-25
87. Shetty GK, Economides PA, Horton ES. et al. Circulating adiponectin and resistin levels in relation to metabolic factors, inflammatory markers, and vascular reactivity in diabetic patients and subjects at risk for diabetes. Diabetes Care. 2004;27:2450-7
88. Pischon T, Bamberger CM, Kratzsch J. et al. Association of plasma resistin levels with coronary heart disease in women. Obes Res. 2005;13:1764-71
89. Senolt L, Housa D, Vernerova Z. et al. Resistin in rheumatoid arthritis synovial tissue, synovial fluid and serum. Ann Rheum Dis. 2007;66:458-63
90. Reilly MP, Lehrke M, Wolfe ML. et al. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111:932-9
91. Stejskal D, Adamovska S, Bartek J. et al. Resistin- concentrations in persons with type 2 diabetes mellitus and in individuals with acute inflammatory disease. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2003;147:63-9
92. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860-7
93. Cancello R, Henegar C, Viguerie N. et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277-86
94. Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241-8
95. Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interlukin-6 the link? Atherosclerosis. 2000;148:209-14
96. Pfeiffer A, Janott J, Mohlig M. et al. Circulating tumor necrosis factor alpha is elevated in male but not in female patients with type II diabetes mellitus. Horm Metab Res. 1997;29:111-4
97. Kellerer M, Rett K, Renn W. et al. Circulating TNF-alpha and leptin levels in offspring of NIDDM patients do not correlate to individual insulin sensitivity. Horm Metab Res. 1996;28:737-43
98. Kern PA, Ranganathan S, Li C. et al. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745-51
99. Mohamed-Ali V, Goodrick S, Rawesh A. et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196-200
100. Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: association with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972-8
101. Engelberts I, Stephens S, Francot GJ. et al. Evidence for different effects of soluble TNF-receptors on various TNF measurements in human biological fluids. Lancet. 1991;338:515-6
102. Vozarova B, Weyer C, Hanson K. et al. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes Res. 2001;9:414-7
103. Bastard JP, Jardel C, Bruckert E. et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab. 2000;85:3338-42
104. Esposito K, Pontillo A, Di Palo C. et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799-804
105. Kopp HP, Kopp CW, Festa A. et al. Impact of weight loss on inflammatory proteins and their association with the insulin resistance syndrome in morbidly obese patients. Arterioscler Thromb Vasc Biol. 2003;23:1042-7
106. Giugliano G, Nicoletti G, Grella E. et al. Effect of liposuction on insulin resistance and vascular inflammatory markers in obese women. Br J Plast Surg. 2004;57:190-4
107. Bermudez EA, Rifai N, Buring J. et al. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler Thromb Vasc Biol. 2002;22:1668-73
108. Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J. 2002;16:1335-47
109. Carey AL, Bruce CR, Sacchetti M. et al. Interleukin-6 and tumor necrosis factor-alpha are not increased in patients with Type 2 diabetes: evidence that plasma interleukin-6 is related to fat mass and not insulin responsiveness. Diabetologia. 2004;47:1029-37
110. Salmenniemi U, Ruotsalainen E, Pihlajamaki J. et al. Multiple abnormalities in glucose and energy metabolism and coordinated changes in levels of adiponectin, cytokines, and adhesion molecules in subjects with metabolic syndrome. Circulation. 2004;110:3842-8
111. Matsushita K, Yatsuya H, Tamakoshi K. et al. Comparison of circulating adiponectin and proinflammatory markers regarding their association with metabolic syndrome in Japanese men. Arterioscler Thromb Vasc Biol. 2006;26:871-6
112. Mortensen RF. C-reactive protein, inflammation, and innate immunity. Immunol Res. 2001;24:163-76
113. Calabro P, Chang DW, Willerson JT, Yeh ET. Release of C-reactive protein in response to inflammatory cytokines by human adipocytes: linking obesity to vascular inflammation. J Am Coll Cardiol. 2005;46:1112-3
114. Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14719 initially healthy American women. Circulation. 2003;107:391-7
115. Lemieux I, Pascot A, Prud'homme D. et al. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol. 2001;21:961-7
116. Freeman DJ, Norrie J, Caslake MJ. et al. C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes. 2002;51:1596-600
117. Heilbronn LK, Noakes M, Clifton PM. Energy restriction and weight loss on very-low-fat diets reduce C-reactive protein concentrations in obese, healthy women. Arteioscler Thromb Vasc Biol. 2001;21:968-70
118. Tchernof A, Nolan A, Sites CK. et al. Weight loss reduces C-reactive protein levels in obese postmenopausal women. Circulation. 2002;105:564-9
119. Gerhardt CC, Romero IA, Cancello R. et al. Chemokines control fat accumulation and leptin secretion by cultured human adipocytes. Mol Cell Endocrinol. 2001;175:81-92
120. Dietze-Schroeder D, Sell H, Uhlig M. et al. Autocrine action of adiponectin on human fat cells prevents the release of insulin resistance-inducing factors. Diabetes. 2005;54:2003-11
121. Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875-80
122. Christiansen T, Richelsen B, Bruun JM. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obes. 2005;29:146-50
123. Kim CS, Park HS, Kawada T. et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes. 2006;30:1347-55
124. Nomura S, Shouzu A, Omoto S. et al. Significance of chemokines and activated platelets in patients with diabetes. Clin Exp Immunol. 2000;121:437-43
125. Piemonti L, Calori G, Mercalli A. et al. Fasting plasma leptin, tumor necrosis factor-alpha receptor 2, and monocyte chemoattracting protein 1 concentration in a population of glucose-tolerant and glucose-intolerant women: impact on cardiovascular mortality. Diabetes Care. 2003;26:2883-9
126. Schernthaner GH, Kopp HP, Kriwanek S. et al. Effect of massive weight loss induced by bariatric surgery on serum levels of interleukin-18 and monocyte-chemoattractant-protein-1 in morbid obesity. Obes Surg. 2006;16:709-15
127. Dahlman I, Kaaman M, Olsson T. et al. A unique role of monocyte chemoattractant protein 1 among chemokines in adipose tissue of obese subjects. J Clin Endocrinol Metab. 2005;90:5834-40
128. Inadera H, Egashira K, Takemoto M. et al. Increase in circulating levels of monocyte chemoattractant protein-1 with aging. J Interferon Cytokine Res. 1999;19:1179-82
129. Parissis JT, Venetsanou KF, Kalantzi MV. et al. Serum profiles of granulocyte-macrophage colony-stimulating factor and C-C chemokines in hypertensive patients with or without significant hyperlipidemia. Am J Cardiol. 2000;85:777-9
130. Garlichs CD, John S, Schmeisser A. et al. Upregulation of CD40 and CD40 ligand (CD154) in patients with moderate hypercholesterolemia. Circulation. 2001;104:2395-400
131. de Lemos JA, Morrow DA, Sabatine MS. et al. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation. 2003;107:690-5
132. Papayianni A, Alexopoulos E, Giamalis P. et al. Circulating levels of ICAM-1, VCAM-1 and MCP-1 are increased in haemodialysis patients: association with inflammation, dyslipidaemia and vascular events. Nephrol Dial Transplant. 2002;17:435-41
133. Bruun JM, Pedersen SB, Richelsen B. Regulation of interleukin 8 production and gene expression in human adipose tissue in vitro. J Clin Endocrinol Metab. 2001;86:1267-73
134. Straczkowski M, Dzienis-Straczkowska S, Stepien A. et al. Plasma interleukin-8 concentrations are increased in obese subjects and related to fat mass and tumor necrosis factor-alpha system. J Clin Endocrinol Metab. 2002;87:4602-6
135. Herder C, Schneitler S, Rathmann W. et al. Low-grade inflammation, obesity, and insulin resistance in adolescents. J Clin Endocrinol Metab. 2007;92:4569-74
136. Alessi MC, Peiretti F, Morange P. et al. Production of plasminogen activator inhibitor 1 by human adipose tissue: possible link between visceral fat accumulation and vascular disease. Diabetes. 1997;46:860-7
137. Loskutoff DJ, Samad F. The adipocyte and hemostatic balance in obesity: studies of PAI-1. Arterioscler Thromb Vasc Biol. 1998;18:1-6
138. Skurk T, Hauner H. Obesity and impaired fibrinolysis: role of adipose production of plasminogen activator inhibitor-1. Int J Obes Relat Metab Disord. 2004;28:1357-64
139. McGill JB, Schneider DJ, Arfken CL. et al. Factors responsible for impaired fibrinolysis in obese subjects and NIDDM patients. Diabetes. 1994;43:104-9
140. Eriksson P, Reynisdottir S, Lonnqvist F. et al. Adipose tissue secretion of plasminogen activator inhibitor-1 in non-obese and obese individuals. Diabetologia. 1998;41:65-71
141. Giltay EJ, Elbers JM, Gooren LJ. et al. Visceral fat accumulation is an important determinant of PAI-1 levels in young, nonobese men and women: modulation by cross-sex hormone administration. Arterioscler Thromb Vasc Biol. 1998;18:1716-22
142. Mertens I, Van der Planken M, Corthouts B. et al. Visceral fat is a determinant of PAI-1 activity in diabetic and non-diabetic overweight and obese women. Horm Metab Res. 2001;33:602-7
143. Primrose JN, Davies JA, Prentice CR. et al. Reduction in factor VII, fibrinogen and plasminogen activator inhibitor-1 activity after surgical treatment of morbid obesity. Thromb Haemost. 1992;68:396-9
144. Hamsten A, Wiman B, de Faire U, Blomback M. Increased plasma levels of a rapid inhibitor of tissue plasminogen activator in young survivors of myocardial infarction. N Engl J Med. 1985;313:1557-63
145. Juhan-Vague I, Roul C, Alessi MC. et al. Increased plasminogen activator inhibitor activity in non insulin dependent diabetic patients- relationship with plasma insulin. Thromb Haemost. 1989;61:370-3
146. Miyazawa-Hoshimoto S, Takahashi K, Bujo H. et al. Elevated serum vascular endothelial growth factor is associated with visceral fat accumulation in human obese subjects. Diabetologia. 2003;46:1483-8
147. Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes. 2005;29:1308-14
148. Rehman J, Considine RV, Bovenkerk JE. et al. Obesity is associated with increased levels of circulating hepatocyte growth factor. J Am Coll Cardiol. 2003;41:1408-13
149. Bell LN, Ward JL, Degawa-Yamauchi M. et al. Adipose tissue production of hepatocyte growth factor contributes to elevated serum HGF in obesity. Am J Physiol Endocrinol Metab. 2006;291:E843-8
150. Taleb S, Lacasa D, Bastard JP. et al. Cathepsin S, a novel biomarker of adiposity: relevance to atherogenesis. FASEB J. 2005;19:1540-2
151. Liu J, Ma L, Yang J. et al. Increased serum cathepsin S in patients with atherosclerosis and diabetes. Atherosclerosis. 2006;186:411-9
152. Taleb S, Cancello R, Poitou C. et al. Weight loss reduces adipose tissue cathepsin S and its circulating levels in morbidly obese women. J Clin Endocrinol Metab. 2006;91:1042-7
153. Weyer C, Funahashi T, Tanaka S. et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resitance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930-5
154. Stefan N, Bunt JC, Salbe AD. et al. Plasma adiponectin concentrations in children: retationships with obesity and insulinemia. J Clin Endocrinol Metab. 2002;87:4652-6
155. Matsubara M, Maruoka S, Katayose S. Decreased plasma adiponectin concentrations in women with dyslipidemia. J Clin Endocrinol Metab. 2002;87:2764-9
156. Shimabukuro M, Higa N, Asahi T. et al. Hypoadiponectinemia is closely linked to endothelial dysfunction in man. J Clin Endocrinol Metab. 2003;88:3236-40
157. Daimon M, Oizumi T, Saitoh T. et al. Decreased serum levels of adiponectin are a risk factor for the progression to type 2 diabetes in the Japanese Population: the Funagata study. Diabetes Care. 2003;26:2015-20
158. Spranger J, Kroke A, Mohlig M. et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226-8
159. Snehalatha C, Mukesh B, Simon M. et al. Plasma adiponectin is an independent predictor of type 2 diabetes in Asian indians. Diabetes Care. 2003;26:3226-9
160. Duncan BB, Scmidt MI, Pankow JS. et al. Adiponectin and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2004;53:2473-8
161. Nakamura Y, Shimada K, Fukuda D. et al. Implications of plasma concentrations of adiponectin in patients with coronary artery disease. Heart. 2004;90:528-33
162. Ranheim T, Haugen F, Staff AC. et al. Adiponectin is reduced in gestational diabetes mellitus in normal weight women. Acta Obstet Gynecol Scand. 2004;83:341-7
163. Abbasi F, Chu JW, Lamendola C. et al. Discrimination between obesity and insulin resistance in the relationship with adiponectin. Diabetes. 2004;53:585-90
164. Xydakis AM, Case CC, Jones PH. et al. Adiponectin, inflammation, and the expression of the metabolic syndrome in obese individuals: the impact of rapid weight loss through caloric restriction. J Clin Endocrinol Metab. 2004;89:2697-703
165. Rothenbacher D, Brenner H, Marz W, Koenig W. Adiponectin, risk of coronary heart disease and correlations with cardiovascular risk markers. Eur Heart J. 2005;26:1640-6
166. Buemann B, Sorensen TI, Pedersen O. et al. Lower-body fat mass as an independent marker of insulin sensitivity - the role of adiponectin. Int J Obes. 2005;29:624-31
167. George J, Patal S, Wexler D. et al. Circulating adiponectin concentrations in patients with congestive heart failure. Heart. 2006;92:1420-4
168. Takashima N, Tomoike H, Iwai N. Retinol-binding protein 4 and insulin resistance. N Engl J Med. 2006;355:1392
169. Erikstrup C, Mortensen OH, Pedersen BK. Retinol-binding protein 4 and insulin resistance. N Engl J Med. 2006;355:1393-4
170. Balagopal P, Graham TE, Kahn BB. et al. Reduction of elevated serum retinol binding protein in obese children by lifestyle intervention: association with subclinical inflammation. J Clin Endocrinol Metab. 2007;92:1971-4
171. Haider DG, Schindler K, Prager G. et al. Serum retinol-binding protein 4 is reduced after weight loss in morbidly obese subjects. J Clin Endocrinol Metab. 2007;92:1168-71
172. Vitkova M, Klimcakova E, Kovacikova M. et al. Plasma levels and adipose tissue messenger ribonucleic acid expression of retinol-binding protein 4 are reduced during calorie restriction in obese subjects but are not related to diet-induced changes in insulin sensitivity. J Clin Endocrinol Metab. 2007;92:2330-5
173. Takebayashi K, Suetsugu M, Wakabayashi S. et al. Retinol binding protein-4 levels and clinical features of type 2 diabetes patients. J Clin Endocrinol Metab. 2007;92:2712-9
174. Tan BK, Chen J, Lehnert H. et al. Raised serum, adipocyte, and adipose tissue retinol-binding protein 4 in overweight women with polycystic ovary syndrome: effects of gonadal and adrenal steroids. J Clin Endocrinol Metab. 2007;92:2764-72
175. Broch M, Vendrell J, Ricart W. et al. Circulating retinol- binding protein-4, insulin sensitivity, insulin secretion, and insulin disposition index in obese and nonobese subjects. Diabetes Care. 2007;30:1802-6
176. Yagmur E, Weiskirchen R, Gressner AM. et al. Insulin resistance in liver cirrhosis is not associated with circulating retinol-binding protein 4. Diabetes Care. 2007;30:1168-72
177. von Eynatten M, Lepper PM, Liu D. et al. Retinol-binding protein 4 is associated with components of the metabolic syndrome, but not with insulin resistance, in men with type 2 diabetes or coronary artery disease. Diabetologia. 2007;50:1930-7
178. Azuma K, Katsukawa F, Oguchi S. et al. Correlation between serum resistin level and adiposity in obese individuals. Obes Res. 2003;11:997-1001
179. Degawa-Yamauchi M, Bovenkerk JE, Juliar BE. et al. Serum resistin (FIZZ3) protein is increased in obese humans. J Clin Endocrinol Metab. 2003;88:5452-5
180. Lee JH, Chan JL, Yiannakouris N. et al. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metab. 2003;88:4848-56
181. Pfutzner A, Langenfeld M, Kunt T. et al. Evaluation of human resistin assays with serum from patients with type 2 diabetes and different degrees of insulin resistance. Clin Lab. 2003;49:571-6
182. Monzillo LU, Hamdy O, Horton ES. et al. Effect of lifestyle modification on adipokine levels in obese subjects with insulin resistance. Obes Res. 2003;11:1048-54
183. Silha JV, Krsek M, Skrha J. et al. Plasma resistin, leptin and adiponectin levels in non-diabetic and diabetic obese subjects. Diabet Med. 2004;21:497-9
184. Heilbronn LK, Rood J, Janderova L. et al. Relationship between serum resistin concentrations and insulin resistance in nonobese, obese, and obese diabetic subjects. J Clin Endocrinol Metab. 2004;89:1844-8
185. Vozarova de Courten B, Degawa-Yamauchi M, Considine RV, Tataranni PA. High serum resistin is associated with an increase in adiposity but not a worsening of insulin resistance in Pima Indians. Diabetes. 2004;53:1279-84
186. Youn BS, Yu KY, Park HJ. et al. Plasma resistin concentrations measured by enzyme-linked immunosorbent assay using a newly developed monoclonal antibody are elevated in individuals with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2004;89:150-6
187. Fujinami A, Obayashi H, Ohta K. et al. Enzyme-linked immunosorbent assay for circulating human resistin: resistin concentrations in normal subjects and patients with type 2 diabetes. Clin Chim Acta. 2004;339:57-63
188. Bajaj M, Suraamornkul S, Hardies LJ. et al. Plasma resistin concentration, hepatic fat content, and hepatic and peripheral insulin resistance in pioglitazone-treated type II diabetic patients. Int J Obes Relat Metab Disord. 2004;28:783-9
189. Ohmori R, Momiyama Y, Kato R. et al. Associations between serum resistin levels and insulin resistance, inflammation, and coronary artery disease. J Am Coll Cardiol. 2005;46:379-80
190. Bo S, Gambino R, Pagani A. et al. Relationships between human serum resistin, inflammatory markers and insulin resistance. Int J Obes. 2005;29:1315-20
191. Katsuki A, Sumida Y, Murashima S. et al. Serum levels of tumor necrosis factor-alpha are increased in obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1998;83:859-62
192. Winkler G, Salamon F, Salamon D. et al. Elevated serum tumour necrosis factor-alpha levels can contribute to the insulin resistance in Type II (non-insulin-dependent) diabetes and in obesity. Diabetologia. 1998;41:860-1
193. Nilsson J, Jovinge S, Niemann A. et al. Relation between plasma tumor necrosis factor-alpha and insulin sensitivity in elderly men with non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1998;18:1199-202
194. Zinman B, Hanley AJ, Harris SB. et al. Circulating tumor necrosis factor-alpha concentrations in a native Canadian population with high rates of type 2 diabetes mellitus. J Clin Endocrinol Metab. 1999;84:272-8
195. Corica F, Allegra A, Corsonello A. et al. Relationship between plasma leptin levels and the tumor necrosis factor-alpha system in obese subjects. Int J Obes Relat Metab Disord. 1999;23:355-60
196. Winkler G, Lakatos P, Salamon F. et al. Elevated serum TNF-alpha level as a link between endothelial dysfunction and insulin resistance in normotensive obese patients. Diabet Med. 1999;16:207-11
197. Ziccardi P, Nappo F, Giugliano G. et al. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105:804-9
198. Maachi M, Pieroni L, Bruckert E. et al. Systemic low-grade inflammation is related to both circulating and adipose tissue TNFalpha, leptin and IL-6 levels in obese women. Int J Obes Relat Metab Disord. 2004;28:993-7
199. Marfella R, Esposito K, Siniscalchi M. et al. Effect of weight loss on cardiac synchronization and proinflammatory cytokines in premenopausal obese women. Diabetes Care. 2004;27:47-52
200. Hak AE, Stehouwer CD, Bots ML. et al. Associations of C-reactive protein with measures of obesity, insulin resistance, and subclinical atherosclerosis in healthy, middle-aged women. Arterioscler Thromb Vasc Biol. 1999;19:1986-91
201. Natali A, Toschi E, Baldeweg S. et al. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes. 2006;55:1133-40
202. Visser M, Bouter LM, McQuillan GM. et al. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131-5
203. McLaughlin T, Abbasi F, Lamendola C. et al. Differentiation between obesity and insulin resistance in the association with C-reactive protein. Circulation. 2002;106:2908-12
204. Escobar-Morreale HF, Villuendas G, Botella-Carretero JI. et al. Obesity, and not insulin resistance, is the major determinant of serum inflammatory cardiovascular risk markers in pre-menopausal women. Diabetologia. 2003;46:625-33
205. Moran LJ, Noakes M, Clifton PM. et al. C-reactive protein before and after weight loss in overweight women with and without polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:2944-51
Author contact
![]() Correspondence to: Hidekuni Inadera, MD and PhD, Department of Public Health, Faculty of Medicine, University of Toyama, 2630 Sugitani, Toyama 930-0194, Japan. Tel: +81 76 434 7275; Fax: +81 76 434 5023; E-mail: inaderau-toyama.ac.jp
Correspondence to: Hidekuni Inadera, MD and PhD, Department of Public Health, Faculty of Medicine, University of Toyama, 2630 Sugitani, Toyama 930-0194, Japan. Tel: +81 76 434 7275; Fax: +81 76 434 5023; E-mail: inaderau-toyama.ac.jp

 Global reach, higher impact
Global reach, higher impact