3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2008; 5(2):62-67. doi:10.7150/ijms.5.62 This issue Cite
Research Paper
A 12 Week, Open Label, Phase I/IIa Study Using Apatone® for the Treatment of Prostate Cancer Patients Who Have Failed Standard Therapy
1. Summa Health System, Department of Urology, Akron, Ohio, USA
2. William Beaumont Hospital, Department of Urology, Royal Oak, Michigan, USA
Received 2008-1-27; Accepted 2008-3-23; Published 2008-3-24
Abstract
Purpose: To evaluate the safety and efficacy of oral Apatone® (Vitamin C and Vitamin K3) administration in the treatment of prostate cancer in patients who failed standard therapy.
Materials and Methods: Seventeen patients with 2 successive rises in PSA after failure of standard local therapy were treated with (5,000 mg of VC and 50 mg of VK3 each day) for a period of 12 weeks. Prostate Specific Antigen (PSA) levels, PSA velocity (PSAV) and PSA doubling times (PSADT) were calculated before and during treatment at 6 week intervals. Following the initial 12 week trial, 15 of 17 patients opted to continue treatment for an additional period ranging from 6 to 24 months. PSA values were followed for these patients.
Results: At the conclusion of the 12 week treatment period, PSAV decreased and PSADT increased in 13 of 17 patients (p ≤ 0.05). There were no dose-limiting adverse effects. Of the 15 patients who continued on Apatone after 12 weeks, only 1 death occurred after 14 months of treatment.
Conclusion: Apatone showed promise in delaying biochemical progression in this group of end stage prostate cancer patients.
Keywords: Prostate, Prostate neoplasms, ascorbic acid, menadione, Vitamin K3, Apatone, Cancer
Introduction
The PSA era has led to a stage migration in the clinical course of prostate cancer. While this success has dramatically lowered the death rate from prostate cancer, it remains the most common cancer in men with 234,460 new cases and 27,350 deaths in the US in 2007.1 While hormonal therapy is typically initiated when the disease has advanced beyond local involvement and delays the time to PSA recurrence, it has not improved overall survival2 of patients with metastatic disease and has significant side effects.3 Newer chemotherapeutic regimens for metastatic prostate cancer show promise,4 but there are few therapy options for androgen independent prostate cancer (AIPC) patients. Therefore, there is a substantial need for new therapeutic options.
Because of their relatively low systemic toxicity, vitamin C (VC) and vitamin K3 (VK3), have been evaluated for their abilities to prevent and treat cancer.5 VC exhibited selective toxicity against a variety of malignant cell lines, prevented the induction of experimental tumors, acted as a chemosensitizer, and acted in vivo as a radiosensitizer. However, variable clinical results were obtained with VC because of the difficulty of attaining clinically active doses.6 VK3 exhibited selective antitumor activity alone and in conjunction with many chemotherapeutic agents in human cancer cell lines. However, while intravenous VK3 acted as a chemosensitizing and radiosensitizing agent in patients, 30% of the patients exhibited hematologic toxicity (at higher doses).7 When VC and VK3 were combined in a ratio of 100:1 (Apatone) and administered to human tumor cell lines, including androgen independent prostate cancer cells (DU145), they exhibited a synergistic inhibition of cell growth and induced cell death by apoptosis at concentrations that were 10 to 50 times lower than for the individual vitamins.8, 9, 10 In addition, oral Apatone significantly (P << 0.01) increased the mean survival time of nude mice inoculated i.p. with DU145 cells and significantly reduced the growth rate of solid tumors in nude mice (P < 0.05) without inducing any significant bone marrow toxicity, changes in organ weight or pathologic changes of these organs.9
The purpose of this study was to evaluate the safety and efficacy of oral Apatone administered throughout the day in prostate cancer in patients who failed standard therapy.
Materials and Methods
Patient selection
Prostate cancer patients who had failed standard therapy were enrolled at William Beaumont, Royal Oak, MI and Summa Health Systems, Akron, OH. Standard therapy was defined to include radical prostatectomy, radiotherapy and hormonal ablation. We did not include docetaxol chemotherapy in our inclusion criteria for failure of standard therapy. A patient was required to have a biopsy with proven prostate cancer and 2 successive rises in PSA to be included in the study. The patient could not be currently undergoing chemotherapy, radiotherapy, or androgen deprivation.
All patients exhibited acceptable renal function with blood urea nitrogen lower than 40 mg/dl and creatinine levels lower than 3 mg/dl and lacked clinical signs of obstructive liver disease as demonstrated by SGOT levels below 75 U/l; SGPT levels below 80 U/l and Alkaline Phosphatase levels below 200 U/l. TPatients using anticoagulants, chemotherapeutic agents, vitamin K, or vitamin C were excluded from the study. This protocol was reviewed and approved by the Institutional Review Board, and all patients provided their voluntary, written informed consent.
Evaluations
Each subject was interviewed by the study coordinator and examined by an urologist. Pretreatment evaluation included: a complete history and physical examination with a digital rectal examination for prostate configuration, size and symmetry; a medication audit; AUA Pain Scale and Symptom Score analysis; a complete blood count with differential, comprehensive chemistry panel including liver and renal panel, coagulation studies and a PSA test. We did not include standard bone scans or other radiographic studies as part of our study protocol. Patients were always seen by the study coordinator and the same examining urologist.
Treatment
All patients were treated with Vitamin C: K3 (5,000 mg. of VC and 50 mg. of VK3 each day, Apatone) for a total of 12 weeks. Apatone in capsular form (500 mg VC as ascorbate and 5mg VK3 as bisulfite) at a dose of 2 capsules on arising, then 1 capsule every two hours for six doses followed by two capsules at bedtime for a total of ten capsules per day. Following the 12 week study, two of the three “non-responders” in the study who had large body mass index values were given double the dose of Apatone by doubling the number of capsules in the previous regimen.
Analysis of PSA changes and Statistics
PSA velocity (PSAV) and doubling time (PSADT) were calculated using the Prostate Cancer Research Institute Algorithms.12 Successful outcome was considered a PSADT increase and a PSAV decrease. The binomial expansion was used to calculate the exact probability of the number of successful outcomes among the enrolled patients. A probability of p <0.05 was taken as indicative of an Apatone effect. Matched t-tests were employed to test for significant difference in PSA velocity and doubling times before and after treatment.13 Linear spline fit analysis was used to measure and compare PSA values before, during and after therapy.12
Results
Of thirty-three patients approached for participation, fourteen were not eligible; one withdrew; and one did not have two documented PSA values prior to enrollment. The characteristics of the remaining seventeen patients are detailed in Table 1. The median patient age was 71.5 (range 56 – 85 years), AUA performance status 6.5 (range 1 – 14) and median number of prior chemotherapy regimens was two.
Patient Characteristics
| N = 17 | |
|---|---|
| Age: median (range) | 74.5 (56 – 85) |
| AUA Symptom Score: median (range) | 6 (1 – 14) |
| Race: | |
| Caucasian | 15 |
| African American | 1 |
| Prior therapies (1 or more treatments): | |
| Hormonal | 10 |
| Radiation | 8 |
| Surgery | 17 |
| Chemotherapy: | |
| None | 0 |
| One | 0 |
| Two | 0 |
| Three or more | 1 |
Pre-treatment PSAV ranged from 1.05 to 696 ng/ml/year (median 21.6 ng/ml/yr), while in-trial PSAV ranged from -12 to 256 ng/ml/year (median 6.39 ng/ml/yr). Conversely, pre-treatment PSADT values ranged from 2.0 to 54.4 months (median 3.12 months), while in-trial PSADT values ranged from -39 to 57.1 months (median 7.88 months).
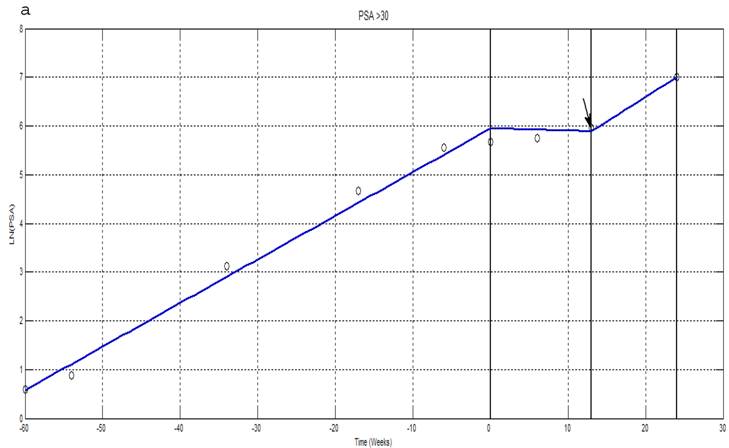
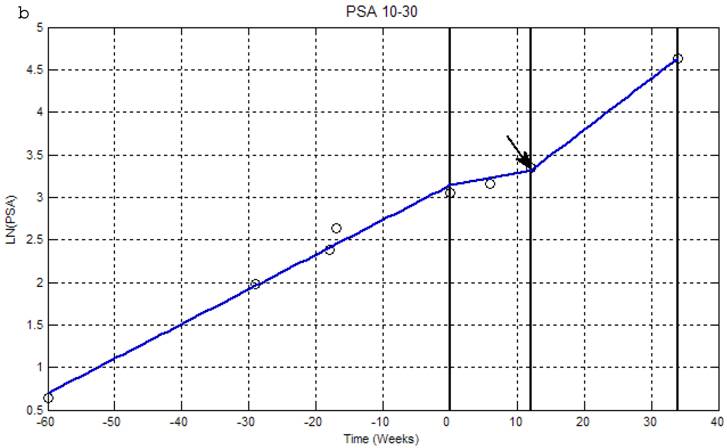
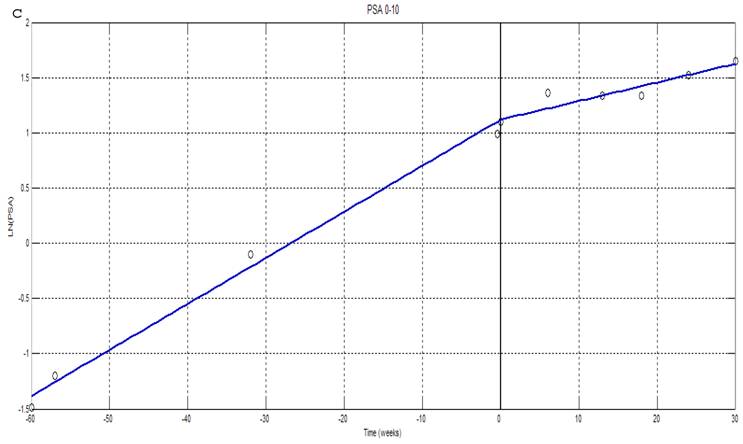
Linear spline fit analysis was performed using PSA levels before treatment, during treatment and following treatment (Figure 1). Representative curves are shown for a patient with a pre-treatment PSA > 30 ng/ml (Fig. 1a), a patient with 30 ng/ml > PSA > 10 ng/ml (Fig. 1b) and for a patient with a PSA < 10 ng/ml (Fig. 1c). In all 3 cases, the rate of PSA increase is significantly decreased during Apatone treatment, but increases at a rate similar to that seen before treatment once treatment ended (Fig. 1a and 1b). Thirteen of the 17 patients had a successful outcome; a decrease in PSAV and a lengthening of PSDT (Table 2). The probability of 13/17 successful outcomes is 0.008 suggesting the 76 % response we observed, was unlikely due to chance. The 3 “non-responders” each volunteered to have their dose of Apatone doubled following the trial. There were no adverse effects and two of these three patients subsequently had a decrease in PSA velocity and increase in PSA doubling time. No patient had a significant decrease in absolute PSA.
Natural Log transformations of PSA measurements for patients with PSA greater than 30ng/ml (a); between 10-30ng/ml (b) and less than 10ng/ml (c). Before and after treatment with Apatone plotted against time in weeks fitted with a linear spline with knots at -60 weeks, the start of Apatone therapy and end of therapy. (→) indicate where patients went off Apatone or started alternative therapy.
PSA Velocity and Doubling Time in Months
| Patient | PSA Velocity | PSA Doubling Time | ||||
|---|---|---|---|---|---|---|
| Pre-trial | In-trial | Change | Pre-trial | In-trial | Change | |
| 1 | 1.74 | - 12.0 | Decreased | 54.4 | - 5.86 | Increased |
| 2 | 26.1 | - 3.01 | Decreased | 2.51 | - 21.2 | Increased |
| 3 | 257 | 158 | Decreased | 3.00 | 6.30 | Increased |
| 4 | 14.6 | 9.14 | Decreased | 27.6 | 57.05 | Increased |
| 5 | 4.38 | 3.65 | Decreased | 2.76 | 9.17 | Increased |
| 6 | 19.3 | - 8.11 | Decreased | 12.1 | - 39.5 | Increased |
| 7 | 9.95 | 2.74 | Decreased | 2.72 | 13.7 | Increased |
| 8 | 1.05 | 0.00 | Decreased | 10.6 | > 60 | Increased |
| 9 | 696 | 256 | Decreased | 2.03 | 9.24 | Increased |
| 10 | 46.5 | 12.6 | Decreased | 3.23 | 20.4 | Increased |
| 11 | 0 | 0 | Unchanged | 0 | 0.00 | Unchanged |
| 12 | 2.09 | 0.00 | Decreased | 5.23 | > 60 | Increased |
| 13 | 352 | 163 | Decreased | 2.79 | 8.90 | Increased |
| 14 | 21.1 | 81.7 | Increased | 7.24 | 4.30 | Decreased |
| 15 | 54.2 | 112 | Increased | 2.91 | 2.88 | Decreased |
| 16 | 22.0 | 30.9 | Increased | 6.54 | 6.58 | Increased |
Following the 12 week trial, 15 of 17 patients opted to continue Apatone therapy. Any decision to remain on Apatone therapy was left entirely to the patient. Anecdotally, most patients reported feeling “better” and more “energetic.” This coupled with stabilization of rising PSA along with no significant side effects led the men to continue therapy. Four continued therapy for 6 months and 11 continued for at least 1 year with one patient continuing for more than 2 years. Therapy was not discontinued in any patient due to vitamin toxicity or for other safety reasons. The PSA values of these patients were checked at various intervals while on treatment and remained stable. Patients terminating Apatone therapy experienced sharp increases in PSA levels as seen in the linear spline fit analysis (Figure 1). Of the 11 patients on therapy for greater than 1 year, only one (initial PSA 256, PSADT= 3 months, and PSAV 157ng/ml/yr) passed away after 14 months.
No noteworthy changes were observed in the patient's complete blood counts, biochemistry panels or coagulation studies. No dose limiting toxicity or adverse events were experienced. Mild intermittent gastro-esophageal reflux symptoms was observed in 16 of 17 patients, but was eliminated when the Apatone was taken with meals or with antacids. The average AUA symptom score prior to beginning therapy was 7.9 (Table 3). This fell to 7.2 upon completion of the 12 week trial (P = .07). The average pain score based on the standard index was 3.2 initially, 2.3 at 6 weeks and returned to 3.2 at twelve weeks (Table 4).
AUA Symptom Scores
| AUA Score In Points | Number of Patients | Initial Visit | Six Week Visit | Twelve Week Visit |
|---|---|---|---|---|
| Mild (0-7) | 7 | 4.14 ± 0.40† | 4.27 ± 0.51 | 3.43 ± 0.53 |
| Moderate (8-19) | 9 | 10.9 ± 0.71 | 12.0 ± 0.21 | 10.0 ± 0.80 |
| Severe (20-35) | 0 | -- | -- | -- |
† = Data expressed as the mean ± standard error of the mean
Pain Scores
| AUA Pain Score In Points | Initial Visit | Six Week Visit | Twelve Week Visit |
|---|---|---|---|
| 3.19 ± 0.79† | 2.31 ± 0.66 | 3.19 ± 0.70 |
† = Data expressed as the mean ± standard error of the mean
Discussion
In a previously published, prospective, randomized trial, patients with pathologically proven prostate cancer in advanced stages (M1), osseous metastasis and resistance to hormone therapy were given two, 7 day courses of oral Apatone (VC at 5 g/m2/day and VK3 at 50 mg/m2/day), VC alone, VK3 alone, or a placebo.14 The 7day courses of treatment occurred during the first and fourth week of the study with two weeks of follow up after each treatment period. For the vitamin combination, homocysteine (a marker of tumor cell death induced by Apatone) assays showed an immediate and statistically significant drop (p<<0.01) in tumor cell numbers, while PSA serum levels rose in the two initial weeks and then fell to levels that were significantly different (p << 0.01) from the control group. For VC and VK3 alone, a non-significant difference was observed between the serum levels of homocysteine and PSA compared to the control group which suggested that the decreased PSA levels were due to tumor cell death.14 In this study, Apatone was administered daily in a single oral dose which was 2.5 to 3 times higher than the dose employed during the initial 12 weeks of our study. This dose resulted in a significant decrease in patient PSA levels which was ascribed to Apatone- induced tumor cell death by autoschizis. Conversely, the lower Apatone doses employed in the current study, led to increased PSADT without decreasing patient PSA levels.
In the previous study, Apatone was given in a single daily dose.14 However, Apatone was designed as an adjunctive therapy for existing treatment regimens with Apatone being administered intravenously in a bolus immediately prior to chemotherapy or radiotherapy and then in daily oral maintenance doses between therapies to prevent tumor growth following washout of the chemotherapeutic agent. In addition, pharmacokinetic studies indicated serum vitamin C levels returned to steady-state values within 5 to 6 hours of oral administration.15 For these reasons, Apatone was given every 5 to 6 hours in this study. During the 12 week course of the study, PSADT was the primary endpoint. Using this criterion, thirteen of 17 patients had significant increases in PSA doubling time. Following the initial 12 week trial, two of the three “non-responders” in the study who had large body mass index values were given increased Apatone doses adjusted to compensate for their elevated BMI values. Both patients subsequently became “responders”. In addition, 15 of 17 patients opted to continue Apatone therapy following the 12 week trial. The PSA values of these patients were checked at various intervals while on treatment and remained stable. Therapy was not discontinued in any patient due to vitamin toxicity or for other safety reasons.
PSADT has been useful in predicting treatment outcome before definitive therapy. For example, PSADT significantly correlated with biochemical recurrence16, linearly correlated with the interval to clinical relapse after PSA failure following radiation therapy for prostate cancer17, and was the most powerful indicator of disease activity in men under observation alone.18 When pretreatment variables in patients with androgen-independent prostate cancer were analyzed to determine the effect on PSA response after initiating maximum androgen blockade, increased PSADT was the only significant predictor of response.19 These results and others have led D'Amico to conclude that PSADT is sufficiently robust as a surrogate marker of prostate cancer survival to serve as a valid endpoint in trials of patients with hormone-refractory disease.17
More recently, PSADT has been used as an effective in vivo method for screening nontoxic agents, such as dihydroxyvitamin D3 (calcitriol), that increase PSADT without concomitantly decreasing PSA and yet become clinically valuable when used in combination with other anticancer agents.11 Our results demonstrate that oral Apatone significantly increased the PSADT of almost all the patients without concomitantly decreasing PSA, while co-administration of Apatone with known chemotherapeutic agents in other cancers resulted in a synergistic increase in antitumor activity.8,20 These results suggest that Apatone may find use in the clinic as a co-adjuvant therapy potentially in addition to docetaxol. Our decision not to include patients with alkaline phosphatase over 200 U/l may have excluded a number of men with osteoblastic bone lesions from metastases. This inherent selection bias does not allow us to examine the potential role of Apatone as salvage therapy, potentially after failure of docetaxol chemotherapy in hormone refractory patients.
Conclusions
Apatone is safe and effective with thirteen of the 17 prostate cancer patients having a statistically significant (P-value < 0.05) increase in PSADT and a decrease in PSADV after taking Apatone for 12 weeks. The long-term impact of Apatone on disease progression is unknown and remains to be demonstrated by further clinical study. Additional studies appear warranted for the use of Apatone as a co–adjuvant, or for emerging salvage chemotherapy in the treatment of late stage prostate cancer.
Acknowledgements
This research was supported by grants from The Beaumont Foundation, Royal Oak, Michigan, IC-MedTech, Inc, San Diego, California and The Summa Health System Foundation, Akron, Ohio.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
1. Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer Statistics, 2006. CA Cancer J Clin. 2006;56:106
2. Newling DW. Early versus late androgen deprivation therapy in metastatic disease. Urology. 2001;58:50
3. Higano C, Shields A, Wood N, Brown J, Tangen C. Bone mineral density in patients with prostate cancer without bony metastases treated with intermittent androgen suppression. Urology. 2004;64:1182
4. Petrylak DP, Tangen CM, Hussain MH, Lara PNJr, Jones JA, Taplin ME. et al. Docetaxel and estramusine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513
5. Lamm D, Riggs D, Shriver J, VanGilder P, Rach J, Dehaven J. Megadose Vitamins in Bladder Cancer: A Double-Blind Clinical Trial. J Urol. 1994;151:21
6. Gonzalez MJ, Miranda-Massari JR, Mora EM, Guzman A, Riordan NH, Riordan HD. et al. Orthomolecular oncology review: ascorbic acid and cancer 25 years later. Integr Cancer Ther. 2005;4:32
7. Lamson DW, Plaza SM. The anticancer effects of vitamin K. Altern Med Rev. 2003;8:303
8. De Loecker W, Janssens J, Bonte J, Taper HS. Effects of sodium ascorbate (vitamin C) and 2-methyl-1,4-naphthoquinone (vitamin K3) treatment on human tumor cell growth in vitro. II. Synergism with combined chemotherapy action. Anticancer Res. 1993;13:103
9. Jamison JM, Gilloteaux J, Taper HS, Buc Calderon P, Perlaky L, Thiry M. et al. The in vitro and in vivo antitumor activity of vitamin C: K3 combinations against prostate cancer. In: (ed.) Lucas JL. Trends in prostate cancer research. Hauppauge, NY: Nova Science Publishers. 2005:189-236
10. Gilloteaux J, Jamison JM, Neal DR, Summers JL. Cell death by autoschizis in TRAMP prostate carcinoma cells as a result of treatment by ascorbate: menadione combination. Ultrastruct Pathol. 2005;29:221
11. Guess BW, Scholz MC, Strum SB, Lam RY, Johnson HJ, Jennrich RI. Modified citrus pectin (MCP) increases the prostate-specific antigen doubling time in men with prostate cancer: a phase II pilot study. Prostate Cancer and Prostatic Dis. 2003;6:301
12. Sokal RR, Rohlf FJ. Introduction to biostatistics. San Francisco, CA: WH Freeman and Company. 1973
13. The MathWorks Inc. MATLAB®, Version 7.4, Spline Toolbox 3.3.2. Novi, MI: The MathWorks Inc. 2007
14. Lasalvia-Prisco E, Cucchi S, Vazquez J, Lasalvia-Galante E, Golomar W, Gordon W. Serum markers variation consistent with autoschizis induced by ascorbic acid-menadione in patients with prostate cancer. Med Oncol. 2003;20:45
15. Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A. et al. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann Int Med. 2004;140:533
16. D'Amico AV, Hanks GE. Linear regressive analysis using prostate-specific antigen doubling time for predicting tumor biology and clinical outcome in prostate cancer. Cancer. 1993;72:2638
17. D'Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen MH. Prostate specific antigen doubling time as a surrogate endpoint for prostate cancer specific mortality following radical prostatectomy or radiation therapy. J Urol. 2004;172:S42
18. McLaren DB, McKenzie M, Duncan G, Pickles T. Watchful waiting or watchful progression? Prostate specific antigen doubling times and clinical behavior in patients with early untreated prostate carcinoma. Cancer. 1998;82:342
19. Shulman MJ, Karam JA, Benaim EA. Prostate-specific antigen doubling time predicts response to deferred antiandrogen therapy in men with androgen-independent prostate cancer. Urology. 2004;63:732
20. Kassouf W, Highshaw R, Nelkin GM, Dinney CP, Kamat AM. Vitamins C and K3 sensitive human urothelial tumors to gemcitabine. J Urol. 2006;176:1642
Author contact
![]() Correspondence to: Basir Tareen, M.D., Department of Urology, New York University, 550 First Avenue, New York, NY 10016. tareennodak.edu
Correspondence to: Basir Tareen, M.D., Department of Urology, New York University, 550 First Avenue, New York, NY 10016. tareennodak.edu

 Global reach, higher impact
Global reach, higher impact